Abstract
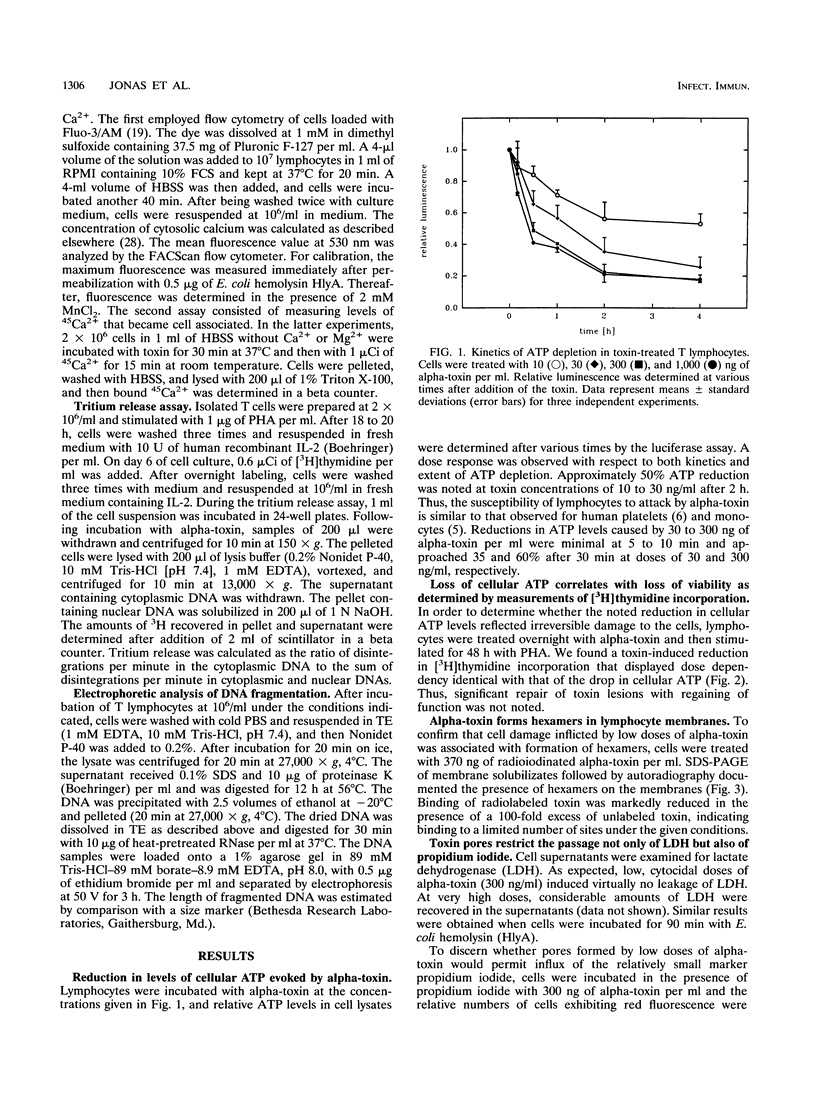
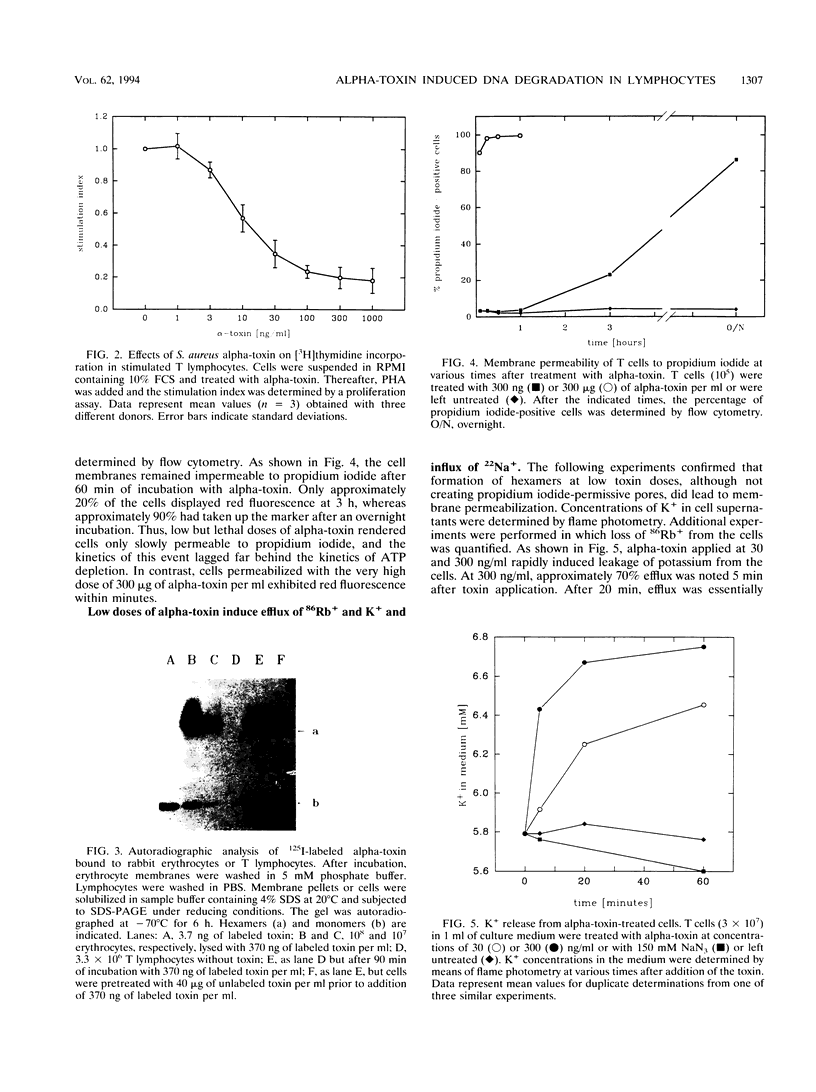
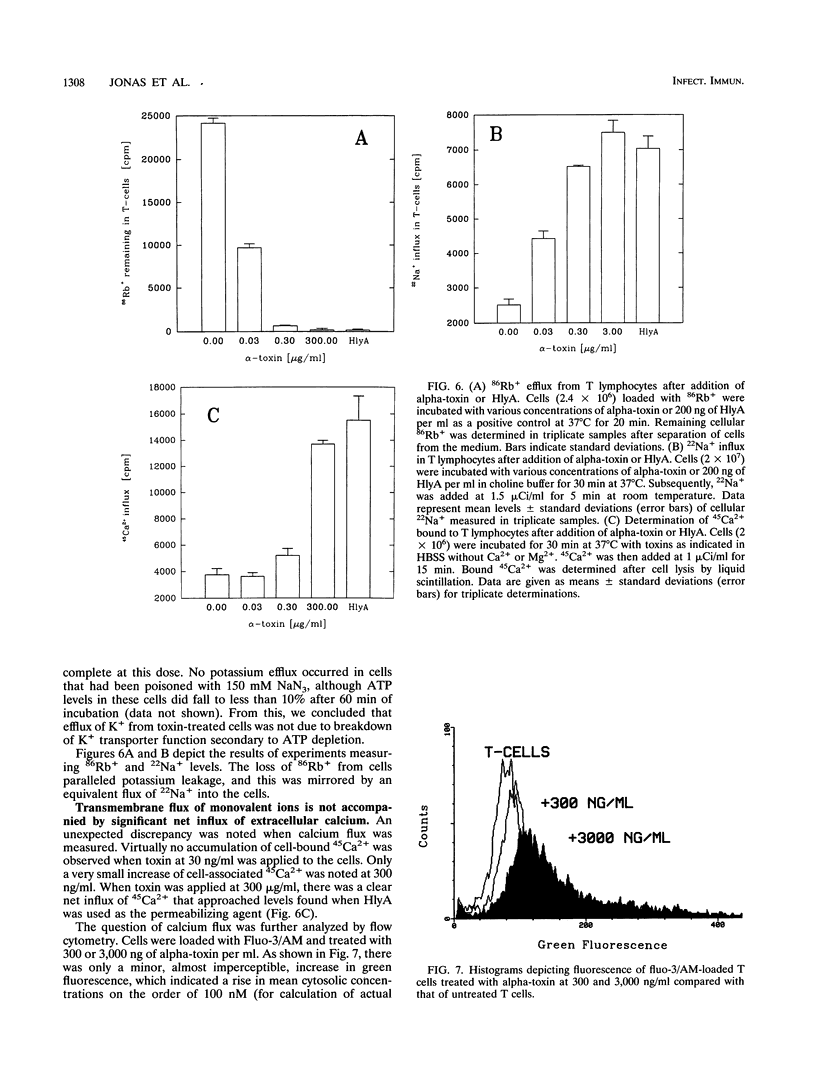
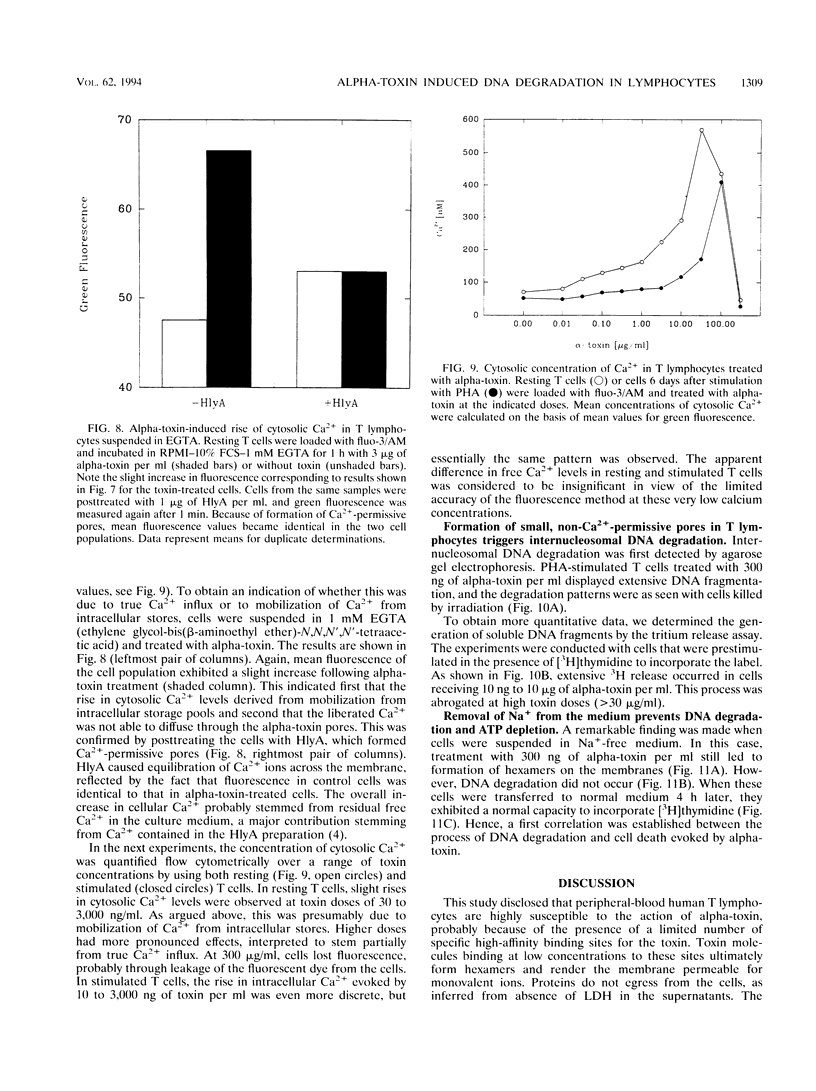
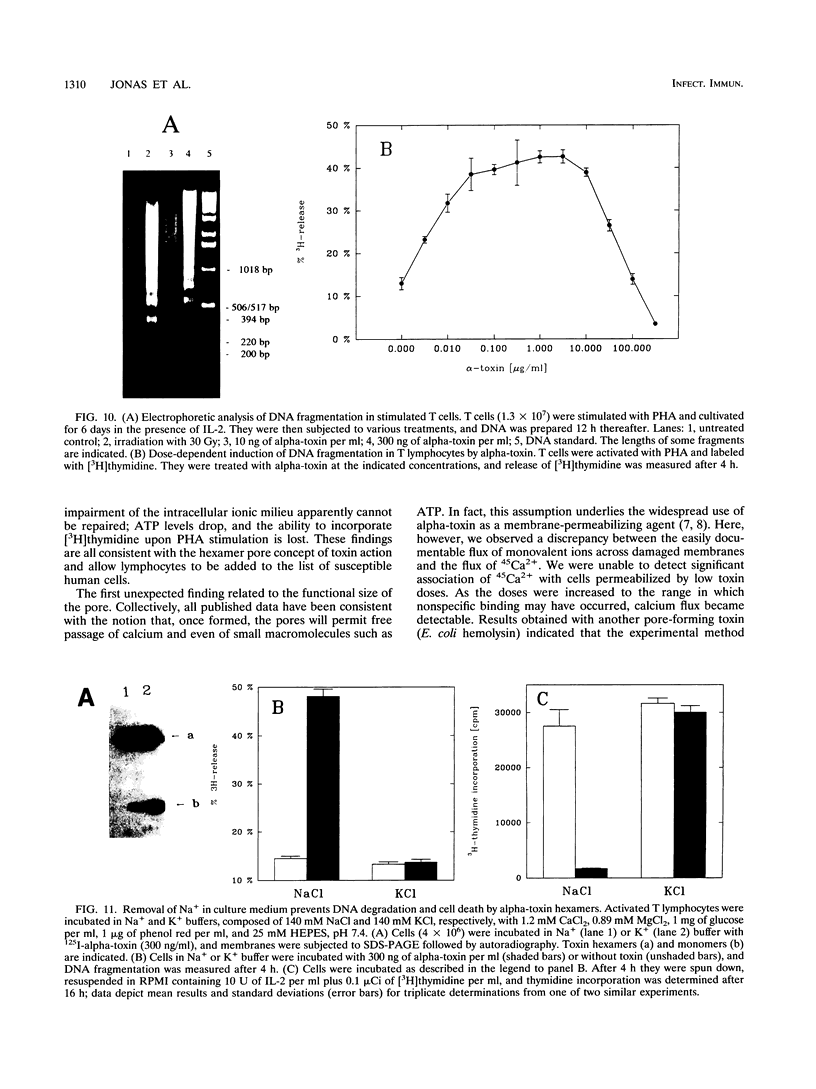
Peripheral-blood human T lymphocytes were treated with Staphylococcus aureus alpha-toxin. Membrane permeabilization was assessed by measuring efflux of K+ and Rb+ and influx of Na+, Ca2+, and propidium iodide. Cellular ATP and [3H]thymidine incorporation following lectin stimulation were measured as parameters for cell viability. Internucleosomal cleavage characteristic of programmed cell death was assessed by agarose gel electrophoresis and by quantifying low-molecular-weight, [3H]thymidine-labeled DNA fragments. Nanomolar concentrations of alpha-toxin evoked protracted, irreversible ATP depletion in both activated and resting T lymphocytes. Toxin-damaged cells also lost their ability to incorporate [3H]thymidine upon subsequent stimulation with phytohemagglutinin. These cells carried toxin hexamers, and their plasma membranes became permeable for monovalent ions but not for Ca2+ and propidium iodide. The permeabilization event was followed by internucleosomal DNA degradation characteristic of programmed cell death. Membranes of cells treated with high toxin doses (> 300 nM) became permeable to both Ca2+ and propidium iodide. In this case, ATP depletion occurred within minutes and no DNA degradation was observed. When cells were suspended in Na(+)-free buffer, alpha-toxin