Abstract
Context
Brain-derived neurotrophic factor (BDNF) and its receptor appear to be important components of the leptin-signaling cascade involved in energy homeostasis, and mice with BDNF or TrkB gene haploinsufficiency have excessive adiposity. Little is known about the relationship between adiposity and BDNF, particularly in children.
Objective
The objective of the study was to study the association of serum BDNF with measures of adiposity in children.
Design/Setting/Patients
BDNF was determined by a sandwich-type ELISA after an overnight fast in convenience sample of 328 subjects, aged 3–19 yr enriched for extreme obesity. In 43, BDNF was also measured before, and again 1 h after, consuming a high-energy content (787 kcal) milkshake.
Main Outcome Measures
Measures included associations between BDNF and measures of adiposity.
Results
There were no significant univariate associations between log BDNF and adiposity measured by body mass index (BMI), BMI-Z score, or fat mass. However, in an analysis of covariance accounting for age, sex, race, pubertal status, and platelet count, BDNF was lower in overweight children (mean ± SD, 39.8 ± 24.8 vs. 47.0 ± 25.4 ng/dl, P = 0.03); in multiple regression analyses with log BDNF as the dependent variable, BMI (P = 0.03), BMI-Z (P = 0.01), and body fat (P < 0.02) were all negatively associated with BDNF once age, pubertal status, and platelet count were included in the model. Ingestion of a meal did not significantly alter serum BDNF 1 h later (P = 0.26).
Conclusions
Serum BDNF is lower in extremely overweight children and adolescents than those of normal weight. It remains to be determined whether obese individuals with low serum BDNF for age and platelet count have mutations that alter BDNF function.
Brain-derived neurotrophic factor (BDNF) is a 119-amino-acid, 13.6-kDa protein (1) belonging to the neurotrophin family of signaling proteins that appears to be involved in the central regulation of energy homeostasis. BDNF and its receptor, tropomyosin-related kinase B (TrkB), are abundantly expressed in hypothalamic regions believed to be important for the maintenance of normal body weight (2, 3). Mice with BDNF haploinsufficiency (4) are hyperphagic and develop obesity, hyperactivity, and aggressiveness. Conditional BDNF mutant mice, in whom BDNF is deleted after birth, develop mature-onset obesity and aspects of the metabolic syndrome (5). Furthermore, central infusion of BDNF in melanocortin 4 receptor-deficient mice suppresses their hyperphagia and reverses their obesity (6). BDNF infusions also ameliorate the hyperglycemia, hyperinsulinemia, and hyperleptinemia seen in mice with BDNF haploinsufficiency (7). In humans, the BDNF gene has been hypothesized to play a role in the obesity found in patients with the Wilm’s tumor, aniridia, genitourinary anomalies, and mental retardation contiguous gene syndrome (8), and a mutation in TrkB has been described in a child with severe obesity and developmental delay (9).
Few studies have examined serum concentrations of BDNF protein. Low serum BDNF has been reported in women with depression (10, 11) or eating disorders such as anorexia nervosa (12), particularly when compared with obese patients (13), but also when women with anorexia nervosa or bulimia nervosa are compared with normal-weight individuals (12–14). Studies evaluating serum BDNF in children are rare (15, 16); to our knowledge, no prior reports have studied serum BDNF concentrations in overweight children.
To establish age-appropriate norms for serum BDNF in children and evaluate possible differences in BDNF in overweight and normal-weight children, we examined fasting serum BDNF in a large cohort of lean and overweight children and studied the relationships between BDNF and body composition. We hypothesized that serum BDNF would be positively related to both body mass index (BMI) and body fat mass.
Subjects and Methods
A convenience sample of generally healthy children and adolescents age 3–19 yr was recruited using newspaper advertisements and mailings to families and physicians for studies of the physiological, metabolic, and molecular basis of childhood obesity as previously described (17). Subjects were recruited for investigations of the natural history of weight gain and weight reduction trials. Both nonoverweight children, with BMI between the fifth and 95th percentile for age and sex (18), and overweight children (BMI ≥ 95th percentile) were studied. Exclusion criteria included recent weight loss, known genetic or endocrine causes of obesity, significant medical illness, and use of medications affecting body weight. These studies were carried out in accordance with the Declaration of Helsinki and were approved by the National Institute of Child Health and Human Development Institutional Review Board, which serves as the ethics committee for pediatric studies conducted by National Institute of Child Health and Human Development investigators. Each child gave written assent, and a parent gave written consent, for protocol participation.
