Abstract
Nanometer scale apolipoprotein A-I stabilized phospholipid disk complexes (nanodisks; ND) have been formulated with the polyene antibiotic amphotericin B (AMB). The present studies were designed to evaluate if a peptide can substitute for the function of the apolipoprotein component of ND with respect to particle formation and stability. An 18-residue synthetic amphipathic α-helical peptide, termed 4F (Ac-D-W-F-K-A-F-Y-D-K-V-A-E-K-F-K-E-A-F-NH2), solubilized vesicles comprised of egg phosphatidylcholine (egg PC), dipentadecanoyl PC or dimyristoylphosphatidylcholine (DMPC) at rates greater than or equal to solubilization rates observed with human apolipoprotein A-I (apoA-I; 243 amino acids). Characterization studies revealed that interaction with DMPC induced a near doubling of 4F tryptophan fluorescence emission quantum yield (excitation 280 nm) and a ~7 nm blue shift in emission wavelength maximum. Inclusion of AMB in the vesicle substrate resulted in formation of 4F AMB-ND. Spectra of AMB containing particles revealed the antibiotic is a highly effective quencher of 4F tryptophan fluorescence emission, giving rise to a Ksv = 7.7 × 104. Negative stain electron microscopy revealed that AMB-ND prepared with 4F possessed a disk shaped morphology similar to ND prepared without AMB or prepared with apoA-I. In yeast and pathogenic fungi growth inhibition assays, 4F AMB-ND was as effective as apoA-I AMB-ND. The data indicate that AMB-ND generated using an amphipathic peptide in lieu of apoA-I form a discrete population of particles that possess potent biological activity. Given their intrinsic versatility, peptides may be preferred for scale up and clinical application of AMB-ND.
Keywords: nanodisk, amphotericin B, dimyristoylphosphatidylcholine, apolipoprotein A-I, peptide, electron microscopy
1. Introduction
Recently we described a novel lipid formulation of the water insoluble antibiotic amphotericin B (AMB), termed nanodisks (ND), comprised of a nanometer scale disk shaped phospholipid bilayer stabilized by recombinant human apolipoprotein A-I (apoA-I) [9]. ND are formed by a reaction between amphipathic apolipoproteins and phospholipid vesicles to which AMB has been added. AMB-ND formulated with apoA-I are approximately 10 nm in diameter and are organized as a disk shaped phospholipid bilayer. The edge of the bilayer is stabilized by the apolipoprotein, which interacts with otherwise solvent exposed phospholipid fatty acyl chains. AMB was stably incorporated into ND particles at a concentration of 2.5 mg AMB per 10 mg phospholipid. Formulation of AMB into apolipoprotein stabilized ND preserves the potent biological activity of AMB against yeast and pathogenic fungi yet attenuates toxicity of this antibiotic toward red blood cells and cultured hepatoma cells [9]. AMB-ND display in vivo efficacy in mice infected with the pathogenic fungi, Candida albicans [9] as well as the protozoal parasite, Leishmania major [8].
In the process of characterizing AMB-ND and evaluating their therapeutic potential in treatment of systemic fungal infections [9] or leishmaniasis [8], it was recognized that the apolipoprotein component of the ND particle represents a limiting factor. Whereas apolipoproteins serve key functions in facilitating ND formation and as a structural component of the product particles, we hypothesized that peptides represent a potential alternative to recombinant apolipoprotein. The requirement of such a peptide would be a reasonably short sequence together with the ability to induce formation of AMB-ND with retention of the stability and biological properties demonstrated for apolipoprotein containing ND. Human apoA-I mimetic peptides have been described that are capable of solubilizing phospholipid vesicles [6].
One such peptide, termed 18A, has been extensively studied [4,5]. Derivatives of this have been made by substituting Phe for Leu residues on the nonpolar face of the amphipathic alpha helix [2]. A helical wheel depiction of the resulting peptide, termed 4F, is shown in Figure 1. The peptide displays characteristic features of a Class A amphipathic alpha helix [13] including localization of positively charged lysine residues at the polar-nonpolar interface and clustering of negatively charged residues at the apex of the polar face. Here, we have examined the ability of 4F to fulfill the role of apoA-I as a component of AMB-ND. The results obtained indicate that 4F efficiently induces formation AMB-ND from AMB enriched phospholipid bilayer vesicles, producing a discrete population of the particles that retain biological activity.
Figure 1. Helical wheel projection of 4F.
