Abstract
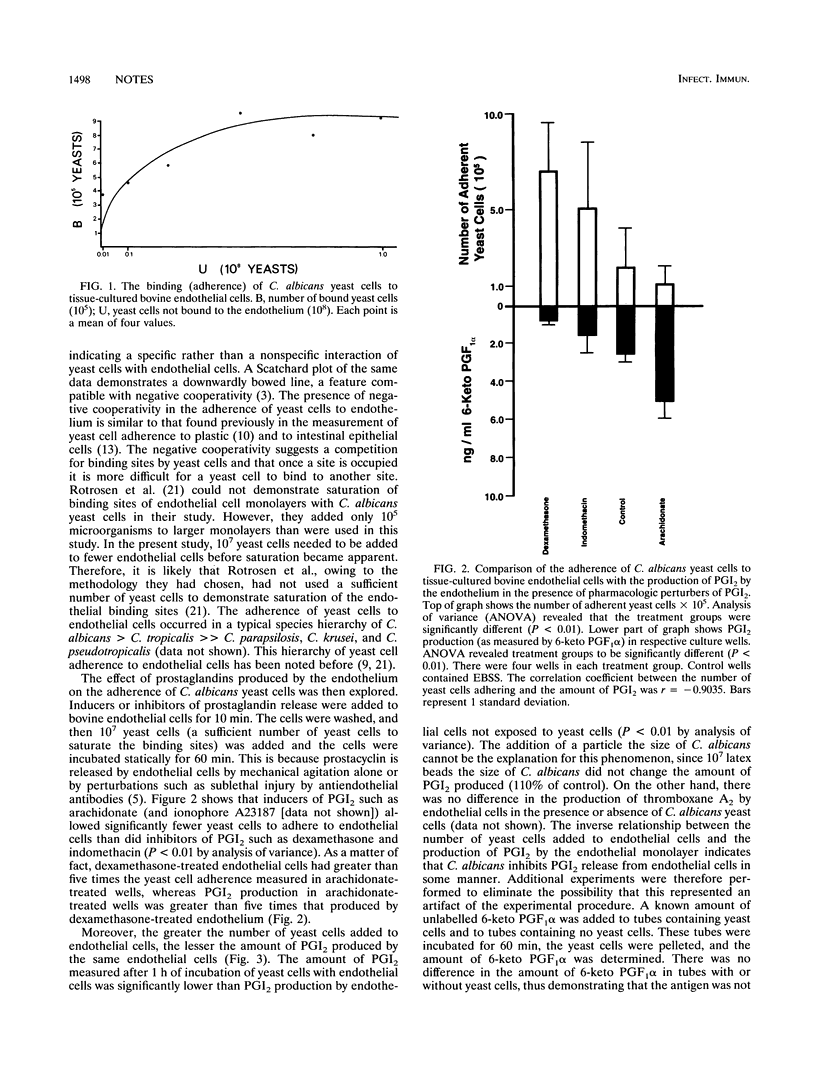
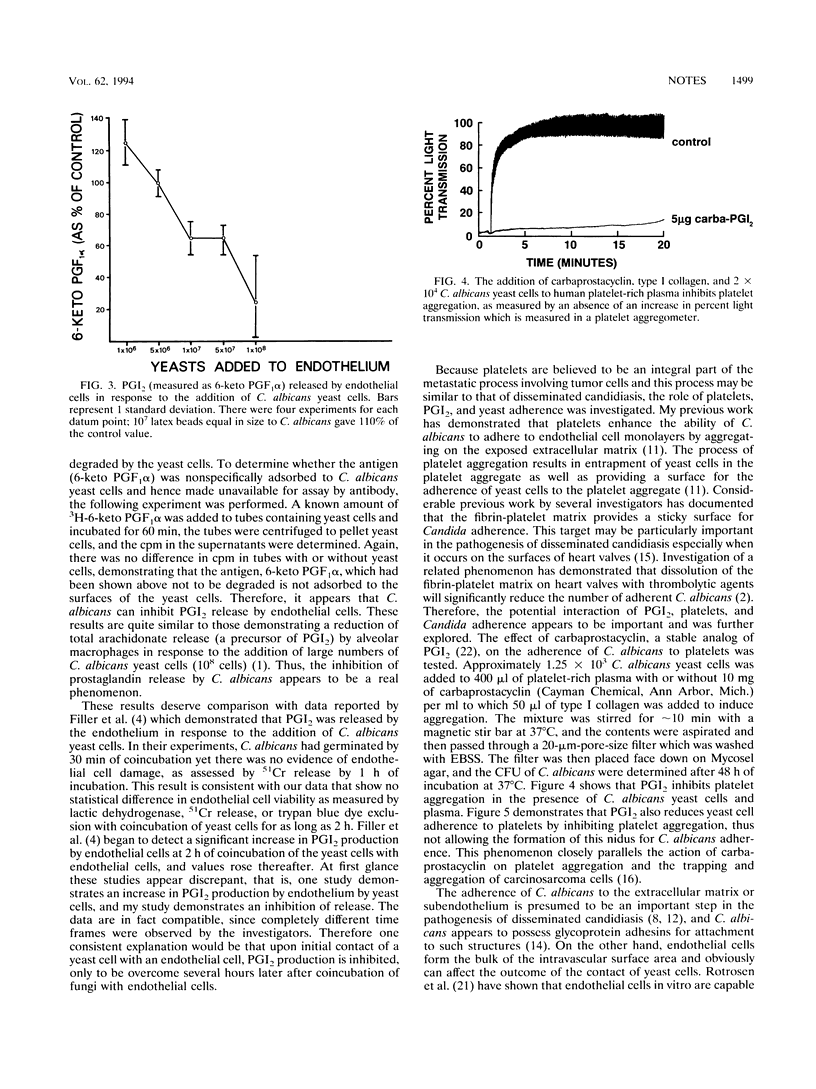
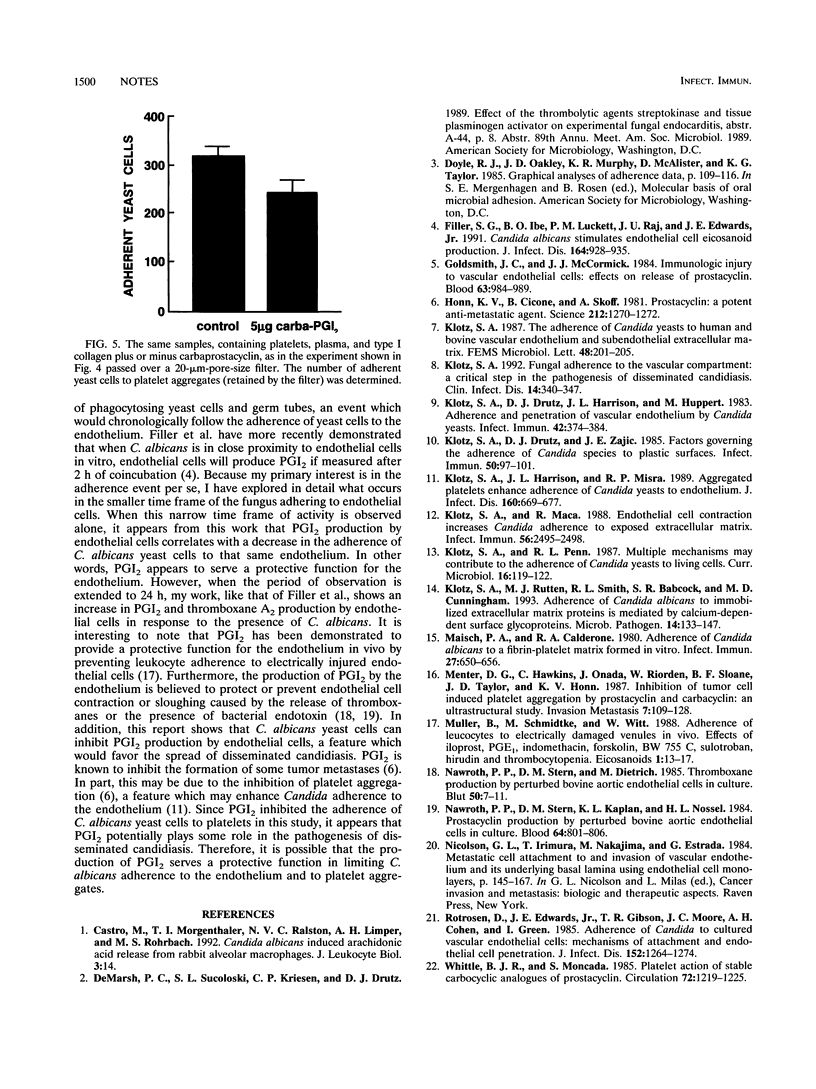
The adherence of Candida species yeast cells to bovine and human endothelium was measured. The adherence phenomenon is a saturable event which can be altered by the release of prostaglandin I2 (PGI2 or prostacyclin) by endothelial cells. Release of PGI2 by endothelium has a protective effect, reducing Candida albicans yeast cell adherence to endothelial cells in a dose-dependent manner. Conversely, C. albicans appears to inhibit the release of PGI2 by endothelial cells and thus perhaps is capable of increasing the number of yeast cells which adhere to endothelium. Furthermore, a long-acting analog of PGI2, carbacyclin, reduced the number of yeast cells adhering to human platelet aggregates. Thus, it is possible that the release of PGI2 by endothelium has a protective effect against the hematogenous dissemination of C. albicans and its ability to adhere to vascular endothelium and fibrin-platelet matrices.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Filler S. G., Ibe B. O., Luckett P. M., Raj J. U., Edwards J. E., Jr Candida albicans stimulates endothelial cell eicosanoid production. J Infect Dis. 1991 Nov;164(5):928–935. doi: 10.1093/infdis/164.5.928. [DOI] [PubMed] [Google Scholar]
- Goldsmith J. C., McCormick J. J. Immunologic injury to vascular endothelial cells: effects on release of prostacyclin. Blood. 1984 May;63(5):984–989. [PubMed] [Google Scholar]