Weight and height were measured as described previously (19) using calibrated electronic instruments so that BMI (kilograms per square meter) could be calculated. BMI SD scores (BMI-Z) and percentile ranks were calculated according to the Centers for Disease Control and Prevention 2000 growth standards (18). Pubertal stage was assessed by a pediatric endocrinologist or trained nurse practitioner, with breast stage according to Tanner assessed for girls and testicular volume measured using an orchidometer for boys. Pubertal staging was then further categorized into three groups for analysis: prepubertal (Tanner 1 breast stage for girls and testis size < 4 ml for boys), midpubertal (Tanner II and III breast stages for girls and testes 4–12 ml for boys), and late pubertal (Tanner IV and V breast stages and testes > 12 ml for boys). Body composition was determined using dual-energy x-ray absorptiometry (DXA; QDR2000 or QDR4500A; Hologic, Bedford, MA) in most subjects. For 59 children (7% prepubertal, 34% midpubertal, and 59% late pubertal) for whom DXA was not available, air displacement plethysmography (Life Measurement Inc., Concord, CA) was performed according to the manufacturer’s instructions and procedures as previously described (17, 19, 20) so that lean body mass and body fat mass could be determined. Measurements of fat mass by air displacement plethysmography and DXA have previously been shown to be well correlated (19, 21).
Fasting blood samples were collected between 0800 and 1100 h in serum separator tubes for BDNF assay and routine chemistry studies. A second sample of heparinized plasma was obtained to determine platelet count because BDNF is stored in and released from platelets (22, 23). To examine the acute impact of food intake on serum BDNF, we also carried out a pilot study of 43 consecutive children studied under one protocol (24), who had a second blood sample collected 1 h after they consumed a standard liquid breakfast (Scandishake; Axcan Pharma Inc., Birmingham, AL; 787 kcal, 52% carbohydrates, 11% protein, 37% fat). All samples were centrifuged at 4 C for 15 min and the serum stored at −20 C until assayed.
Assays
Serum BDNF was measured using a commercial kit (BDNF Emax immunoassay system; Promega, Madison, WI). Briefly, 96-well flat-bottom immunoplates (Nalge Nunc International, Rochester, NY) were coated with an anti-BDNF monoclonal antibody overnight at 4 C for 16–18 h. The sandwich enzyme immunoassay was then performed using aliquots of subjects’ serum samples diluted in the range of 1:25 to 1:2000. BDNF concentration in serum was calculated based on a standard curve, which was linear between 7.8 and 125 pg/ml. Intra- and interassay variability were less than 9 and less than 18%, respectively; the lower limit of detection was 7.8 pg/ml. Platelet count was measured in heparinized samples by automated two-dimensional optical analysis with automatic verification by focused-flow impedance (CELL-DYN 4000; Abbott Laboratories, Abbott Park, IL) using standard methodology in the National Institutes of Health clinical laboratory.
Statistical analysis
Statistical analyses were conducted using SuperAnova (version 1.11; Abacus Concepts, Inc., Berkeley, CA) and StatView (version 5.01; SAS Institute Inc., Cary, NC) software. The BDNF concentrations of overweight and nonoverweight children were log transformed and then compared by analysis of covariance. Age, sex, race, pubertal status, and platelet count were used as covariates, and least squares means retransformed to conventional units are reported from this analysis. Multiple linear regressions were also used to determine whether BMI-Z score, body fat mass, or lean body mass significantly contributed to the prediction of the log of BDNF after the aforementioned demographic factors and platelet count were taken into account. Statistical significance was set at P = 0.05. Mean (SD) is reported unless otherwise noted.