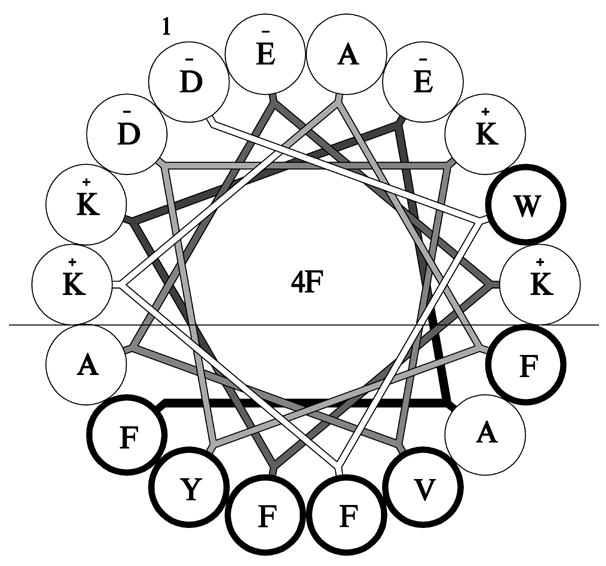
The amphipathic nature of 4F is illustrated with the line denoting the polar – nonpolar interface. Bold font indicates hydrophobic residues. The figure was prepared using AutoCAD LT 2007.
2. Material and Methods
2.1 Materials
AMB (USP grade) was obtained from Research Organics Inc. Dimyristoylphosphatidylcholine (DMPC) and dipentadecanoyl phosphatidylcholine (PC) were from Avanti Polar Lipids Inc. and egg PC was from Sigma. 4F peptide was synthesized by the solid phase method with a Protein Technologies PS-3 automated peptide synthesizer as described previously [2]. Recombinant human apoA-I was produced according to Ryan et al. [12].
2.2 AMB-ND preparation
ND particles were prepared essentially as described by Oda et al. [9]. Briefly, 10 mg DMPC was dissolved in chloroform: methanol (3:1 v/v) and dried under a stream of nitrogen gas, coating the vessel wall with the phospholipid. The tube was then lyophilized for a minimum of 2 h to remove residual organic solvent. Following this the lipids were dispersed in 1 ml phosphate buffered saline (PBS; 20 mM sodium phosphate, pH 7.0, 150 mM sodium chloride) by vortexing. To the dispersed lipid 2.5 mg AMB from a stock solution (30 mg/ml in dimethylsulfoxide; DMSO) was added. Subsequently, 4 mg 4F peptide or apoA-I (in PBS) was added and the solution incubated at 24 °C. Following bath sonication to induce sample clarification, the respective ND solutions were dialyzed overnight against PBS. Empty 4F-ND and empty apoA-I ND were prepared as described above except AMB was omitted. ND were sterilized by 0.22 μm syringe filtration prior to use.
2.3 Analytical procedures
Protein concentrations were determined by the bicinchoninic acid assay (Pierce Chemical Co.) with bovine serum albumin as standard. Peptide concentrations were determined spectrophotometrically. Absorbance spectroscopy was performed on a Perkin- Elmer Lambda 20 spectrophotometer. AMB levels were determined using an extinction coefficient at 416 nm = 1.214 × 105 M−1 cm−1 in DMSO [1].
2.4 Lipid binding studies
Bilayer vesicles of egg PC, dipentadecanoyl PC or DMPC were prepared in 20 mM sodium phosphate, pH 7.0 by extrusion through a 200 nm filter as described by Weers et al. [16]. Unless otherwise specified, 100 μg phospholipid was incubated at a given temperature in a thermostated cell holder in the absence or presence of 40 μg 4F peptide or apoA-I (sample volume = 400 μl). Sample right angle light scattering intensity was monitored as a function of time on a Perkin-Elmer LS 50B luminescence spectrometer, with the excitation and emission monochromaters set at 600 nm (3.6 nm slit width).
2.5 Fluorescence studies
Fluorescence spectra were obtained on a Perkin-Elmer LS 50B luminescence spectrometer. Samples were excited at 280 nm and emission was monitored from 300 – 500 nm. Quenching studies were performed by addition of exogenous AMB to a solution of empty 4F ND with determination of AMB concentration dependent changes in 4F Trp fluorescence emission intensity. Quenching data were analyzed by the Stern-Volmer equation: F0/F = 1 + Ksv [Q] where F0 and F represent the emission maximum in the absence and presence of quencher, respectively. The collisional quenching constant was estimated from the slope of plots of F0/F versus [Q].
