Abstract
In the Japanese quail, normally only males crow, but treatment of adult females with testosterone (T) facilitates the behavior. The sternotrachealis muscles are thought to adjust the length of the trachea during inspiration and/or expiration and control rigidity of the cartilages of the vocal organ (syrinx) during phonation. These muscles are heavier in males than females, and T increases their mass in females [1,2]. To investigate sex differences in morphology and potential effects of T in more detail, we examined several components of male, female, and T-treated female quail syrinx. No group effects were detected on overall tracheal size, size of the tracheal lumen, quantity of cartilage, overall muscle volume, or cross-sectional muscle area. However, the area and estimated volume of the muscles were greater on the right than left, due to increased fiber number. The similarity across groups suggests that if the sternotrachealis muscles are critical for crowing, morphology in females is sufficient, and the sex difference in behavior has another source. In contrast, these muscles may not play as large a role as was hypothesized. If the increased number of fibers on the right has a functional consequence, it likely reflects one similar in the two sexes, for example a common role in vocalizations they each produce – the male’s crow and the female’s cricket call.
Terms for Indexing: Sex difference, Testosterone, Asymmetry, Vocalization
1. Introduction
Avian vocalization starts in the brain, but requires the syrinx, a specialized structure located where the trachea bifurcates into the two primary bronchi [3–5]. The syrinx of Japanese quail consists of a series of ossified cartilages, both tracheal and bronchial, internal labia, and bilateral extrinsic syringeal muscles. For example, the sternotrachealis muscle (M. St.) attaches cranially to the lateral wall of the trachea and travels laterally to the sternum [5]. It has been hypothesized that this muscle could play an important role in the modifying the length of the trachea during inspiration and/or expiration, as well as controlling the rigidity of the syringeal cartilages during phonation [6–9].
In the Japanese quail (Coturnix japonica), males but not females crow [1,10]. The crow of a male quail consists of a few short introductory notes and a longer one that fluctuates in amplitude and frequency. This crow is accompanied by a synchronized postural display with the neck extended, followed by several patterned “head bobs” [11]. These behaviors attract females and thus aid in reproductive success [10].
While work has been conducted on a range of avian species [12], most research on syrinx structure and function has been conducted in songbirds (passerines). Sex differences exist in various aspects of their syrinxes. Male songbirds vocalize much more than females, and tracheal lumen size, overall mass, and size of muscle fibers are increased in adult males compared to females [13–16]. In addition, while variability can exist within a species and across types of sounds produced, some evidence exists for an average lateral asymmetry in the production of vocalizations. For example, in male canaries, the majority of song components are controlled by the left side of the syrinx [17], and in female canaries treated with testosterone (T), which facilitates their singing, a left dominance is displayed as well [18]. In the brown-headed cowbird, the right side produces higher frequency notes than the left [19]. Zebra finches show a right side dominance in muscle fiber size in both sexes [15]. To our knowledge, no lateralization has been documented for the Japanese quail. However, Balthazart el al. [1] reported that the M. St. of male quail are significantly heavier than those of females; no difference in weight was found between the left and right muscles.
The syrinxes and behavior of some female birds can be masculinized through treatment with T. For example, crowing and other male-typical behaviors can be facilitated by treating adult female Japanese quail with T [2,10–11]. This same manipulation increases syrinx muscle mass [2]. In zebra finches, adult T can increase the weight of the entire female syrinx, as well as the cross-sectional area of individual muscle fibers [13–15]. In the Grey partridge, T treatment during development yields larger tracheal and bronchial lumina, thicker external membranes, and a significantly greater number of syringeal muscle fibers [20].
The present study was designed to examine sex differences in and effects of adult T on Japanese quail in more detail by evaluating the morphology of a variety of syringeal and tracheal components.
2. Methods
2.1 Treatments
The animals in this study were sexually mature male and female Japanese quail that were raised, housed, and treated at Cornell University. All birds were housed separately in individual metal cages, and were kept under long days (16L: 8D). Animals were divided into three groups: Testosterone propionate (TP) implanted females (n=8), blank implanted females (controls; n=5), and males that received no implants (n=7). These birds were also used in Experiment 3 of Adkins-Regan and Leung [22]. Female quail in the experimental condition received two subcutaneous Silastic implants (25mm long, 1.6mm i.d. × 2.4mm o.d.) of TP placed under the skin where the neck meets the back. These implants reliably elevated T levels to those seen in males [22]. Each control female received an empty implant in the same location.
