Summary
Context Both obesity (body mass index, BMI ≥ 30 kg/m2) and Black race are associated with a higher risk of vitamin D deficiency and secondary hyperparathyroidism. We hypothesized the risk of hypovitaminosis D would therefore be extraordinarily high in obese Black adults.
Objective
To study the effects of race and adiposity on 25-hydroxyvitamin D [25(OH)D] and parathyroid hormone (iPTH).
Design, Setting and Participants
Cross-sectional study of 379 Black and White adults from the Washington D.C. area. BMI ranged from 19.9 to 58.2 kg/m2.
Main Outcome Measures
Prevalence of hypovitaminosis D [25(OH)D < 37.5 nmol/l] and secondary hyperparathyroidism [25(OH)D < 37.5 nmol/l with iPTH > 4.2 pmol/l].
Results
Obese Black subjects had lower mean 25(OH)D, 40.3 (SD, 20.3) nmol/l, compared with obese Whites, 64.5 (29.7), P < 0.001, nonobese Blacks, 53.3 (26.0), P = 0.0025 and nonobese Whites, 78.0 (33.5), P < 0.001. The prevalence of hypovitaminosis D increased with increasing BMI, and was greater (P < 0.001) in Blacks than Whites within all BMI categories examined. Among subjects with BMI ≥ 35 kg/m2, 59% of Blacks vs 18% of Whites had hypovitaminosis D (odds ratio 6.5, 95% confidence interval 3.0–14.2). iPTH was negatively correlated with 25(OH)D (r = −0.31, P < 0.0001), suggesting those with hypovitaminosis D had clinically important vitamin D deficiency with secondary hyperparathyroidism. For secondary hyperparathyroidism 35.2% of Blacks met the criteria, compared to 9.7% of Whites (OR 3.6, CI 1.5–98.8).
Conclusions
Obese Black Americans are at particularly high risk for vitamin D deficiency and secondary hyperparathyroidism. Physicians should consider routinely supplementing such patients with vitamin D or screening them for hypovitaminosis D.
Introduction
Obesity, defined as a body mass index (BMI) ≥ 30 kg/m2, is one of the most prevalent health problems worldwide.1 Being overweight (BMI 25–29.9 kg/m2) or obese is associated with decreased serum concentrations of 25-hydroxyvitamin D [25(OH)D] and high intact parathyroid hormone (iPTH).2–8 High iPTH indicates that the lower 25(OH)D observed in obesity is physiologically suboptimal, because it induces a compensatory (secondary) hyperparathyroidism.
Serum 25(OH)D concentrations are also influenced by skin colour because melanin interferes with ultraviolet light-induced conversion of 7-dehydrocholesterol in the skin into previtamin D3.9 Compared to Whites, Blacks have lower mean serum 25(OH)D, higher iPTH and a markedly increased prevalence of vitamin D deficiency.10–12
Although osteoporosis is uncommon in obese persons and in Blacks, vitamin D deficiency is likely a risk factor for the development of a number of chronic diseases. Epidemiologic studies have associated hypovitaminosis D with an increased risk of a number of cancers, autoimmune diseases, heart disease and hypertension,13 which are more prevalent in African Americans.
Black adults are potentially at particularly high risk for vitamin D deficiency because of two factors: their greater skin melanin and their greater prevalence of being overweight or obese.14 However, whether or not obesity has an additional effect on vitamin D in Black individuals is unclear.10,15,16 Because lower vitamin D in individuals with dark skin and in those with obesity are likely caused by different mechanisms, we hypothesized that both obesity and Black race would affect serum 25(OH)D concentrations independently, such that obese Black adults would have an extraordinarily high prevalence of hypovitaminosis D. To test this hypothesis, we studied the effects of race and adiposity on vitamin D and parathyroid hormone in a sample of Black and White adults with a wide range of BMI.
Subjects and methods
Subjects were recruited through advertisements inviting ‘healthy volunteers’ or ‘overweight’ adults to participate in a calcium-supplement study. One thousand nine hundred nineteen potential subjects inquired; 1800 were screened by telephone, 1349 potential subjects were excluded for reporting a significant medical illness such as diabetes, bone, kidney, liver or gallbladder disease, were taking medications that affect vitamin D and iPTH concentrations or body weight, had intentional weight change of more than 3% of body weight in the preceding 3 months, were of a self-identified race/ethnicity other than non-Hispanic White or non-Hispanic Black, or declined to participate. Five subjects were excluded for reporting intake exceeding 3.5 g/day of calcium or 400 IU/day of vitamin D supplements. Sixty-seven subjects were excluded for not completing required laboratory testing. Included and excluded subjects did not differ in age, although Black individuals comprised 34% of those participating, but 42% of those excluded (P = 0.02), and men comprised 26% of those participating, but 17% of those excluded (P = 0.0007). Volunteers were from the Washington D.C. area between latitudes 36° to 40°N. The research protocol was approved by the National Institute of Child Health and Human Development Institutional Review Board, and signed consent was obtained from all subjects. Financial compensation was provided for subjects’ inconvenience.