Results
A total of 328 healthy children and adolescents, age 3–19 yr, were studied, including 224 overweight and 104 nonoverweight children (Table 1). As expected, the mean BMI, BMI-Z score, and body fat mass of the two groups differed significantly. Among the overweight group, 45% had BMI in excess of the 99th percentile for age and sex. The mean age and the gender distribution of the overweight and nonoverweight children were not significantly different. As expected (25), pubertal development was significantly more advanced among overweight children. The racial/ethnic distribution of the two groups was also significantly different in the anticipated direction (26), with more African-American children in the overweight group (50.9 vs. 22.1%, P < 0.001). As has been found previously among overweight children (27) cross-sectionally and among overweight adults who are gaining weight rapidly (28), platelet count was significantly greater among overweight children (P < 0.001).
TABLE 1.
Subject characteristics
| Not overweight: BMI fifth to 95th percentile (n = 104) | Overweight: BMI ≥ 95th percentile (n = 224) | |
|---|---|---|
| Age (yr) | 12.5 (3.1) | 12.7 (2.7) |
| Range | 4.1–19.1 | 3.5–19.1 |
| Sex (%) | ||
| Female | 52.9 | 59.4 |
| Male | 47.1 | 40.6 |
| Pubertal stage (%)a | ||
| Prepubertal | 25.0 | 12.5 |
| Midpubertal | 22.1 | 39.7 |
| Late pubertal | 52.9 | 47.8 |
| Race/ethnicity (%)a | ||
| Black/African-American | 22.1 | 50.9 |
| White/Caucasian | 67.3 | 44.6 |
| Other | 10.6 | 4.5 |
| BMI (kg/m2)a | 19.9 (3.6) | 36.2 (8.5) |
| BMI-Z scorea | 0.378 (0.805) | 2.441 (0.362) |
| Range | −2.24–1.59 | 1.67–3.43 |
| Body fat mass (%)a | 22.3 (7.8) | 42.2 (9.3) |
| Platelet count (× 1000/μl)a | 270 (77) | 306 (62) |
Mean (SD) unless otherwise indicated. For body fat mass, n = 286; for platelet count, n = 254.
P < 0.001, overweight vs. normal-weight children.
There were no significant univariate associations between BDNF and BMI (Fig. 1A), BMI-Z (Fig. 1B) or percent body fat mass (Fig. 1C). There was a small but significant association between age and BDNF (r = +0.11, P = 0.05). Serum BDNF was, however, significantly associated with platelet count (r = 0.16, P < 0.006; Fig. 1D). However, by analysis of covariance, serum BDNF adjusted for sex, age, pubertal status, and platelet count was significantly lower in overweight subjects (39.8 ± 24.8 vs. 47.0 ± 25.4 ng/dl, P = 0.03; Fig. 2). In a multiple regression model with fasting serum BDNF as the dependent variable (model r2 = 0.065, P = 0.002), there were no significant contributions from sex (P = 0.61), race (P = 0.38), or adiposity expressed as BMI, BMI-Z, or percentage body fat (all P ≥ 0.16), but platelet count (beta = +0.21, P < 0.001) and age (beta = +0.14, P = 0.006) were significant predictors, with each accounting for approximately 3% of the variance in fasting serum BDNF. Separate analyses seeking a relationship between BDNF and BMI for white children, black children, and children of other races or ethnicities found no significant differences in the relationships between BMI and BDNF for any group. Removal of the three subjects with BMI between the fifth and 10th percentiles and the 12 subjects with BMI between the 90th and 95th percentiles did not alter these results (data not shown).
Fig. 1.
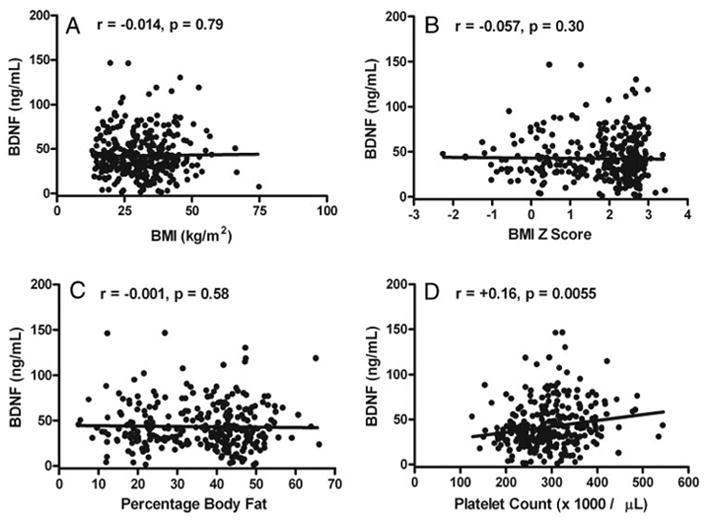
Univariate associations in 328 children between fasting serum BDNF concentrations and BMI (A), BMI-Z score for age and sex (B), percentage body fat (C), and platelet count (D).