2.2 Tissue Collection and Histology
Tissue from each female was collected after 16 days of treatment, and males were collected at the same time. A single block of tissue was obtained from each bird that included the entire trachea, bronchi, syrinx, and the sternotrachealis muscles. Each block of tissue was rinsed several times in phosphate-buffered saline (PBS), and blood was cleared from the trachea using cold PBS. Tissue was placed in Bouin’s fixative for 7 days and then transferred into 70% ethanol for shipment from Cornell University to Michigan State University (MSU). Once tissue arrived at MSU, the portion of the throats containing the trachea, syrinx, and rostral end of the bronchi was dissected, dehydrated, cleared in xylene, and embedded in paraffin. Cross-sections of 20μm were obtained and stained with hemotoxylin and eosin.
2.3 Measurements and Analyses
By tracing every 20th section, reconstructions based upon an interval of 400μm were created through the use of Neurolucida (v. 6.02.1) and Neuroexplorer (v. 4.01.1; Microbrightfield Inc.). For each tracing, delineations were made of the inner and outer perimeters of the trachea, as well as the associated cartilages. These measurements allowed for the computation of volume estimates for the tracheal lumina (outer diameter volume minus inner diameter volume), as well as average area estimates of syringeal cartilage. Estimates of the volume of the M. St., average cross-sectional area of the muscle as a whole and of individual fibers, as well as fiber counts were made separately on the left and right sides. Each region of interest was outlined with a different marker permitting estimates to be computed individually. All sections for each reconstruction in which the left and right M. St. were running parallel to the trachea were recorded for each individual, and a series of common sections (total of 19 sections for each reconstruction) were used for all analyses. The tissue section used for M. St. fiber area estimates was located in the middle of these 19 sections, and the 13th traced section rostral to where the bronchi merge into the trachea was used for the muscle fiber area estimates (see figure 1).
Figure 1.
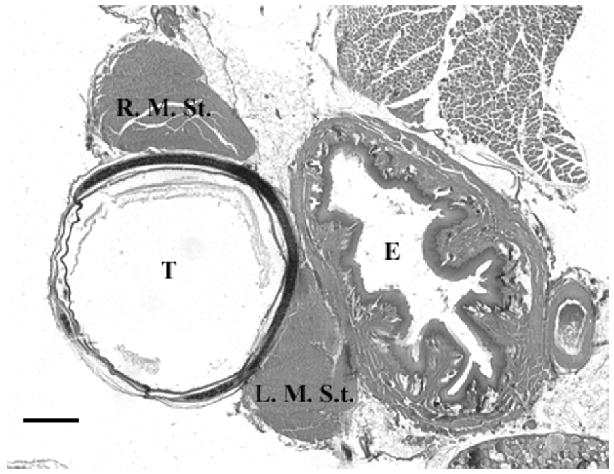
Cross-section through syrinx showing left and right sternotrachealis muscles (L. M. St. and R. M. St. respectively) in relation to esophagus (E) and trachea (T). Scale bar = 500μm.
Muscle fiber cross-sectional area estimates were computed with the fractionator probe in Stereo Investigator (v. 6.02.1; Microbrightfield Inc.). This method provided a means to randomly sample approximately 20 muscle fibers within each M. St. At each sampling site, the muscle fiber closest to the upper right corner of the counting frame was traced, and these measurements were analyzed in Neuroexplorer. Next, volume estimates were computed for each M. St. For each animal, a contour summary was performed in Neurolucida in which the areas of each respective muscle tracing are used to estimate a total volume for the entire muscle.
Finally, estimates of total fiber counts were computed for each M. St. A fractionator probe was used in Stereo Investigator to randomly sample each muscle in three rost-caudal locations. Fiber count estimates at each of these levels were then averaged separately for the left and right muscle.