2.6 Electron microscopy
ND samples for electron microscopy (EM) were prepared as described above using Tris buffered saline (TBS) as solvent. Three μl of a 0.02 mg/ml ND solution was applied to a carbon-coated 400 mesh grid (Ted Pella, Tustin, CA) previously rendered hydrophilic by glow-discharge and blotted dry with Whatman filter paper. Negative staining was conducted using 3 μl of a 2 % solution of uranyl acetate, and again blotted with Whatman paper. Grids were transferred to a JEOL 1230 electron microscope, operating at 120 keV acceleration.
2.7 Yeast growth inhibition assays
Cultures of the yeast, Saccharomyces cerevisiae, were grown in yeast extract peptone glucose broth media (YEPD; Teknova, Hollister, CA). Twenty μl of a saturated overnight culture (OD600 = 13) was used to inoculate 5 ml YEPD media in the presence of indicated amounts of AMB-ND, empty ND, 4F peptide alone or apoA-I alone. Cultures were grown for 16 h at 30 °C with rotation and the extent of culture growth monitored by measuring sample turbidity at 600 nm.
2.8 Pathogenic fungi growth inhibition assays
Microtiter broth growth inhibition assays were conducted with three species of pathogenic fungi, Aspergillus fumigatus (ATCC#: 16424), Candida albicans (ATCC #: 90028) and Cryptococcus neoformans (isolate H99, ATCC #: 208821). Fungi were cultured in RPMI 1640 medium buffered with MOPS to pH 7.0. The final inoculum was 1 × 105 spores/ml (A. fumigatus), 1 × 104 cells/ml (C. albicans), and 5 × 105 cells/ml (C. neoformans). All experiments were performed in triplicate at 35º C for 48 h (except C. albicans, which were conducted for 24 h) according to established protocols [10, 15]. All samples tested were soluble in the standard RPMI medium used and no precipitation or interference was seen in any of the samples tested against either fungal species. Inhibitory activity of given ND formulations were determined from cultures grown in the absence or presence of AMB concentrations ranging from 0.01 – 8.4 μg/ml. Minimal inhibitory concentration (MIC) values were determined visually using a colorimetric indicator that changes hue in the presence of metabolically active organisms.
3. Results
3.1 Phospholipid vesicle solubilization studies
A critical aspect of AMB-ND formation relates to the ability of apolipoproteins to disrupt AMB containing phospholipid vesicles, transforming them into disk-shaped bilayers. 4F and apoA-I were characterized with respect to their relative ability to induce a time-dependent decrease in the light scattering intensity of candidate phospholipid vesicle substrates (Figure 2), including egg PC (Panel A) dipentadecanoyl PC (Panel B) and DMPC (Panel C). Incubation of phospholipid vesicles alone had no effect on the light scattering intensity of the sample. In the case of substrate vesicles prepared with egg PC, although apoA-I was ineffective at solubilizing the vesicle substrate, 4F induced a rapid, albeit partial, decrease in vesicle light scatter intensity at 40 μg peptide / 100 μg phospholipid. Doubling the amount of peptide to 80 μg resulted in complete egg PC vesicle solubilization. In the case of the synthetic saturated chain phospholipid, dipentadecanoyl PC, apoA-I was unable to induce a significant decrease in vesicle light scattering intensity upon incubation at 33 °C (the gel to liquid transition temperature of this phospholipid). On the other hand, 4F induced dipentadecanoyl PC vesicle solubilization as a function of time. Consistent with the known properties of apoA-I [11,13], it was able to disrupt DMPC vesicles upon incubation at the gel to liquid phase transition temperature of this lipid (23.9 °C). At the same time, however, 4F was equally effective, indicating that 4F and apoA-I are equivalent in terms of their ability to solubilize DMPC vesicles. Based on relative vesicle solubilization activity of 4F with different phospholipids, subsequent studies were carried out with DMPC.
Figure 2. Effect of 4F peptide or apoA-I on the light scattering intensity of phospholipid vesicles.
