Abstract
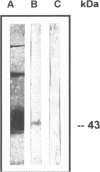
Extracellular matrix protein laminin binds specifically to yeast forms of Paracoccidioides brasiliensis and enhances adhesion of the fungus to the surface of epithelial Madin-Darby canine kidney cells in vitro. Immunoblotting of fungal extracts showed that the gp43 glycoprotein is responsible for adhesion. This was confirmed by binding assays using purified gp43, with a Kd of 3.7 nM. The coating of P. brasiliensis yeast forms with laminin before injection into hamster testicles enhanced the fungus virulence, resulting in a faster and more severe granulomatous disease. These results indicate that interaction of fungi with extracellular matrix elements may constitute a basis for the evolution of fungal infection toward regional spreading and dissemination.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barsky S. H., Rao C. N., Hyams D., Liotta L. A. Characterization of a laminin receptor from human breast carcinoma tissue. Breast Cancer Res Treat. 1984;4(3):181–188. doi: 10.1007/BF01806483. [DOI] [PubMed] [Google Scholar]
- Blotta M. H., Camargo Z. P. Immunological response to cell-free antigens of Paracoccidioides brasiliensis: relationship with clinical forms of paracoccidioidomycosis. J Clin Microbiol. 1993 Mar;31(3):671–676. doi: 10.1128/jcm.31.3.671-676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brummer E., Castaneda E., Restrepo A. Paracoccidioidomycosis: an update. Clin Microbiol Rev. 1993 Apr;6(2):89–117. doi: 10.1128/cmr.6.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant G., Rao C. N., Brentani M., Martins W., Lopes J. D., Martin S. E., Liotta L. A., Schiffmann E. A role for the laminin receptor in leukocyte chemotaxis. J Leukoc Biol. 1987 Mar;41(3):220–227. doi: 10.1002/jlb.41.3.220. [DOI] [PubMed] [Google Scholar]
- Camargo Z. P., Taborda C. P., Rodrigues E. G., Travassos L. R. The use of cell-free antigens of Paracoccidioides brasiliensis in serological tests. J Med Vet Mycol. 1991;29(1):31–38. [PubMed] [Google Scholar]
- Casta e Silva Filho F., de Souza W., Lopes J. D. Presence of laminin-binding proteins in trichomonads and their role in adhesion. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8042–8046. doi: 10.1073/pnas.85.21.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Franco M. Host-parasite relationships in paracoccidioidomycosis. J Med Vet Mycol. 1987 Feb;25(1):5–18. doi: 10.1080/02681218780000021. [DOI] [PubMed] [Google Scholar]
- Gold L. I., Pearlstein E. Fibronectin-collagen binding and requirement during cellular adhesion. Biochem J. 1980 Feb 15;186(2):551–559. doi: 10.1042/bj1860551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover A., Andrews G., Adamson E. D. Role of laminin in epithelium formation by F9 aggregates. J Cell Biol. 1983 Jul;97(1):137–144. doi: 10.1083/jcb.97.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard T. K., Malinoff H. L., Wicha M. S. Macrophages express a plasma membrane receptor for basement membrane laminin. Am J Pathol. 1986 May;123(2):365–370. [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., Cannon F. B., Laurie G. W., Hassell J. R., Aumailley M., Terranova V. P., Martin G. R., DuBois-Dalcq M. Biological activities of laminin. J Cell Biochem. 1985;27(4):317–325. doi: 10.1002/jcb.240270402. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Liotta L. A., Robey P. G., Tryggvason K., Martin G. R. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982 Nov 23;21(24):6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Barsky S. H. Tumor invasion and the extracellular matrix. Lab Invest. 1983 Dec;49(6):636–649. [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986 Jan;46(1):1–7. [PubMed] [Google Scholar]