2.4 Statistics
For M. St. analyses (volume as well as fiber size and number estimates) and estimated volumes of the trachea, its lumen and cartilage components, effects of group (between animals) and side (within each animal) were calculated with mixed model ANOVAs. All statistics were conducted by using Statview (SAS Institute, Cary, NC).
3. Results
No effects of group were detected for the estimates of tracheal volume (F(2,17) = 0.22, P = 0.80), size of the tracheal lumen (F(2,17) = 0.94, P = 0.41), or cartilage area (F(2,17) = 2.53, P = 0.11), or any muscle measure (all F(2,17) < 1.84, P > 0.189). However, estimates of volume and overall cross-sectional area of the M. St. were greater on the right than left (both F(1,17) > 9.60, P < 0.007; Fig. 2A & 2B). This effect was due to an increased number of fibers in the right muscle (F(1,17) = 15.55, P = 0.001; Fig. 2C), but not average fiber size (F(1,17) = 0.47, P = 0.5044; Fig. 2D). No significant interactions between group and side were observed for any of these measures (all F(2,17) < 1.43, P > 0.266).
Figure 2.
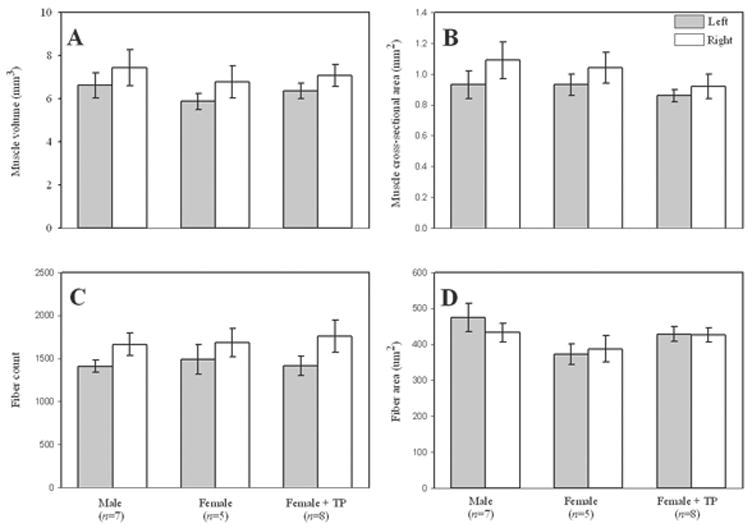
Left and right sternotrachealis muscle measures in male, female, and TP-treated Japanese quail (A = overall volume estimate, B = overall cross-sectional area, C = total fiber number, D = fiber cross-sectional area). With the exception of fiber area, each was greater on the right than left.
4. Discussion
Morphology of the M. St. is lateralized in Japanese quail. Estimates of volume and cross-sectional area were significantly increased on the right compared to the left side, and this difference was due to an increased number of fibers. Interestingly, an increased fiber number in the right vs. left M. St. was present in every animal in each group used in this study. No differences were found in other morphological measures, such as overall trachea size, size of the tracheal lumen or cartilage area.
These results are somewhat consistent with data from songbirds. For example, male and T-treated female canaries show a left dominance in the production of vocalizations and/or syrinx morphology, whereas the brown-headed cowbird and zebra finch show a right dominance [15, 17–19, 21]. However, the laterality in songbirds is generally seen in the intrinsic syringeal muscles, which Japanese quail do not posses. To our knowledge, the extrinsic muscles, including the M. St., have not been investigated in this way in songbirds.
Our data are not as consistent with the little work that has been done on this muscle in the Japanese quail. For example, Balthazart et al. [1] documented increased mass of the M. St. in males compared to females, and Schumacher and Balthazart [2] reported that castrated male and female quail treated with T yielded heavier M. St. than controls. We did not weigh the muscles and quantified morphology (e.g., overall volume, cross-sectional area, fiber size and number) using the majority of, but not the entire, extent. It is therefore possible that we did not capture the specific characteristic(s) responsible for the effects of sex and T on muscle mass. For example, the length of the muscle could have differed across the groups. While we cannot be sure of the reasons for the differences among studies, lack of effective T exposure is not one of them. That is, the individuals treated with T in the present study showed plasma levels equivalent to the males and had enlarged foam glands, which depends on androgen [22].