Visits were conducted between March 2002 and December 2003. Subjects reported after a 10- h overnight fast to undergo a detailed history, physical examination and anthropometric measurements. Subjects were weighed in hospital gowns using a digital scale (Life Measurement Instruments, Concord, CA) that was calibrated with a known weight before each subject’s measurement. Height was measured using a stadiometer also calibrated before each set of measurements (Holtain Ltd, Crymych, UK). Whole body dual energy X-ray absorptiometry (DXA) for estimation of fat mass (Delphi A, LX 20 Beckman, Bedford, MA, software version 11.2) was performed in 306 subjects.
Laboratory assays
Subjects underwent measurements of calcitropic hormones and routine chemistries by venous blood sampling. 25(OH)D was measured using a competitive binding assay (Nichols Advantage, Nichols Diagnostic, San Clemente, CA).17 Functional sensitivity of the assay was < 16.8 nmol/l and mean interassay coefficient of variation (CV) was 5.7%. 1,25 Dihydroxyvitamin D [1,25(OH)2D] was measured using cartridge extraction and radioimmunoassay (Mayo Medical Laboratories, Rochester, MN) having 24 pmol/l sensitivity and mean interassay CV 11.4%.18 Intact PTH measurements were performed using two-site immunochemiluminometric assays. One hundred eighty-six samples were analysed with Nichols Advantage Intact PTH (Nichols Diagnostics),19 which had mean interassay CV of 4.7% and 193 samples with Nichols Advantage Bio-Intact PTH (1–84) with mean interassay CV of 6.7%. The reported Pearson’s correlation coefficient between the two assays was 0.97 (95% confidence interval 0.96–0.98), and least squares linear regression analysis of the two assays performed on the same samples by the NIH clinical laboratory yielded an equation of Bio-Intact PTH = 0.6(Intact PTH) + 4.2. Those samples measured using the Intact PTH assay were converted using this equation into Bio-Intact assay concentrations before statistical analysis. The Bio-Intact PTH assay does not detect the large, biologically inactive C-terminal fragments that may be detected by the Intact PTH assay.20 Results were not altered in direction or significance when a variable for PTH assay was included in analyses. Ionized calcium was measured by an ion-selective electrode method using the Roche AVL 988-4 Analyser (Roche Diagnostics Corp., Indianapolis, IN). The sensitivity for the assay was 0.1 mmol/l, the upper limit of linearity was 6 mmol/l and the interassay CV was 2.3%. Other blood chemistries including alkaline phosphatase and phosphorus were obtained using standard methods.
Recorded food intake
Subjects were given written instructions to record all foods and beverages consumed over seven consecutive days. Food records were reviewed in person with subjects by a registered dietician to maximize accuracy and completeness, and analysed for dietary vitamin D intake using the Nutrition Data System for Research (NDS-R) software versions 4.04_32 and 4.05_33, developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis.21 The dietary vitamin D (calciferol) data for database version 4.04_32 were 99.6% complete. In version 4.05_33 the dietary vitamin D data were 99.0% complete. Information about intake of vitamin D supplements, including that from multivitamins, was confirmed through interview by the dietician. Dietary and supplemental calcium intake was also measured with these methods.
Statistical analysis
Contingency table analyses and analyses of covariance (ANCOVA) were performed using STATVIEW version 5.01 (SAS Institute, Inc. Cary, NC). Logistic regression with Newton-Raphson iterative fitting was carried out using EGRET 2.0.31 (Cytel Software Corporation, Cambridge, MA). The primary analysis examined 25(OH)D concentrations with race and level of obesity (obese vs nonobese) as the categorical factors of interest, with age, sex, season and socioeconomic status included in the analysis. Results were not altered in direction or significance when subjects taking vitamin D supplementation were excluded. To examine seasonal variation of 25(OH)D levels, visit dates were grouped into four categories (spring 21 March through 20 June, summer 21 June through 20 September, fall 21 September through 20 December and winter 21 December through 20 March). Socioeconomic status was determined by Hollingshead score,22 grouped as follows: categories I and II were ‘high’ (n = 150), category III was ‘mid’ (n = 165) and categories IV and V constituted ‘low’ socioeconomic status (n = 64). Tests were performed for co-linearity, overfitting and second-degree interactions. In subsequent analyses, obesity was subdivided into those with class I (BMI 30–34.99 kg/m2), class II (BMI 35–39.99 kg/m2), and class III obesity (BMI ≥ 40 kg/m2). Although multiple definitions for hypovitaminosis D have been proposed,13 we chose a conservative definition of 25(OH)D < 37.5 nmol/l (< 15 ng/ml) in accordance with data showing that iPTH begins to rise at this level of 25(OH)D.10 High iPTH was defined as serum iPTH > 4.2 pmol/l (40 pg/ml) based on manufacturer’s guidelines. With alpha set at 0.05, a power analysis (using the Fleiss correction) suggested that comparisons between subgroups having > 34 subjects would have 80% power to detect 35% differences in the proportion of subjects with hypovitaminosis D or hyperparathyroidism. No subject was found to have the combination of abnormally high iPTH, serum ionized calcium and urinary calcium consistent with primary hyperparathyroidism. Unadjusted odds ratios are presented, as they were not greatly altered by covariates. Results are presented as mean (SD) unless otherwise specified, with statistical significance set at P = 0.05. Posthoc pairwise comparisons were performed using unpaired t-tests or contingency table analysis as appropriate using the Bonferonni–Hochberg adjustment for multiple comparisons.