- Honn K. V., Cicone B., Skoff A. Prostacyclin: a potent antimetastatic agent. Science. 1981 Jun 12;212(4500):1270–1272. doi: 10.1126/science.7015512. [DOI] [PubMed] [Google Scholar]
- Klotz S. A., Drutz D. J., Harrison J. L., Huppert M. Adherence and penetration of vascular endothelium by Candida yeasts. Infect Immun. 1983 Oct;42(1):374–384. doi: 10.1128/iai.42.1.374-384.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz S. A., Drutz D. J., Zajic J. E. Factors governing adherence of Candida species to plastic surfaces. Infect Immun. 1985 Oct;50(1):97–101. doi: 10.1128/iai.50.1.97-101.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz S. A. Fungal adherence to the vascular compartment: a critical step in the pathogenesis of disseminated candidiasis. Clin Infect Dis. 1992 Jan;14(1):340–347. doi: 10.1093/clinids/14.1.340. [DOI] [PubMed] [Google Scholar]
- Klotz S. A., Harrison J. L., Misra R. P. Aggregated platelets enhance adherence of Candida yeasts to endothelium. J Infect Dis. 1989 Oct;160(4):669–677. doi: 10.1093/infdis/160.4.669. [DOI] [PubMed] [Google Scholar]
- Klotz S. A., Maca R. D. Endothelial cell contraction increases Candida adherence to exposed extracellular matrix. Infect Immun. 1988 Sep;56(9):2495–2498. doi: 10.1128/iai.56.9.2495-2498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz S. A., Rutten M. J., Smith R. L., Babcock S. R., Cunningham M. D. Adherence of Candida albicans to immobilized extracellular matrix proteins is mediated by calcium-dependent surface glycoproteins. Microb Pathog. 1993 Feb;14(2):133–147. doi: 10.1006/mpat.1993.1014. [DOI] [PubMed] [Google Scholar]