Fig. 2.
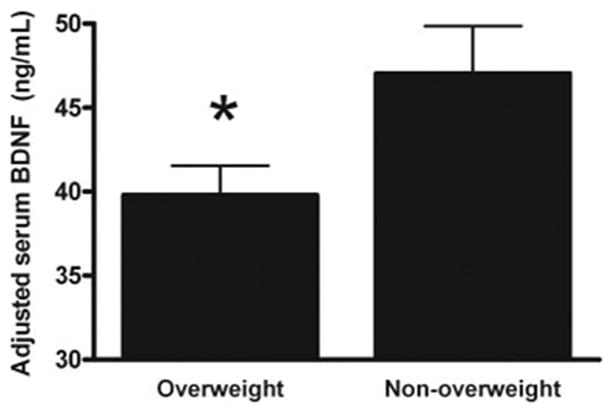
Serum BDNF adjusted for covariates in overweight (n = 224) and nonoverweight (n = 104) children and adolescents. *, P = 0.03.
Compared with fasting values, postprandial serum BDNF concentrations measured in 43 children 1 h after drinking a high-calorie milkshake (Fig. 3) were not significantly different from baseline values in either nonoverweight (P = 0.24) or overweight children (P = 0.39).
Fig. 3.
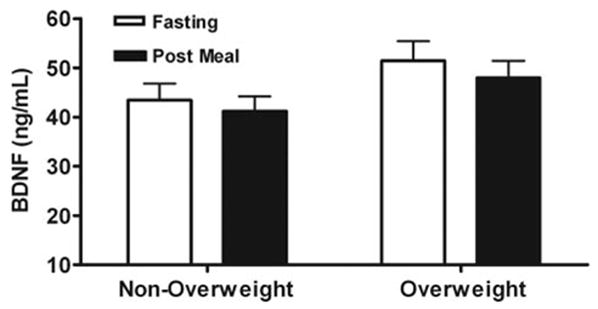
Children’s serum BDNF concentrations in the fasted state and 1 h after consumption of a 787-kcal milkshake meal. There were no significant differences in BDNF after the meal for either nonoverweight (n = 19) or overweight (n = 24) children.
Discussion
We measured serum BDNF in a large cohort of nonoverweight and overweight children to examine the importance of demographic and auxologic factors as predictors of circulating BDNF. Because conditions that are often associated with energy restriction, such as anorexia nervosa (12), bulimia nervosa (13), and untreated major depression (10, 11), have been found to be associated with low serum BDNF and because serum BDNF has been reported to reflect central BDNF levels in rats (29, 30), we hypothesized that serum concentrations of BDNF would be positively related to BMI or body adiposity. Contrary to our initial hypothesis, children’s serum BDNF concentrations were not significantly associated with BMI, BMI-Z, or fat mass in univariate models, and in multivariate models, in which chronological age and platelet count (22, 23) were the strongest factors associated with fasting serum BDNF concentrations, we found a negative association between BDNF and measures of adiposity, such that BDNF concentrations, adjusted for sex, race, pubertal status, age, and platelet count, were 15% lower in severely overweight youth than in those of normal weight. These results differ from those of Nakazato et al. (12), who reported a significant, positive relationship between BMI and serum BDNF in adults but only when patients with anorexia nervosa were included in the analysis. Within the group of normal-weight individuals studied, a positive relationship between BMI and BDNF was not found. Interestingly, adults in the study by Nakazato et al. who had bulimia nervosa, whose weight did not differ from those of his healthy controls, had lower BDNF, a result that may be consistent with the findings reported in the present investigation for severely obese children. Eating-disordered psychopathology involving loss of control over eating may be reported by as many as 30% of such children (31–36). Alternatively, it is possible that some extremely overweight children may have relative deficiencies of BDNF and, like the BDNF haploinsufficient mouse (4), may have hyperphagia as a consequence of BDNF insufficiency. Further studies seeking function-altering mutations in the BDNF gene among severely overweight children with low serum BDNF would thus seem indicated.