Results
Two hundred thirty-two obese and 147 nonobese subjects were enrolled (Table 1). One hundred twenty-three were Black (65% obese) and 256 were White (59% obese). Black and White subjects were not significantly different in mean age, although men were older than women overall and had lower mean body fat mass (P < 0.005). There were significant differences between Black and White subjects in socioeconomic status (P < 0.0001); therefore, socioeconomic status was included as a covariate in analyses. Black and White subjects were not significantly different in mean BMI or body fat mass. However, when examined categorically, a greater proportion of Black than White women had BMI ≥ 40 kg/m2 (P < 0.005).
Table 1.
Subjects*
| Characteristics | Black women (n = 106) | White women (n = 168) | Black men (n = 17) | White men (n = 88) |
|---|---|---|---|---|
| Age (years)† (range) | 37 (10) (19–61) | 37 (9) (18–64) | 42 (13) (21–70) | 42 (12) (21–71) |
| Socioeconomic status (%)†,‡ | ||||
| High | 24 | 41 | 37.5 | 58 |
| Mid | 53 | 43 | 37.5 | 34 |
| Low | 23 | 16 | 25.0 | 8 |
| BMI (kg/m2) (range) | 34.2 (8.6) (19.9–58.2) | 33.5 (6.6) (20.0–53.6) | 33.9 (8.6) (22.7–54.3) | 31.3 (5.7) (22.2–51.4) |
| Body fat (kg)† (n = 306) (range) | 35.5 (13.9) (8.7–68.7) | 37.0 (11.7) (12.4–68.3) | 20.3 (6.1) (9.8–26.2) | 26.2 (10.2) (8.1–49.0) |
| Sample Size (%)§ | ||||
| BMI 18–24.99 kg/m2 | 17 (16) | 9 (5) | 1 (6) | 6 (7) |
| BMI 25–29.99 kg/m2 | 19 (18) | 50 (30) | 6 (35) | 39 (44) |
| BMI ≥30 kg/m2 | 70 (66) | 109 (65) | 10 (59) | 43 (49) |
| BMI 30–34.99 kg/m2 | 21 (20) | 48 (29) | 5 (29) | 22 (25) |
| BMI 35–39.99 kg/m2 | 23 (22) | 35 (21) | 1 (6) | 13 (15) |
| BMI ≥40 kg/m2 | 26 (24) | 26 (15) | 4 (24) | 8 (9) |
men vs. women, P < 0.005.
Black vs. White, P < 0.0001.
Black vs. White women, P < 0.005.
Data are presented as mean (SD) unless otherwise specified.
Mean serum 25(OH)D (nmol/l) varied significantly by season (P = 0.006): spring 56.5 (29.7), summer 60.5 (29.7), fall 69.0 (32.7) and winter 63.3 (35.0). Neither 1,25(OH)2D (P = 0.44) nor iPTH (P = 0.31) varied significantly with season.
In obese subjects, 25(OH)D (Table 2) was significantly lower than in nonobese subjects (P < 0.0001), and 25(OH)D was lower in Black than White subjects regardless of body weight (P < 0.0001). Furthermore, 25(OH)D was significantly lower in obese Black subjects than nonobese Black (P = 0.0025), or obese White (P < 0.0001) subjects. In obese White subjects, 25(OH)D was also significantly lower than in nonobese White subjects (P = 0.0008).
Table 2.
Calcitropic hormone serum concentrations and vitamin D intake
| Race | Nonobese (BMI < 30 kg/m2) N = 147 | Obese (BMI ≥30 kg/m2) N = 232 | All subjects | |
|---|---|---|---|---|
| 25(OH)D (nmol/l) | B | 53.3 (26.0)† | 40.3 (20.3)†,‡ | 44.7 (23.3)† |
| W | 78.0 (33.5) | 64.5 (29.7)‡ | 70.0 (32.0) | |
| B + W | 70.7 (33.3) | 56.0 (29.3)‡ | ||
| 1,25(OH)2D (pmol/l) | B | 131.0 (54.7) | 106.3 (37.9)‡ | 115.2 (46.1) |
| W | 122.4 (36.2) | 114.2 (38.6) | 117.6 (37.9) | |
| B + W | 125.0 (42.5) | 111.6 (38.6)‡ | ||
| iPTH (pmol/l) | B | 3.3 (1.0) | 4.3 (1.3)†,‡ | 3.9 (1.3)† |
| W | 3.1 (1.0) | 3.6 (1.2)‡ | 3.4 (1.1) | |
| B + W | 3.2 (1.0) | 3.8 (1.3)‡ | ||
| Vitamin D intake (IU/d) | B | 164 (95) | 160 (97) | 163 (95) |
| Food record | W | 210 (156) | 209 (340) | 209 (276) |
| (N = 284) | B + W | 196 (140) | 192 (279) | |
| Supplemental vitamin D | B | 111.8 (152.8) | 84.1 (140.5) | 93.4 (144.5) |
| intake (IU/d) | W | 113.6 (115.8) | 88.5 (139.4) | 99.7 (147.1) |
| (N = 154) | B + W | 113.1 (154.3) | 86.9 (139.4) |
B, Black; W, White.
