Abstract
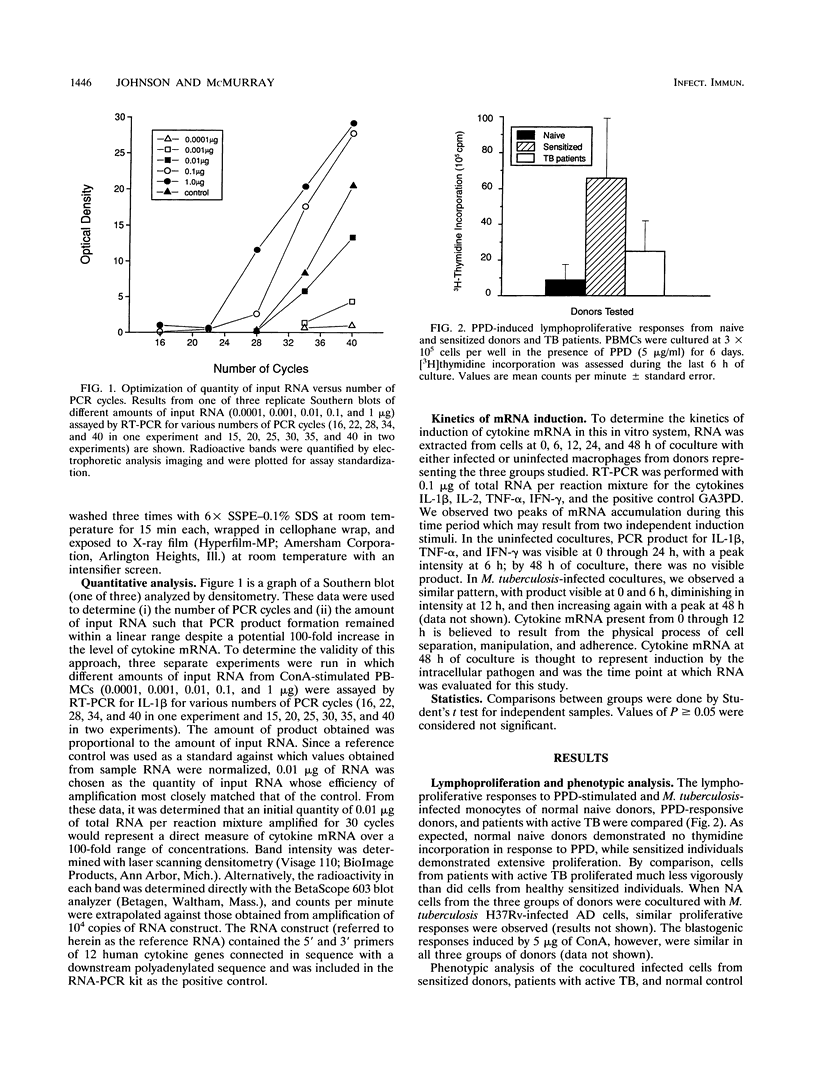
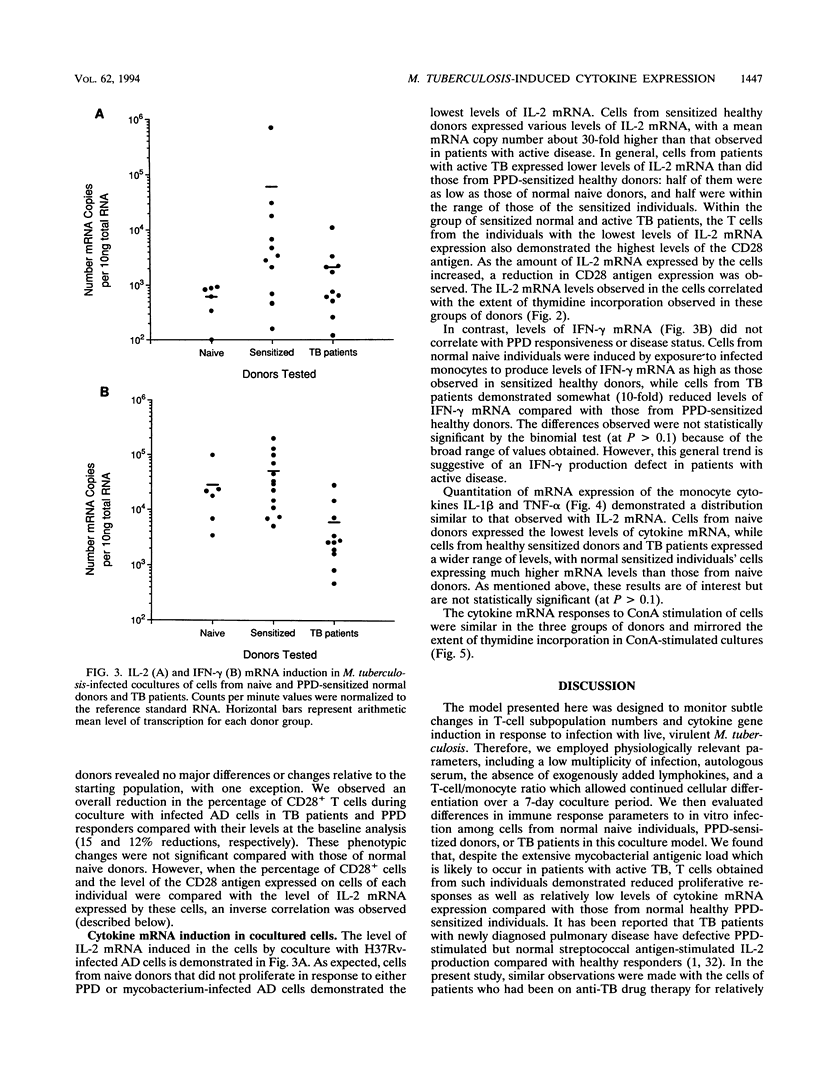
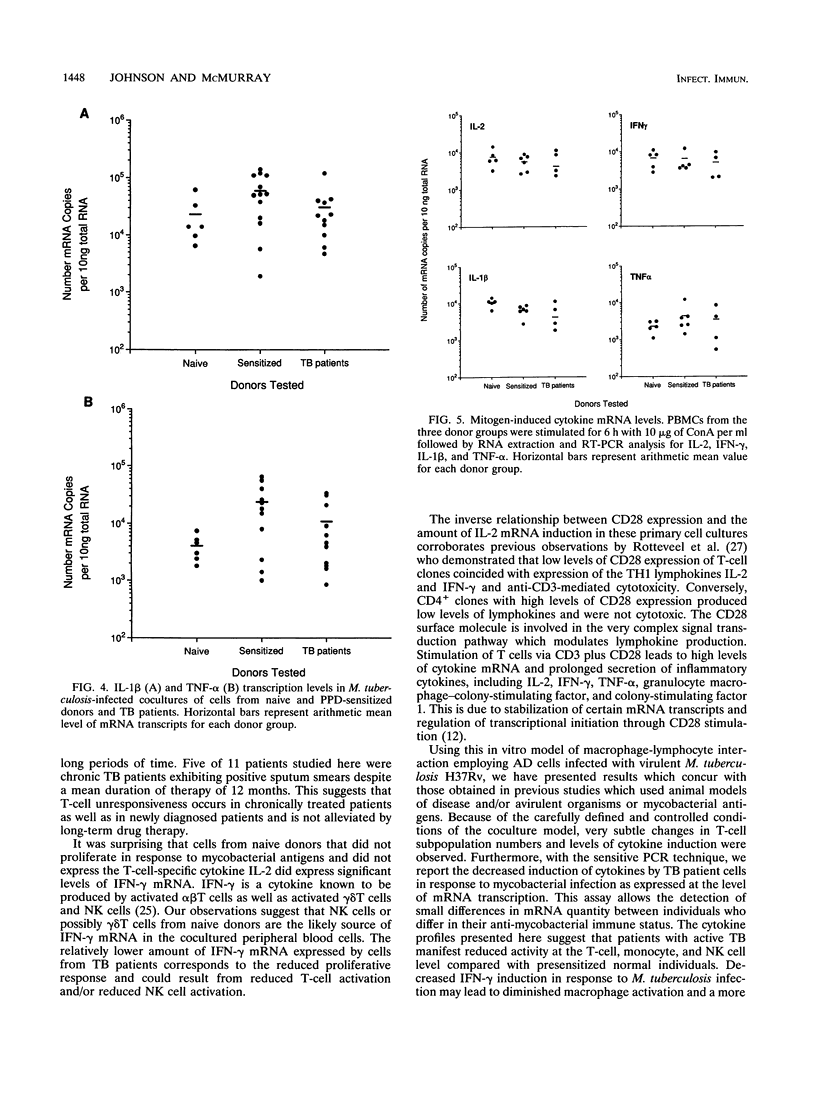
In order to better understand the immunoregulation following Mycobacterium tuberculosis infection, cytokine mRNA induction in response to in vitro infection of human monocytes with live virulent M. tuberculosis H37Rv cocultured with autologous lymphocytes was quantitated by reverse transcriptase-PCR. Induced levels of interleukin 1 beta (IL-1 beta), IL-2, tumor necrosis factor alpha, and gamma interferon (IFN-gamma) were compared among groups of individuals representing three phases of immunity to infection with M. tuberculosis: naive normal control subjects, purified protein derivative (PPD)-reactive normal donors, and individuals with active tuberculosis (TB [diseased]). Levels of IL-1 beta and tumor necrosis factor alpha mRNA in cocultured cells from TB patients were 51 and 45%, respectively, of those obtained in cells from sensitized healthy volunteers and were comparable to those from naive normal donors. Lymphoproliferative responses to M. tuberculosis and induction of the T-cell cytokine IL-2 were predictably high in the cells of PPD-sensitized donors, low in normal naive individuals, and variable among TB patients. In contrast, the induced level of another lymphokine, IFN-gamma, did not follow the pattern seen in IL-2 induction. Infection with live M. tuberculosis induced high levels of IFN-gamma mRNA in lymphocytes of both PPD-sensitized and normal naive donors compared with those of TB patients. Interestingly, polyclonal stimulation with the mitogen concanavalin A induced similar IFN-gamma levels in cells from all three donor groups. The high level of IFN-gamma induced by the infection of monocytes from naive normal donors suggests a role for natural killer (NK) cells in the production of IFN-gamma in this coculture system. This response appears independent of the role performed by T cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade-Arzabe R., Machado I. V., Fernandez B., Blanca I., Ramirez R., Bianco N. E. Cellular immunity in current active pulmonary tuberculosis. Am Rev Respir Dis. 1991 Mar;143(3):496–500. doi: 10.1164/ajrccm/143.3.496. [DOI] [PubMed] [Google Scholar]