applied at low doses still bound and formed hexamers. However, these cells displayed neither DNA degradation nor loss of viability. The data indicate that formation of very small but not of large alpha-toxin pores may trigger programmed cell death in lymphocytes and that uncontrolled flux of Na+ ions may be an important event precipitating the suicide cascade.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnert-Hilger G., Bhakdi S., Gratzl M. Minimal requirements for exocytosis. A study using PC 12 cells permeabilized with staphylococcal alpha-toxin. J Biol Chem. 1985 Oct 15;260(23):12730–12734. [PubMed] [Google Scholar]
- Belmonte G., Cescatti L., Ferrari B., Nicolussi T., Ropele M., Menestrina G. Pore formation by Staphylococcus aureus alpha-toxin in lipid bilayers. Dependence upon temperature and toxin concentration. Eur Biophys J. 1987;14(6):349–358. doi: 10.1007/BF00262320. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Martin E. Superoxide generation by human neutrophils induced by low doses of Escherichia coli hemolysin. Infect Immun. 1991 Sep;59(9):2955–2962. doi: 10.1128/iai.59.9.2955-2962.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Muhly M., Korom S., Hugo F. Release of interleukin-1 beta associated with potent cytocidal action of staphylococcal alpha-toxin on human monocytes. Infect Immun. 1989 Nov;57(11):3512–3519. doi: 10.1128/iai.57.11.3512-3519.