significantly different from W, P < 0.005.
significantly different from nonobese, P < 0.005.
Hypovitaminosis D was present in 43.1% of all Black, vs 11.7% of all White, subjects (P < 0.0001, odds ratio [OR] 5.7, CI 3.4–9.6). Examined by BMI category (Fig. 1), 28% of overweight Black subjects had hypovitaminosis D vs 9.0% of overweight White subjects (OR 3.8, CI 1.3–11.0). Among subjects with class I obesity (OR 4.8, CI 1.5–14.7), class II obesity (OR 8.2, CI 2.6–25.7) and class III obesity (OR 4.9, CI 1.7–14.2), Black subjects had significantly higher likelihood of hypovitaminosis D (P < 0.01). For all subjects with BMI ≥ 35 kg/m2, 59% of Black participants had hypovitaminosis D, compared to 18% of White subjects (OR 6.5, CI 3.0–14.2). Both BMI (P < 0.001) and race (P < 0.001) had similarly independent effects in the prediction of 25OHD levels when a less conservative definition of vitamin D deficiency (< 80 nmol/l) was used in logistic regression.
Fig. 1.
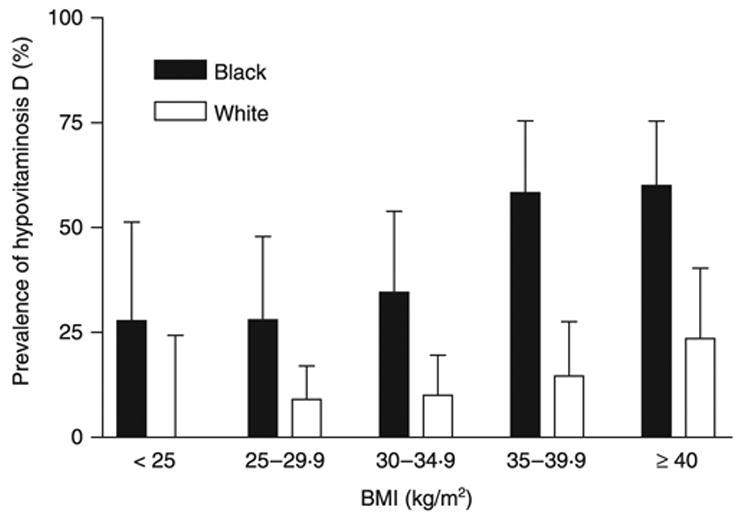
Prevalence of hypovitaminosis D defined as serum 25(OH)D concentration less than 37.5 nmol/l (15 ng/ml). Prevalence was greater in Blacks than Whites independent of BMI (P < 0.001). The 95% confidence interval is shown as the error bar.
For the 284 subjects who completed food records (Table 2), mean daily dietary vitamin D intake was not different according to race (P > 0.1) or body mass (P > 0.9), nor was there a race by BMI interaction. There was no seasonal variation in reported dietary vitamin D intake (P > 0.6). There was no difference in mean daily supplemental vitamin D intake between Black and White subjects (P = 0.73) nor between obese and nonobese subjects (P = 0.13). In logistic regression analyses with 25(OH)D as the dependent measure and adiposity (BMI or DXA body fat), race, sex, age, season, socioeconomic status and vitamin D intake as the independent measures, adiposity expressed either as BMI (odds ratio 1.08, CI 1.04–1.13, P < 0.001) or percentage body fat (odds ratio 1.07, CI 1.02–1.12, P = 0.004) and race (odds ratio 6.8, CI 3.5–13.2, P < 0.001) were significantly and independently related to likelihood for a low 25(OH)D.
Mean iPTH (Table 2) was significantly higher in obese than nonobese subjects (P < 0.0001) and in Black compared to White subjects (P < 0.0001). Mean iPTH was also significantly greater in obese vs nonobese Black subjects (P = 0.0001) and in obese vs nonobese White individuals (P < 0.005). The prevalence of secondary hyperparathyroidism, defined as iPTH > 4.2 pmol/l (40 pg/ml) when 25(OH)D was below 37.5 nmol/l (15 ng/ml), was significantly greater in all Black (21.9%) than in all White subjects (4.3%, P < 0.0001, OR 6.9, CI 3.2–14.8) and in obese Black (28.7%) than in obese White subjects (7.2%, OR 5.2, CI 2.4–11.3). The prevalence of secondary hyperparathyroidism differed in Black and White subjects according to BMI (Fig. 2). Among subjects with class III obesity, Black subjects had a greater likelihood of secondary hyperparathyroidism (OR 7.9, CI 2.0–31.7). For all subjects with BMI ≥ 35 kg/m2, 35.2% of Black, vs 9.7% of White subjects, had secondary hyperparathyroidism (OR 3.6, CI 1.5–98.8).