We also conducted a pilot investigation of the effect of food intake on serum BDNF 1 h after food consumption. In rats that have undergone a percussive head injury, a 4-wk exposure to a high-fat sucrose diet suppresses BDNF synthesis in some hippocampal neurons (37). However, in the present study of children, eating a meal did not acutely change serum BDNF. We therefore conclude that circulating BDNF concentrations in children are not rapidly affected by food intake. We hypothesize that the reason we saw relatively little impact of food intake on BDNF concentrations is because serum BDNF most closely reflects platelet stores rather than neuronal secretion of BDNF. It remains possible, however, that serum BDNF might require a longer time period before the effects of food intake might be detected. It is also possible that some individuals have substantial circulating quantities of pro-BDNF, which cross-reacts with all currently available BDNF assays and might thus have affected these results (38).
BDNF serves as a neurotransmitter modulator and participates in plasticity mechanisms such as long-term potentiation (39) and learning (40). Serum BDNF has therefore previously been investigated in children with autistic disorders and mental retardation (15, 16). Miyazaki et al. (15) reported high serum BDNF in a small group of children with autistic disorders and mental retardation, compared with adult controls. Additional studies comparing serum BDNF in overweight children without neurocognitive difficulties and age- and BMI-matched children who have well-characterized learning and/or mood problems are required to elucidate the relationship between serum BDNF and neurocognitive deficiencies in children.
In conclusion, serum BDNF is not positively related to body adiposity in youth aged 3–19 yr. The current investigation suggests that serum BDNF is positively associated with platelet count and negatively associated with both BMI and age. Thus, serum BDNF concentrations in children may need to be interpreted with age-specific and platelet count-specific standards.
Prospective studies of individuals with phenotypes that include childhood-onset obesity in combination with learning or mood disorders are needed to define the role of serum BDNF as a biological marker for abnormalities in the BDNF-TrkB signal transduction system. Mutations in the BDNF and TrkB genes that can be shown to be associated with overweight appear to be rare in obese children (9, 41). It remains to be determined whether individuals with mutations expected to change BDNF expression or function have significant alterations in serum BDNF or whether serum BDNF will be useful to identify individuals anticipated to have resistance to the actions of BDNF at its receptor.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, Grant HD-000641 from the National Institute of Child Health and Human Development, National Institutes of Health (to J.A.Y.). J.A.Y. is a Commissioned Officer in the U.S. Public Health Service, Department of Health and Human Services.
Abbreviations
- BDNF
Brain-derived neurotrophic factor
- BMI
body mass index
- BMI-Z
BMI SD score
- DXA
dual-energy x-ray absorptiometry
- TrkB
tropomyosin-related kinase B
References
- 1.Mowla SJ, Farhadi HF, Pareek S, Atwal JK, Morris SJ, Seidah NG, Murphy RA. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J Biol Chem. 2001;276:12660–12666. doi: 10.1074/jbc.M008104200. [DOI] [PubMed] [Google Scholar]
- 2.Tapia-Arancibia L, Rage F, Givalois L, Arancibia S. Physiology of BDNF: focus on hypothalamic function. Front Neuroendocrinol. 2004;25:77–107. doi: 10.1016/j.yfrne.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci USA. 1999;96:15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, Lechan RM, Jaenisch R. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- 6.Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6:736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakagawa T, Tsuchida A, Itakura Y, Nonomura T, Ono M, Hirota F, Inoue T, Nakayama C, Taiji M, Noguchi H. Brain-derived neurotrophic factor regulates glucose metabolism by modulating energy balance in diabetic mice. Diabetes. 2000;49:436–444. doi: 10.2337/diabetes.49.3.436. [DOI] [PubMed] [Google Scholar]