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Muhly M., Mannhardt U., Hugo F., Klapettek K., Mueller-Eckhardt C., Roka L. Staphylococcal alpha toxin promotes blood coagulation via attack on human platelets. J Exp Med. 1988 Aug 1;168(2):527–542. doi: 10.1084/jem.168.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991 Dec;55(4):733–751. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Weller U., Walev I., Martin E., Jonas D., Palmer M. A guide to the use of pore-forming toxins for controlled permeabilization of cell membranes. Med Microbiol Immunol. 1993 Sep;182(4):167–175. doi: 10.1007/BF00219946. [DOI] [PubMed] [Google Scholar]
- Cassidy P., Harshman S. Studies on the binding of staphylococcal 125I-labeled alpha-toxin to rabbit erythrocytes. Biochemistry. 1976 Jun 1;15(11):2348–2355. doi: 10.1021/bi00656a016. [DOI] [PubMed] [Google Scholar]
- Duke R. C., Persechini P. M., Chang S., Liu C. C., Cohen J. J., Young J. D. Purified perforin induces target cell lysis but not DNA fragmentation. J Exp Med. 1989 Oct 1;170(4):1451–1456. doi: 10.1084/jem.170.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Füssle R., Bhakdi S., Sziegoleit A., Tranum-Jensen J., Kranz T., Wellensiek H. J. On the mechanism of membrane damage by Staphylococcus aureus alpha-toxin. J Cell Biol. 1981 Oct;91(1):83–94. doi: 10.1083/jcb.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Clarke C. A., Dupre A., Rothstein A. Volume-induced increase of anion permeability in human lymphocytes. J Gen Physiol. 1982 Dec;80(6):801–823. doi: 10.1085/jgp.80.