Fig. 2.
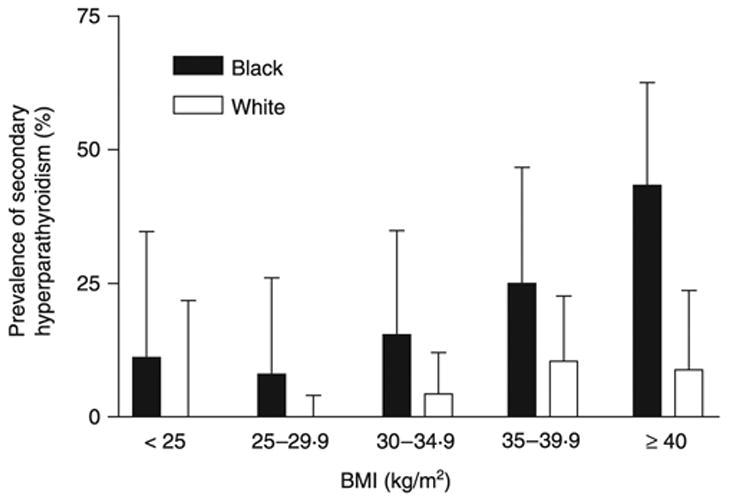
Prevalence of secondary hyperparathyroidism, defined as 25(OH)D less than 37.5 nmol/l (15 ng/ml) plus iPTH greater than 4.2 pmol/l (40 pg/ml). Prevalence was greater in Blacks than Whites independent of BMI (P < 0.001). The 95% confidence interval is shown as the error bar.
Serum concentrations of iPTH and 25(OH)D were negatively associated in both Black and White subjects (Fig. 3, r = −0.31, P < 0.0001). Ionized calcium was negatively correlated with iPTH (r = −0.16, P = 0.0024), and ionized calcium was significantly lower in obese subjects, 1.285 (0.05), than in nonobese subjects, 1.298 (0.05) mmol/l, P < 0.01. Mean daily calcium intake including supplements was not correlated with serum iPTH concentrations (P = 0.18). Mean phosphorus was negatively correlated with iPTH (r = −0.27, P < 0.0001), whereas there was no difference in phosphorus between obese and nonobese subjects (P = 0.9). Alkaline phosphatase was significantly higher in obese subjects, 69.6 (18.5), vs 58.9 (17.0) U/l, P < 0.0001 and was positively correlated with iPTH (r = 0.16, P = 0.002) and with BMI (r = 0.34, P < 0.0001).
Fig. 3.
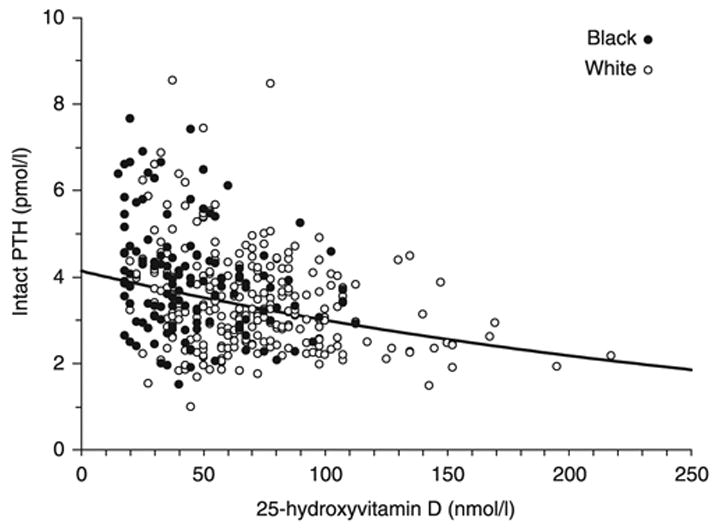
Association between serum concentrations of iPTH and 25(OH)D in Black and White subjects (r = −0.31, P < 0.0001).
Discussion
Both vitamin D deficiency and obesity are nutritional disorders of global importance. In the United States, both conditions disproportionately affect Black individuals.14 Our data suggest that both high adiposity and Black race are independent predictors of hypovitaminosis D. In the current study, for each one-point increase in BMI there was an 8% increased risk of vitamin D deficiency. Furthermore, Black adults with BMI ≥ 35 kg/m2 had a prevalence of hypovitaminosis D of greater than 50% and sixfold increased relative odds of having hypovitaminosis D vs similarly obese White subjects. The relatively large sample of severely obese subjects studied allowed us to demonstrate that the prevalence of hypovitaminosis D within each BMI category was greater in Black than in White adults. The effects of adiposity and race on vitamin D status were independent of dietary vitamin D intake, socioeconomic status, sex, age and season. The low 25(OH)D levels appeared to represent functional vitamin D deficiency, as the odds of secondary hyperparathyroidism were significantly greater in obese Black than obese White subjects. In obese subjects, 1,25(OH)2D was also lower than in nonobese subjects, and lowest in obese Black subjects.