- Baldari C., Murray J. A., Ghiara P., Cesareni G., Galeotti C. L. A novel leader peptide which allows efficient secretion of a fragment of human interleukin 1 beta in Saccharomyces cerevisiae. EMBO J. 1987 Jan;6(1):229–234. doi: 10.1002/j.1460-2075.1987.tb04743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Mycobacterial disease, immunosuppression, and acquired immunodeficiency syndrome. Clin Microbiol Rev. 1989 Oct;2(4):360–377. doi: 10.1128/cmr.2.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch I. E., Kaufmann S. H. Mechanisms involved in mycobacterial growth inhibition by gamma interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect Immun. 1991 Sep;59(9):3213–3218. doi: 10.1128/iai.59.9.3213-3218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H., Kleinhenz M. E., Wallis R. S., Ellner J. J. Increased interleukin-1 production and monocyte suppressor cell activity associated with human tuberculosis. Am Rev Respir Dis. 1986 Jan;133(1):73–77. doi: 10.1164/arrd.1986.133.1.73. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Kretschmer C., Zahn G., Ernst M., Jones D. B., Flad H. D. Immunoenzymatic assessment of interferon-gamma in Hodgkin and Sternberg-Reed cells. Cytokine. 1990 Jul;2(4):307–310. doi: 10.1016/1043-4666(90)90033-p. [DOI] [PubMed] [Google Scholar]
- Huygen K., Van Vooren J. P., Turneer M., Bosmans R., Dierckx P., De Bruyn J. Specific lymphoproliferation, gamma interferon production, and serum immunoglobulin G directed against a purified 32 kDa mycobacterial protein antigen (P32) in patients with active tuberculosis. Scand J Immunol. 1988 Feb;27(2):187–194. doi: 10.1111/j.1365-3083.1988.tb02338.x. [DOI] [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Linsley P. S., Thompson C. B. Role of the CD28 receptor in T-cell activation. Immunol Today. 1990 Jun;11(6):211–216. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- Kaplan G., Britton W. J., Hancock G. E., Theuvenet W. J., Smith K. A., Job C. K., Roche P. W., Molloy A., Burkhardt R., Barker J. The systemic influence of recombinant interleukin 2 on the manifestations of lepromatous leprosy. J Exp Med. 1991 Apr 1;173(4):993–1006. doi: 10.1084/jem.173.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Flesch I. E. Antimycobacterial functions in bone-marrow-derived macrophages. Res Microbiol. 1990 Feb;141(2):244–248. doi: 10.1016/0923-2508(90)90038-r. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Flesch I. E. The role of T cell--macrophage interactions in tuberculosis. Springer Semin Immunopathol. 1988;10(4):337–358. doi: 10.1007/BF02053845. [DOI] [PubMed] [Google Scholar]