Taken together, these data indicate that the M. St. of Japanese quail could play a different role in aiding vocalization than has been previously suggested. Consistent with this idea, the M. St. of castrated males given 5α-dihydrotestosterone were as heavy as those of castrated males given T, yet their calls were weaker than those of T-males [2]. The authors suggest that the relationship between the size of this muscle and crowing may not be as strong as has been previously thought. It is possible that the greater number of fibers contained within the right muscle (and the resulting larger volume of the right muscle) may reflect some other behavior engaged in equally by the three groups of quail used in this study. In the chicken, EMG recordings of the M. St. show activity not only during vocalizations, but also during other times when a bird moves its head or neck [23]. It is possible that a behavior such as pecking or feeding may slightly favor the use of the right muscle, and this would explain the increase in fibers on this side.
An alternative explanation is that the M. St. may help modulate the volume of calls emitted by both sexes in the quail, and that as in songbirds, the influence of critical muscles is lateralized. Though untreated female quail do not crow, they do vocalize, including the production of “cricket calls”, which may serve the purpose of establishing contact with a mate [24]. It may be possible that these calls require the action of the M. St., although the role in female vocalizations is presently unclear. If this muscle is important for modulating the vocalizations of both sexes, then this may help to explain why a sex difference was not seen in any measure of these muscles. It is also possible that the laterality seen in the syringeal muscle morphology of male and female Japanese quail may be indicative of a right dominance in the production and/or projection of both male and female vocalizations. The right M. St. may adjust the rigidity of the right side of the syrinx, and in turn help modulate syringeal airflow, which would ultimately alter the volume of a given call. Alternatively, perhaps the left and right muscles attach differently to the sternum, and the increased fibers on the right side are required to equalize the force of the bilateral muscles at the level of the syrinx. Future studies should attempt to identify the role of M. St. in facilitating both male and female vocalizations, as well as investigate the possible role of the right side of the syrinx in modulating the overall volume of these phonations.
Acknowledgments
We thank Jennifer Stynoski and Casey Bartrem for help with sectioning and staining of tissue, and John Morris assistance with Microbrightfield software and subsequent analyses. This work was supported by the grants of Juli Wade (NIH grants MH55488 and MH065907) and Elizabeth Adkins-Regan (NSF grant IBN-0130986).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Balthazart J, Schumacher M, Otttinger MA. Sexual differences in the japanese quail: Behavior, morphology, and intracellular metabolism of testosterone. Gen Comp Endocrinol. 1983;51:191–207. doi: 10.1016/0016-6480(83)90072-2. [DOI] [PubMed] [Google Scholar]
- 2.Schumacher M, Balthazart J. The effects of testosterone and its metabolites on sexual behavior and morphology in male and female japanese quail. Physiol Behav. 1983;30:335–339. doi: 10.1016/0031-9384(83)90135-x. [DOI] [PubMed] [Google Scholar]
- 3.Goller F, Larsen ON. New perspectives on mechanisms of sound generation in songbirds. J Comp Physiol A. 2002;188:841–850. doi: 10.1007/s00359-002-0350-6. [DOI] [PubMed] [Google Scholar]
- 4.King AS, McLelland J. Birds: Their structure and function. London: Bailliere Tindall; 1984. Respiratory system, chapter 7; pp. 110–144. [Google Scholar]
- 5.King AS, McLelland J. Functional anatomy of the syrinx. In: King AS, McLelland J, editors. Form and function in birds. London: Academic Press; 1989. pp. 105–192. [Google Scholar]