The consequences of vitamin D deficiency include a broad range of health problems. Some reports suggest the potential importance of vitamin D in the prevention of certain cancers, autoimmune diseases, heart disease and hypertension.13,23 It has been proposed,24 because of the association between lower UVB radiation exposure and malignancy, that premature deaths from several different types of internal cancers, including some with higher incidence and mortality in Black subjects,25,26 could be prevented if adequate serum vitamin D concentrations could be achieved. Therefore, we believe there is potential public health benefit from identifying high-risk populations for routine supplementation or for screening for vitamin D deficiency. Black adults with BMI in excess of 35 kg/m2 are a particularly high-risk population for whom routine screening appears justified. This is important because roughly 40% of non-Hispanic Black Americans have a BMI ≥ 30 kg/m2 and close to 10% of non-Hispanic Black Americans have a BMI ≥ 40 kg/m2.14 However, to our knowledge, no cost-benefit analysis has been performed to consider the relative advantages of screening vs vitamin D supplementation.
Our results are consistent with previous investigations reporting a relationship between lower vitamin D concentrations and increasing body weight,2,3 body fat mass5,8 and Black race.11,12,27 Unique to our study was the analysis of subjects with classes II and III obesity to demonstrate that the relative risk of hypovitaminosis D continues to increase with greater BMI, an analysis that was not performed in a recent NHANES study.10
The relationship we found between iPTH and BMI is also consistent with previous studies.4,6,8,28–30 Additionally, we found a positive relationship between iPTH and alkaline phosphatase, a marker of bone turnover. These findings are consistent with previous studies in which obesity was shown to be a risk factor for metabolic bone disease.3,4 Although we did not use bone-specific alkaline phosphatase measurements, none of our subjects had clinically significant renal, liver or gallbladder disease, or elevated transaminases. The possibility is raised then, that the higher alkaline phosphatase in obese subjects is related to bone metabolism, although the effects of hepatic fatty infiltration (unaccompanied by elevation of transaminases) on alkaline phosphatase cannot be ruled out. The lower ionized calcium observed in obese subjects might then reflect their lower vitamin D status, and be the proximal cause of their greater iPTH.
The primary mechanism for the greater prevalence of hypovitaminosis D in Black individuals is considered to be darker skin pigmentation, concurrent with reduced sunlight exposure.9 The need for a greater time of exposure to ultraviolet B rays in order to maximize the epidermal synthesis of previtamin D3 has been shown not only in Black individuals but also in other groups with greater amounts of melanin in the skin, including Pakistani and Indian immigrants to Great Britain.31–33 Similarly, such groups have been shown to have a higher prevalence of secondary hyperparathyroidism.34
The mechanism underlying the lower 25(OH)D levels in obesity is less clear. Greater storage of vitamin D as a result of a larger fat mass has been suggested,35 as adipose tissue has been shown to be a major storage site of vitamin D3 in rats36 and humans.37 Consistent with this hypothesis, higher fat mass was reported to be a better predictor than BMI for low vitamin D status in healthy nonobese women.5 Alternatively, Compston et al.3 have proposed that obese individuals may spend less time outdoors and therefore be exposed to less UV radiation. The efficiency of epidermal vitamin D production does not, however, appear to be impaired in obesity.35
Among the limitations of this study is the relatively small number of normal-weight individuals and Black men included. However, there were no interaction effects between race, sex and obesity, and both race and adiposity were independent predictors of vitamin D status. Bias from 25(OH)D commercial assay kits might also affect results. The competitive binding assay used in this study may not detect vitamin D made from ergocalciferol. However, as ergocalciferol intake was low and was not different among study groups, we do not believe the results were greatly affected by this potential difficulty. It should also be noted that dietary vitamin D intake was assessed by food records that may not be equivalent to actual subject dietary intake. There may be a greater tendency for obese subjects to under-report food intake,38 which could further bias food record data. However, if our obese subjects under-reported their vitamin D intake, this would tend to strengthen our finding of hypovitaminosis D independent of vitamin D intake in obese subjects. Also, because sun exposure was not assessed in our subjects, it is possible that the obese Black subjects had less sun exposure. Although this might, in part, account for the differences in vitamin D concentrations among our subjects, it would not change the clinical importance of the results. Lastly, our subjects resided in the Washington, D.C. region and might not represent subjects living at other latitudes. Additional studies are also needed to address how 25(OH)D may vary in Black and White individuals at different times of year.