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Goetz J. D., Rothstein A. 22Na+ fluxes in thymic lymphocytes. I. Na+/Na+ and Na+/H+ exchange through an amiloride-insensitive pathway. J Gen Physiol. 1984 Oct;84(4):565–584. doi: 10.1085/jgp.84.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromkowski S. H., Brown T. C., Masson D., Tschopp J. Lack of DNA degradation in target cells lysed by granules derived from cytolytic T lymphocytes. J Immunol. 1988 Aug 1;141(3):774–778. [PubMed] [Google Scholar]
- Hameed A., Olsen K. J., Lee M. K., Lichtenheld M. G., Podack E. R. Cytolysis by Ca-permeable transmembrane channels. Pore formation causes extensive DNA degradation and cell lysis. J Exp Med. 1989 Mar 1;169(3):765–777. doi: 10.1084/jem.169.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand A., Pohl M., Bhakdi S. Staphylococcus aureus alpha-toxin. Dual mechanism of binding to target cells. J Biol Chem. 1991 Sep 15;266(26):17195–17200. [PubMed] [Google Scholar]
- Ikigai H., Nakae T. Interaction of the alpha-toxin of Staphylococcus aureus with the liposome membrane. J Biol Chem. 1987 Feb 15;262(5):2150–2155. [PubMed] [Google Scholar]
- Jonas D., Schultheis B., Klas C., Krammer P. H., Bhakdi S. Cytocidal effects of Escherichia coli hemolysin on human T lymphocytes. Infect Immun. 1993 May;61(5):1715–1721. doi: 10.1128/iai.61.5.1715-1721.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao J. P., Harootunian A. T., Tsien R. Y. Photochemically generated cytosolic calcium pulses and their detection by fluo-3. J Biol Chem. 1989 May 15;264(14):8179–8184. [PubMed] [Google Scholar]