- 6.Larsen ON, Goller F. Direct observation of syringeal muscle function in songbirds and a parrot. J Exp Biol. 2002;205:25–35. doi: 10.1242/jeb.205.1.25. [DOI] [PubMed] [Google Scholar]
- 7.Daley M, Goller F. Tracheal length changes during zebra finch song and their possible role in upper vocal tract filtering. J Neurobiol. 2003;59:319–330. doi: 10.1002/neu.10332. [DOI] [PubMed] [Google Scholar]
- 8.Goller F, Suthers RA. Role of syringeal muscles in gating airflow and sound production in singing brown thrashers. J Neurophysiol. 1996;75:867–876. doi: 10.1152/jn.1996.75.2.867. [DOI] [PubMed] [Google Scholar]
- 9.Fitch WT. Acoustic exaggeration of size in birds via tracheal elongation: Comparative and theoretical analyses. J Zool. 1999;248:31–48. [Google Scholar]
- 10.Balthazart J, Adkins-Regan E. Sexual differentiation of brain and behavior in birds. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, brain and behavior. Vol. 4 San Diego: Academic Press; 2002. pp. 223–301. [Google Scholar]
- 11.Shaw BK. Involvement of a midbrain vocal nucleus in the production of both the acoustic and postural components of crowing behavior in japanese quail. J Comp Physiol A. 2000;186:747–757. doi: 10.1007/s003590000128. [DOI] [PubMed] [Google Scholar]
- 12.Suthers RA, Zollinger SA. Producing song: The vocal apparatus. Ann NY Acad Sci. 2004;1016:109–129. doi: 10.1196/annals.1298.041. [DOI] [PubMed] [Google Scholar]
- 13.Springer ML, Wade J. The effects of testicular tissue and prehatching inhibition of estrogen synthesis on the development of courtship and copulatory behavior in zebra finches. Horm Behav. 1997;32:46–59. doi: 10.1006/hbeh.1997.1406. [DOI] [PubMed] [Google Scholar]
- 14.Arnold AP. Sexual differentiation of the zebra finch song system: Positive evidence, negative evidence, null hypotheses, and a paradigm shift. J Neurobiol. 1997;33:572–584. [PubMed] [Google Scholar]
- 15.Wade J, Buhlman L. Lateralization and effects of adult androgen in a sexually dimorphic neuromuscular system controlling song in zebra finches. J Comp Neurol. 2000;426:154–164. [PubMed] [Google Scholar]
- 16.Bleisch W, Luine VN, Nottebohm F. Modification of synapses in androgen-sensitive muscle. Hormonal regulation of acetylcholine receptor number in the songbird syrinx. J Neurosci. 1983;4:786–792. doi: 10.1523/JNEUROSCI.04-03-00786.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, serinus canarius. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- 18.Hartley RS, Chinn MS, Ullrich NFE. Left syringeal dominance in testosterone-treated female canaries. Neurobiol Learn Mem. 1997;67:248–253. doi: 10.1006/nlme.1996.3759. [DOI] [PubMed] [Google Scholar]
- 19.Allan SE, Suthers RA. Lateralization and motor stereotypy of song production in the brown-headed cowbird. J Neurobiol. 1994;25:1154–1166. doi: 10.1002/neu.480250910. [DOI] [PubMed] [Google Scholar]
- 20.Beani L, Panzica G, Briganti F, Persichella P, Dessi-Fulgheri F. Testosterone-induced changes of call structure, midbrain and syrinx anatomy in partridges. Physiol Behav. 1995;58:1149–1157. doi: 10.1016/0031-9384(95)02060-8. [DOI] [PubMed] [Google Scholar]
- 21.Luine V, Nottebohm F, Harding C, McEwen BS. Androgen affects cholinergic enzymes in syringeal motor neurons and muscle. Brain Res. 1980;192:89–107. doi: 10.1016/0006-8993(80)91011-2. [DOI] [PubMed] [Google Scholar]
- 22.Adkins-Regan E, Leung CH. Hormonal and social modulation of cloacal muscle activity in female japanese quail. Physiol Behav. 2006;87:82–87. doi: 10.1016/j.physbeh.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 23.Gaunt AS, Gaunt LL. Mechanics of the syrinx in gallus gallus. II. Electromyographic studies of ad libitum vocalizations. J Morphol. 1977;152:1–19. doi: 10.1002/jmor.1051520102. [DOI] [PubMed] [Google Scholar]
- 24.Mills AD, Crawford LL, Domjan M, Faure JM. The behavior of the Japanese or domestic quail Coturnix japonica. Neurosci Biobehav Rev. 1997;21:261–281. doi: 10.1016/s0149-7634(96)00028-0. [DOI] [PubMed] [Google Scholar]