In conclusion, both BMI and Black race appear to be important and independent risk factors for hypovitaminosis D. As a result, obese Black adults are at significantly greater risk of vitamin D deficiency than obese White or normal-weight Black adults. In view of recent recognition of the multitude of diseases linked to hypovitaminosis D, we propose that obese Black adults, particularly those with BMI ≥ 35 kg/m2, either be routinely supplemented with vitamin D or be routinely screened for vitamin D deficiency.
Footnotes
Supported by ZO1 HD-00641 (to JAY) from the National Institute of Child Health and Human Development and by Y2-OD-2067 (to JAY) from the Office of Dietary Supplements, National Institutes of Health, DHHS.
References
- 1.Rosenthal AM. WHO names top 10 health risks. Environmental Health Perspectives. 2003;111:A456. doi: 10.1289/ehp.111-a456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell NH, Epstein S, Greene A, Shary J, Oexmann MJ, Shaw S. Evidence for alteration of the vitamin D–endocrine system in obese subjects. Journal of Clinical Investigation. 1985;76:370–373. doi: 10.1172/JCI111971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Compston JE, Vedi S, Ledger JE, Webb A, Gazet JC, Pilkington TR. Vitamin D status and bone histomorphometry in gross obesity. American Journal of Clinical Nutrition. 1981;34:2359–2363. doi: 10.1093/ajcn/34.11.2359. [DOI] [PubMed] [Google Scholar]
- 4.Zamboni G, Soffiati M, Giavarina D, Tato L. Mineral metabolism in obese children. Acta Paediatrica Scandinavica. 1988;77:741–746. doi: 10.1111/j.1651-2227.1988.tb10740.x. [DOI] [PubMed] [Google Scholar]
- 5.Arunabh S, Pollack S, Yeh J, Aloia JF. Body fat content and 25-hydroxyvitamin D levels in healthy women. Journal of Clinical Endocrinology and Metabolism. 2003;88:157–161. doi: 10.1210/jc.2002-020978. [DOI] [PubMed] [Google Scholar]
- 6.Andersen T, McNair P, Fogh-Andersen N, Nielsen TT, Hyldstrup L, Transbol I. Increased parathyroid hormone as a consequence of changed complex binding of plasma calcium in morbid obesity. Metabolism. 1986;35:147–151. doi: 10.1016/0026-0495(86)90116-2. [DOI] [PubMed] [Google Scholar]
- 7.Hey H, Stokholm KH, Lund B, Sorensen OH. Vitamin D deficiency in obese patients and changes in circulating vitamin D metabolites following jejunoileal bypass. International Journal of Obesity. 1982;6:473–479. [PubMed] [Google Scholar]
- 8.Snijder MB, van Dam RM, Visser M, Deeg DJ, Dekker JM, Bouter LM, Seidell JC, Lips P. Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. Journal of Clinical Endocrinology and Metabolism. 2005;90:4119–4123. doi: 10.1210/jc.2005-0216. [DOI] [PubMed] [Google Scholar]
- 9.Holick MF. The cutaneous photosynthesis of previtamin D3: a unique photoendocrine system. Journal of Investigative Dermatology. 1981;77:51–58. doi: 10.1111/1523-1747.ep12479237. [DOI] [PubMed] [Google Scholar]
- 10.Nesby-O’Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA. Hypovitaminosis D prevalence and determinants among African American and White women of reproductive age: third National Health and Nutrition Examination Survey, 1988–94. American Journal of Clinical Nutrition. 2002;76:187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 11.Dawson-Hughes B. Racial/ethnic considerations in making recommendations for vitamin D for adult and elderly men and women. American Journal of Clinical Nutrition. 2004;80:1763S–1766S. doi: 10.1093/ajcn/80.6.1763S. [DOI] [PubMed] [Google Scholar]
- 12.Bell NH, Greene A, Epstein S, Oexmann MJ, Shaw S, Shary J. Evidence for alteration of the vitamin D–endocrine system in blacks. Journal of Clinical Investigation. 1985;76:470–473. doi: 10.1172/JCI111995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. American Journal of Clinical Nutrition. 2004;79:362–371. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 14.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. Journal of the American Medical Association. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 15.Epstein S, Bell NH, Shary J, Shaw S, Greene A, Oexmann MJ. Evidence that obesity does not influence the vitamin D–endocrine system in blacks. Journal of Bone Mineral Research. 1986;1:181–184. doi: 10.1002/jbmr.5650010203. [DOI] [PubMed] [Google Scholar]
- 16.Looker AC. Body fat and vitamin D status in black versus white women. Journal of Clinical Endocrinology and Metabolism. 2004;90:635–640. doi: 10.1210/jc.2004-1765. [DOI] [PubMed] [Google Scholar]
- 17.Roth HJ, Zahn I, Alkier R, Schmidt H. Validation of the first automated chemiluminescence protein-binding assay for the detection of 25-hydroxycalciferol. Clinical Laboratory. 2001;47:357–365. [PubMed] [Google Scholar]