- Menestrina G. Ionic channels formed by Staphylococcus aureus alpha-toxin: voltage-dependent inhibition by divalent and trivalent cations. J Membr Biol. 1986;90(2):177–190. doi: 10.1007/BF01869935. [DOI] [PubMed] [Google Scholar]
- Ojcius D. M., Zychlinsky A., Zheng L. M., Young J. D. Ionophore-induced apoptosis: role of DNA fragmentation and calcium fluxes. Exp Cell Res. 1991 Nov;197(1):43–49. doi: 10.1016/0014-4827(91)90477-c. [DOI] [PubMed] [Google Scholar]
- Palmer M., Jursch R., Weller U., Valeva A., Hilgert K., Kehoe M., Bhakdi S. Staphylococcus aureus alpha-toxin. Production of functionally intact, site-specifically modifiable protein by introduction of cysteine at positions 69, 130, and 186. J Biol Chem. 1993 Jun 5;268(16):11959–11962. [PubMed] [Google Scholar]
- Peitsch M. C., Polzar B., Stephan H., Crompton T., MacDonald H. R., Mannherz H. G., Tschopp J. Characterization of the endogenous deoxyribonuclease involved in nuclear DNA degradation during apoptosis (programmed cell death). EMBO J. 1993 Jan;12(1):371–377. doi: 10.1002/j.1460-2075.1993.tb05666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter C. Pro-oxidants and mitochondrial Ca2+: their relationship to apoptosis and oncogenesis. FEBS Lett. 1993 Jun 28;325(1-2):104–107. doi: 10.1016/0014-5793(93)81423-w. [DOI] [PubMed] [Google Scholar]