- Maisch P. A., Calderone R. A. Adherence of Candida albicans to a fibrin-platelet matrix formed in vitro. Infect Immun. 1980 Feb;27(2):650–656. doi: 10.1128/iai.27.2.650-656.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menter D. G., Harkins C., Onoda J., Riorden W., Sloane B. F., Taylor J. D., Honn K. V. Inhibition of tumor cell induced platelet aggregation by prostacyclin and carbacyclin: an ultrastructural study. Invasion Metastasis. 1987;7(2):109–128. [PubMed] [Google Scholar]
- Müller B., Schmidtke M., Witt W. Adherence of leucocytes to electrically damaged venules in vivo. Effects of iloprost, PGE1, indomethacin, forskolin, BW 755 C, sulotroban, hirudin, and thrombocytopenia. Eicosanoids. 1988;1(1):13–17. [PubMed] [Google Scholar]
- Nawroth P. P., Stern D. M., Dietrich M. Thromboxane production by perturbed bovine aortic endothelial cells in culture. Blut. 1985 Jan;50(1):7–11. doi: 10.1007/BF00319763. [DOI] [PubMed] [Google Scholar]
- Nawroth P. P., Stern D. M., Kaplan K. L., Nossel H. L. Prostacyclin production by perturbed bovine aortic endothelial cells in culture. Blood. 1984 Oct;64(4):801–806. [PubMed] [Google Scholar]
- Rotrosen D., Edwards J. E., Jr, Gibson T. R., Moore J. C., Cohen A. H., Green I. Adherence of Candida to cultured vascular endothelial cells: mechanisms of attachment and endothelial cell penetration. J Infect Dis. 1985 Dec;152(6):1264–1274. doi: 10.1093/infdis/152.6.1264. [DOI] [PubMed] [Google Scholar]
- Whittle B. J., Moncada S. Platelet actions of stable carbocyclic analogues of prostacyclin. Circulation. 1985 Dec;72(6):1219–1225. doi: 10.1161/01.cir.72.6.1219. [DOI] [PubMed] [Google Scholar]