- 8.Marlin S, Couet D, Lacombe D, Cessans C, Bonneau D. Obesity: a new feature of WAGR (del 11p) syndrome. Clin Dysmorphol. 1994;3:255–257. [PubMed] [Google Scholar]
- 9.Yeo GS, Connie Hung CC, Rochford J, Keogh J, Gray J, Sivaramakrishnan S, O’Rahilly S, Farooqi IS. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat Neurosci. 2004;7:1187–1189. doi: 10.1038/nn1336. [DOI] [PubMed] [Google Scholar]
- 10.Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109:143–148. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, Nakazato M, Watanabe H, Shinoda N, Okada S, Iyo M. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry. 2003;54:70–75. doi: 10.1016/s0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- 12.Nakazato M, Hashimoto K, Shimizu E, Kumakiri C, Koizumi H, Okamura N, Mitsumori M, Komatsu N, Iyo M. Decreased levels of serum brain-derived neurotrophic factor in female patients with eating disorders. Biol Psychiatry. 2003;54:485–490. doi: 10.1016/s0006-3223(02)01746-8. [DOI] [PubMed] [Google Scholar]
- 13.Monteleone P, Tortorella A, Martiadis V, Serritella C, Fuschino A, Maj M. Opposite changes in the serum brain-derived neurotrophic factor in anorexia nervosa and obesity. Psychosom Med. 2004;66:744–748. doi: 10.1097/01.psy.0000138119.12956.99. [DOI] [PubMed] [Google Scholar]
- 14.Monteleone P, Fabrazzo M, Martiadis V, Serritella C, Pannuto M, Maj M. Circulating brain-derived neurotrophic factor is decreased in women with anorexia and bulimia nervosa but not in women with binge-eating disorder: relationships to co-morbid depression, psychopathology and hormonal variables. Psychol Med. 2005;35:897–905. doi: 10.1017/s0033291704003368. [DOI] [PubMed] [Google Scholar]
- 15.Miyazaki K, Narita N, Sakuta R, Miyahara T, Naruse H, Okado N, Narita M. Serum neurotrophin concentrations in autism and mental retardation: a pilot study. Brain Dev. 2004;26:292–295. doi: 10.1016/S0387-7604(03)00168-2. [DOI] [PubMed] [Google Scholar]
- 16.Nelson KB, Grether JK, Croen LA, Dambrosia JM, Dickens BF, Jelliffe LL, Hansen RL, Phillips TM. Neuropeptides and neurotrophins in neonatal blood of children with autism or mental retardation. Ann Neurol. 2001;49:597–606. [PubMed] [Google Scholar]
- 17.Elberg J, McDuffie JR, Sebring NG, Salaita C, Keil M, Robotham D, Reynolds JC, Yanovski JA. Comparison of methods to assess change in children’s body composition. Am J Clin Nutr. 2004;80:64–69. doi: 10.1093/ajcn/80.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. Adv Data. 2000. CDC growth charts: United States; pp. 1–27. [PubMed] [Google Scholar]
- 19.Nicholson JC, McDuffie JR, Bonat SH, Russell DL, Boyce KA, McCann S, Michael M, Sebring NG, Reynolds JC, Yanovski JA. Estimation of body fatness by air displacement plethysmography in African American and white children. Pediatr Res. 2001;50:467–473. doi: 10.1203/00006450-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Dempster P, Aitkens S. A new air displacement method for the determination of human body composition. Med Sci Sports Exerc. 1995;27:1692–1697. [PubMed] [Google Scholar]
- 21.Fields DA, Goran MI, McCrory MA. Body-composition assessment via air-displacement plethysmography in adults and children: a review. Am J Clin Nutr. 2002;75:453–467. doi: 10.1093/ajcn/75.3.453. [DOI] [PubMed] [Google Scholar]
- 22.Fujimura H, Altar CA, Chen R, Nakamura T, Nakahashi T, Kambayashi J, Sun B, Tandon NN. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost. 2002;87:728–734. [PubMed] [Google Scholar]
- 23.Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, Virchow JC. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26:115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Anonymous . NIH clinical research studies. Bethesda, MD: National Institute of Child Health and Human Development; 2006. Eating behavior in children. [Google Scholar]