- Suttorp N., Seeger W., Dewein E., Bhakdi S., Roka L. Staphylococcal alpha-toxin-induced PGI2 production in endothelial cells: role of calcium. Am J Physiol. 1985 Jan;248(1 Pt 1):C127–C134. doi: 10.1152/ajpcell.1985.248.1.C127. [DOI] [PubMed] [Google Scholar]
- Suttorp N., Seeger W., Zucker-Reimann J., Roka L., Bhakdi S. Mechanism of leukotriene generation in polymorphonuclear leukocytes by staphylococcal alpha-toxin. Infect Immun. 1987 Jan;55(1):104–110. doi: 10.1128/iai.55.1.104-110.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobkes N., Wallace B. A., Bayley H. Secondary structure and assembly mechanism of an oligomeric channel protein. Biochemistry. 1985 Apr 9;24(8):1915–1920. doi: 10.1021/bi00329a017. [DOI] [PubMed] [Google Scholar]
- Vandenberghe P. A., Ceuppens J. L. Flow cytometric measurement of cytoplasmic free calcium in human peripheral blood T lymphocytes with fluo-3, a new fluorescent calcium indicator. J Immunol Methods. 1990 Mar 9;127(2):197–205. doi: 10.1016/0022-1759(90)90069-8. [DOI] [PubMed] [Google Scholar]
- Walev I., Martin E., Jonas D., Mohamadzadeh M., Müller-Klieser W., Kunz L., Bhakdi S. Staphylococcal alpha-toxin kills human keratinocytes by permeabilizing the plasma membrane for monovalent ions. Infect Immun. 1993 Dec;61(12):4972–4979. doi: 10.1128/iai.61.12.4972-4979.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker B., Krishnasastry M., Zorn L., Bayley H. Assembly of the oligomeric membrane pore formed by Staphylococcal alpha-hemolysin examined by truncation mutagenesis. J Biol Chem. 1992 Oct 25;267(30):21782–21786. [PubMed] [Google Scholar]