- Kunkel S. L., Chensue S. W., Strieter R. M., Lynch J. P., Remick D. G. Cellular and molecular aspects of granulomatous inflammation. Am J Respir Cell Mol Biol. 1989 Dec;1(6):439–447. doi: 10.1165/ajrcmb/1.6.439. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Dejana E. Cytokines as communication signals between leukocytes and endothelial cells. Immunol Today. 1989 Nov;10(11):370–375. doi: 10.1016/0167-5699(89)90270-3. [DOI] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Muñoz-Fernández M. A., Pimentel-Muiños F. X., Alonso M. A., Campanero M., Sánchez-Madrid F., Silva A., Alonso J. L., Fresno M. Synergy of tumor necrosis factor with protein kinase C activators on T cell activation. Eur J Immunol. 1990 Mar;20(3):605–610. doi: 10.1002/eji.1830200321. [DOI] [PubMed] [Google Scholar]
- Nishi T., Fujita T., Nishi-Takaoka C., Saito A., Matsumoto T., Sato M., Oka T., Itoh S., Yip Y. K., Vilcek J. Cloning and expression of a novel variant of human interferon-gamma cDNA. J Biochem. 1985 Jan;97(1):153–159. doi: 10.1093/oxfordjournals.jbchem.a135039. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Uchida H., Kusumoto Y., Mori Y., Yamamura Y., Hamada S. Increase in tumor necrosis factor alpha- and interleukin-6-secreting cells in peripheral blood mononuclear cells from subjects infected with Mycobacterium tuberculosis. Infect Immun. 1991 Sep;59(9):3021–3025. doi: 10.1128/iai.59.9.3021-3025.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onwubalili J. K., Scott G. M., Robinson J. A. Deficient immune interferon production in tuberculosis. Clin Exp Immunol. 1985 Feb;59(2):405–413. [PMC free article] [PubMed] [Google Scholar]