- 18.Enders DB, Rude RK. Vitamin D and its Metabolites. In: Burtis CA, Ashwood ER, editors. Tietz textbook of clinical chemistry. W.B. Saunders; Philadelphia, PA: 1999. pp. 1417–1423. [Google Scholar]
- 19.Ratcliffe WA, Heath DA, Ryan M, Jones SR. Performance and diagnostic application of a two-site immunoradiometric assay for parathyrin in serum. Clinical Chemistry. 1989;35:1957–1961. [PubMed] [Google Scholar]
- 20.Santini SA, Carrozza C, Vulpio C, Capoluongo E, Luciani G, Lulli P, Giardina B, Zuppi C. Assessment of parathyroid function in clinical practice: which parathyroid hormone assay is better? Clinical Chemistry. 2004;50:1247–1250. doi: 10.1373/clinchem.2003.030759. [DOI] [PubMed] [Google Scholar]
- 21.Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. Journal of the American Dietic Association. 1988;88:1268–1271. [PubMed] [Google Scholar]
- 22.Hollingshead A. Four Factor Index of Social Status. Yale University; New Haven: 1975. [Google Scholar]
- 23.Heaney RP. Long-latency deficiency disease: insights from calcium and vitamin D. American Journal of Clinical Nutrition. 2003;78:912–919. doi: 10.1093/ajcn/78.5.912. [DOI] [PubMed] [Google Scholar]
- 24.Grant WB. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer. 2002;94:1867–1875. doi: 10.1002/cncr.10427. [DOI] [PubMed] [Google Scholar]
- 25.Jemal A, Clegg LX, Ward E, Ries LA, Wu X, Jamison PM, Wingo PA, Howe HL, Anderson RN, Edwards BK. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101:3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 26.Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, Thun M. Cancer disparities by race/ethnicity and socioeconomic status. CA: A Cancer Journal for Clinicians. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 27.Harkness L, Cromer B. Low levels of 25-hydroxy vitamin D are associated with elevated parathyroid hormone in healthy adolescent females. Osteoporosis International. 2005;16:109–113. doi: 10.1007/s00198-004-1656-8. [DOI] [PubMed] [Google Scholar]
- 28.Stein MS, Flicker L, Scherer SC, Paton LM, O’Brien ML, Walton SC, Chick P, Di Carlantonio M, Zajac JD, Wark JD. Relationships with serum parathyroid hormone in old institutionalized subjects. Clinical Endocrinology. 2001;54:583–592. doi: 10.1046/j.1365-2265.2001.01182.x. [DOI] [PubMed] [Google Scholar]
- 29.Kamycheva E, Sundsfjord J, Jorde R. Serum parathyroid hormone level is associated with body mass index. The 5th Tromso study. European Journal of Endocrinology. 2004;151:167–172. doi: 10.1530/eje.0.1510167. [DOI] [PubMed] [Google Scholar]
- 30.Parikh SJ, Edelman M, Uwaifo GI, Freedman RJ, Semega-Janneh M, Reynolds J, Yanovski JA. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. Journal of Clinical Endocrinology and Metabolism. 2004;89:1196–1199. doi: 10.1210/jc.2003-031398. [DOI] [PubMed] [Google Scholar]
- 31.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1:74–76. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 32.Holick MF, MacLaughlin JA, Doppelt SH. Regulation of cutaneous previtamin D3 photosynthesis in man: skin pigment is not an essential regulator. Science. 1981;211:590–593. doi: 10.1126/science.6256855. [DOI] [PubMed] [Google Scholar]
- 33.Lo CW, Paris PW, Holick MF. Indian and Pakistani immigrants have the same capacity as Caucasians to produce vitamin D in response to ultraviolet irradiation. American Journal of Clinical Nutrition. 1986;44:683–685. doi: 10.1093/ajcn/44.5.683. [DOI] [PubMed] [Google Scholar]
- 34.Meyer HE, Falch JA, Sogaard AJ, Haug E. Vitamin D deficiency and secondary hyperparathyroidism and the association with bone mineral density in persons with Pakistani and Norwegian background living in Oslo, Norway: The Oslo Health Study. Bone. 2004;35:412–417. doi: 10.1016/j.bone.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. American Journal of Clinical Nutrition. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 36.Rosenstreich SJ, Rich C, Volwiler W. Deposition in and release of vitamin D3 from body fat: evidence for a storage site in the rat. Journal of Clinical Investigation. 1971;50:679–687. doi: 10.1172/JCI106538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mawer EB, Backhouse J, Holman CA, Lumb GA, Stanbury SW. The distribution and storage of vitamin D and its metabolites in human tissues. Clinical Science. 1972;43:413–431. doi: 10.1042/cs0430413. [DOI] [PubMed] [Google Scholar]
- 38.Lafay L, Basdevant A, Charles MA, Vray M, Balkau B, Borys JM, Eschwege E, Romon M. Determinants and nature of dietary underreporting in a free-living population: the Fleurbaix Laventie Ville Sante (FLVS) Study. International Journal of Obesity and Related Metabolic Disorders. 1997;21:567–573. doi: 10.1038/sj.ijo.0800443. [DOI] [PubMed] [Google Scholar]