- 25.Kaplowitz PB, Slora EJ, Wasserman RC, Pedlow SE, Herman-Giddens ME. Earlier onset of puberty in girls: relation to increased body mass index and race. Pediatrics. 2001;108:347–353. doi: 10.1542/peds.108.2.347. [DOI] [PubMed] [Google Scholar]
- 26.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among U.S. children and adolescents, 1999–2000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 27.Cindik N, Baskin E, Agras PI, Kinik ST, Turan M, Saatci U. Effect of obesity on inflammatory markers and renal functions. Acta Paediatr. 2005;94:1732–1737. doi: 10.1111/j.1651-2227.2005.tb01845.x. [DOI] [PubMed] [Google Scholar]
- 28.Barzilay JI, Forsberg C, Heckbert SR, Cushman M, Newman AB. The association of markers of inflammation with weight change in older adults: the Cardiovascular Health Study. Int J Obes (Lond) 2006 doi: 10.1038/sj.ijo.0803306. [DOI] [PubMed] [Google Scholar]
- 29.Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett. 2002;328:261–264. doi: 10.1016/s0304-3940(02)00529-3. [DOI] [PubMed] [Google Scholar]
- 30.Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 31.Decaluwe V, Braet C. Prevalence of binge-eating disorder in obese children and adolescents seeking weight-loss treatment. Int J Obes Relat Metab Disord. 2003;27:404–409. doi: 10.1038/sj.ijo.0802233. [DOI] [PubMed] [Google Scholar]
- 32.Greenfield D, Quinlen D, Harding P, Glass E, Bliss A. Eating behavior in an adolescent population. Int J Eat Disord. 1987;6:99–111. [Google Scholar]
- 33.Isnard P, Michel G, Frelut ML, Vila G, Falissard B, Naja W, Navarro J, Mouren-Simeoni MC. Binge eating and psychopathology in severely obese adolescents. Int J Eat Disord. 2003;34:235–243. doi: 10.1002/eat.10178. [DOI] [PubMed] [Google Scholar]
- 34.Morgan CM, Yanovski SZ, Nguyen TT, McDuffie J, Sebring NG, Jorge MR, Keil M, Yanovski JA. Loss of control over eating, adiposity, and psychopathology in overweight children. Int J Eat Disord. 2002;31:430–441. doi: 10.1002/eat.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanofsky-Kraff M, Yanovski SZ, Wilfley DE, Marmarosh C, Morgan CM, Yanovski JA. Eating-disordered behaviors, body fat, and psychopathology in overweight and normal-weight children. J Consult Clin Psych. 2004;72:53–61. doi: 10.1037/0022-006X.72.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanofsky-Kraff M, Faden D, Yanovski SZ, Wilfley DE, Yanovski JA. The perceived onset of dieting and loss of control eating behaviors in overweight children. Int J Eat Disord. 2005;38:112–122. doi: 10.1002/eat.20158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu A, Molteni R, Ying Z, Gomez-Pinilla F. A saturated-fat diet aggravates the outcome of traumatic brain injury on hippocampal plasticity and cognitive function by reducing brain-derived neurotrophic factor. Neuroscience. 2003;119:365–375. doi: 10.1016/s0306-4522(03)00154-4. [DOI] [PubMed] [Google Scholar]
- 38.Fayard B, Loeffler S, Weis J, Vogelin E, Kruttgen A. The secreted brain-derived neurotrophic factor precursor pro-BDNF binds to TrkB and p75NTR but not to TrkA or TrkC. J Neurosci Res. 2005;80:18–28. doi: 10.1002/jnr.20432. [DOI] [PubMed] [Google Scholar]
- 39.Gartner A, Staiger V. Neurotrophin secretion from hippocampal neurons evoked by long-term-potentiation-inducing electrical stimulation patterns. Proc Natl Acad Sci USA. 2002;99:6386–6391. doi: 10.1073/pnas.092129699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 41.Friedel S, Horro FF, Wermter AK, Geller F, Dempfle A, Reichwald K, Smidt J, Bronner G, Konrad K, Herpertz-Dahlmann B, Warnke A, Hemminger U, Linder M, Kiefl H, Goldschmidt HP, Siegfried W, Remschmidt H, Hinney A, Hebebrand J. Mutation screen of the brain derived neurotrophic factor gene (BDNF): identification of several genetic variants and association studies in patients with obesity, eating disorders, and attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2005;132:96–99. doi: 10.1002/ajmg.b.30090. [DOI] [PubMed] [Google Scholar]