- Lopes J. D., dos Reis M., Brentani R. R. Presence of laminin receptors in Staphylococcus aureus. Science. 1985 Jul 19;229(4710):275–277. doi: 10.1126/science.3160113. [DOI] [PubMed] [Google Scholar]
- Marquezini M. V., Chammas R., Sonohara S., Brentani M. M. Multiple laminin receptors--a radioligand binding approach. Mem Inst Oswaldo Cruz. 1991;86 (Suppl 3):119–120. doi: 10.1590/s0074-02761991000700023. [DOI] [PubMed] [Google Scholar]
- McCarthy J. B., Palm S. L., Furcht L. T. Migration by haptotaxis of a Schwann cell tumor line to the basement membrane glycoprotein laminin. J Cell Biol. 1983 Sep;97(3):772–777. doi: 10.1083/jcb.97.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen J. G., Bedoya V., Patiño M. M., Salazar M. E., Restrepo A. Experimental murine paracoccidiodomycosis induced by the inhalation of conidia. J Med Vet Mycol. 1987 Jun;25(3):165–175. doi: 10.1080/02681218780000231. [DOI] [PubMed] [Google Scholar]
- Puccia R., Schenkman S., Gorin P. A., Travassos L. R. Exocellular components of Paracoccidioides brasiliensis: identification of a specific antigen. Infect Immun. 1986 Jul;53(1):199–206. doi: 10.1128/iai.53.1.199-206.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puccia R., Travassos L. R. 43-kilodalton glycoprotein from Paracoccidioides brasiliensis: immunochemical reactions with sera from patients with paracoccidioidomycosis, histoplasmosis, or Jorge Lobo's disease. J Clin Microbiol. 1991 Aug;29(8):1610–1615. doi: 10.1128/jcm.29.8.1610-1615.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puccia R., Travassos L. R. The 43-kDa glycoprotein from the human pathogen Paracoccidioides brasiliensis and its deglycosylated form: excretion and susceptibility to proteolysis. Arch Biochem Biophys. 1991 Sep;289(2):298–302. doi: 10.1016/0003-9861(91)90475-x. [DOI] [PubMed] [Google Scholar]
- San-Blas G., Restrepo A., Clemons K., Stevens D. A., San-Blas F., Puccia R., Travassos L. R., Figueroa J. I., Hamilton A. J., Bartholomew M. A. Paracoccidioidomycosis. J Med Vet Mycol. 1992;30 (Suppl 1):59–71. doi: 10.1080/02681219280000771. [DOI] [PubMed] [Google Scholar]
- Speziale P., Hök M., Wadström T., Timpl R. Binding of the basement membrane protein laminin to Escherichia coli. FEBS Lett. 1982 Sep 6;146(1):55–58. doi: 10.1016/0014-5793(82)80704-7. [DOI] [PubMed] [Google Scholar]
- Stambuk B. U., Puccia R., de Almeida M. L., Travassos L. R., Schenkman S. Secretion of the 43 kDa glycoprotein antigen by Paracoccidioides brasiliensis. J Med Vet Mycol. 1988;26(6):367–373. doi: 10.1080/02681218880000521. [DOI] [PubMed] [Google Scholar]
- Switalski L. M., Murchison H., Timpl R., Curtiss R., 3rd, Hök M. Binding of laminin to oral and endocarditis strains of viridans streptococci. J Bacteriol. 1987 Mar;169(3):1095–1101. doi: 10.1128/jb.169.3.1095-1101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switalski L. M., Speziale P., Hök M., Wadström T., Timpl R. Binding of Streptococcus pyogenes to laminin. J Biol Chem. 1984 Mar 25;259(6):3734–3738. [PubMed] [Google Scholar]
- Taborda C. P., Camargo Z. P. Diagnosis of paracoccidioidomycosis by passive haemagglutination assay of antibody using a purified and specific antigen-gp43. J Med Vet Mycol. 1993;31(2):155–160. doi: 10.1080/02681219380000171. [DOI] [PubMed] [Google Scholar]
- Terranova V. P., Rao C. N., Kalebic T., Margulies I. M., Liotta L. A. Laminin receptor on human breast carcinoma cells. Proc Natl Acad Sci U S A. 1983 Jan;80(2):444–448. doi: 10.1073/pnas.80.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]