- Robertson M. J., Soiffer R. J., Wolf S. F., Manley T. J., Donahue C., Young D., Herrmann S. H., Ritz J. Response of human natural killer (NK) cells to NK cell stimulatory factor (NKSF): cytolytic activity and proliferation of NK cells are differentially regulated by NKSF. J Exp Med. 1992 Mar 1;175(3):779–788. doi: 10.1084/jem.175.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Taverne J., Leveton C., Steele J. The role of gamma-interferon, vitamin D3 metabolites and tumour necrosis factor in the pathogenesis of tuberculosis. Immunology. 1987 Oct;62(2):229–234. [PMC free article] [PubMed] [Google Scholar]
- Rotteveel F. T., Kokkelink I., van Lier R. A., Kuenen B., Meager A., Miedema F., Lucas C. J. Clonal analysis of functionally distinct human CD4+ T cell subsets. J Exp Med. 1988 Nov 1;168(5):1659–1673. doi: 10.1084/jem.168.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takashima T., Ueta C., Tsuyuguchi I., Kishimoto S. Production of tumor necrosis factor alpha by monocytes from patients with pulmonary tuberculosis. Infect Immun. 1990 Oct;58(10):3286–3292. doi: 10.1128/iai.58.10.3286-3292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T., Matsui H., Fujita T., Takaoka C., Kashima N., Yoshimoto R., Hamuro J. Structure and expression of a cloned cDNA for human interleukin-2. Nature. 1983 Mar 24;302(5906):305–310. doi: 10.1038/302305a0. [DOI] [PubMed] [Google Scholar]
- Teppler H., Kaplan G., Smith K., Cameron P., Montana A., Meyn P., Cohn Z. Efficacy of low doses of the polyethylene glycol derivative of interleukin-2 in modulating the immune response of patients with human immunodeficiency virus type 1 infection. J Infect Dis. 1993 Feb;167(2):291–298. doi: 10.1093/infdis/167.2.291. [DOI] [PubMed] [Google Scholar]
- Toossi Z., Kleinhenz M. E., Ellner J. J. Defective interleukin 2 production and responsiveness in human pulmonary tuberculosis. J Exp Med. 1986 May 1;163(5):1162–1172. doi: 10.1084/jem.163.5.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilcek J., Klion A., Henriksen-DeStefano D., Zemtsov A., Davidson D. M., Davidson M., Friedman-Kien A. E., Le J. Defective gamma-interferon production in peripheral blood leukocytes of patients with acute tuberculosis. J Clin Immunol. 1986 Mar;6(2):146–151. doi: 10.1007/BF00918747. [DOI] [PubMed] [Google Scholar]
- Villiger P. M., Cronin M. T., Amenomori T., Wachsman W., Lotz M. IL-6 production by human T lymphocytes. Expression in HTLV-1-infected but not in normal T cells. J Immunol. 1991 Jan 15;146(2):550–559. [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]