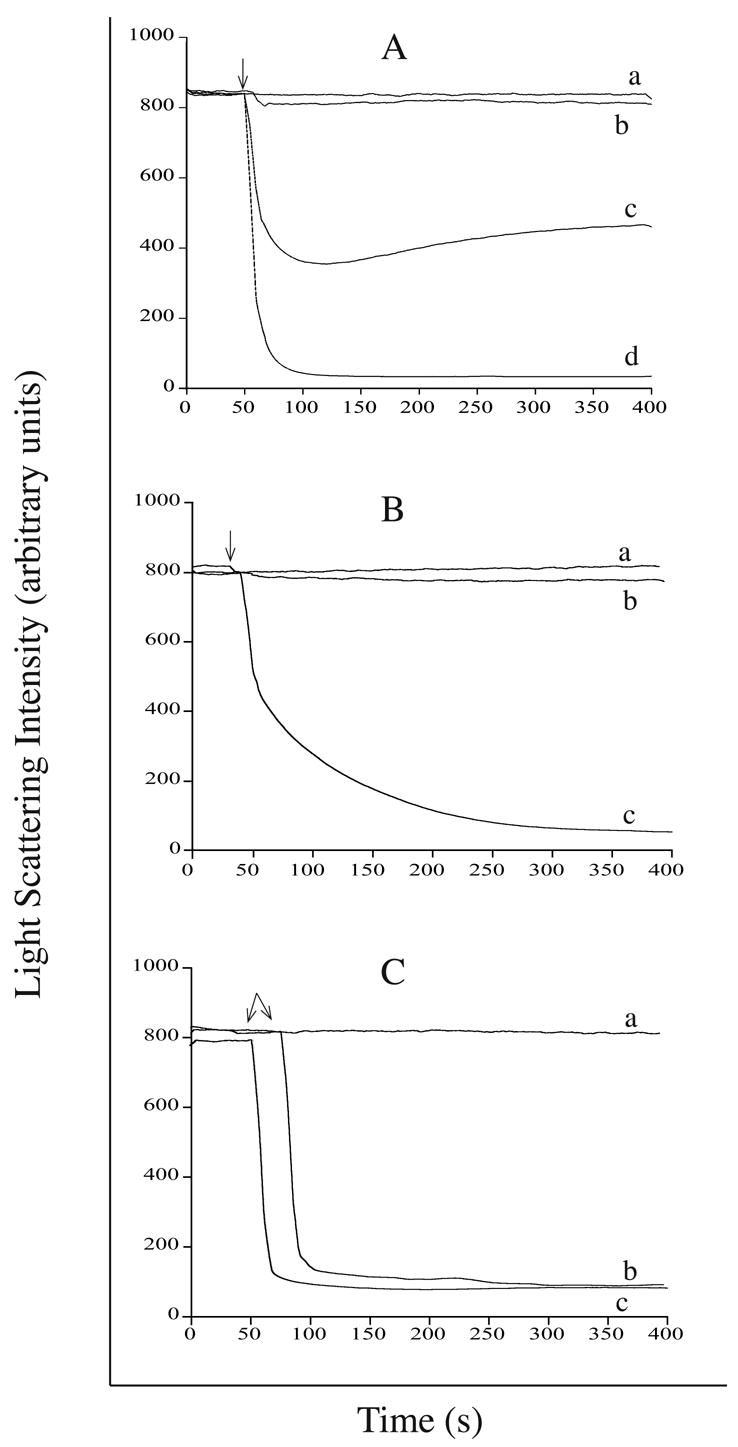
Panel A) egg PC vesicles (100 μg) were incubated with a) buffer alone; b) 40 μg apoA-I c) 40 μg 4F peptide or d) 80 μg 4F peptide. Panel B) dipentadecanoyl PC vesicles (100 μg) were incubated in a) buffer alone; b) 40 μg apoA-I or c) 40 μg 4F peptide. Panel C) DMPC vesicles (100 μg) were incubated with a) buffer alone; b) 40 μg apoA-I or c) 40 μg 4F peptide. Arrows indicate the time at which protein was added to the lipid substrate. Right angle light scattering intensity was monitored as a function of time on a Perkin-Elmer LS 50B luminescence spectrometer with excitation and emission monochromaters set at 600 nm (3.6 nm slit width).
3.2 Fluorescence studies
4F contains a single Trp residue that provides a potentially useful intrinsic fluorescent probe. Indeed, whereas 4F in buffer has a wavelength of maximum Trp fluorescence emission of 350 nm (excitation 280 nm), binding to DMPC to generate empty 4F ND induced a ~7 nm blue shift in emission wavelength maximum together with a near doubling of Trp fluorescence emission quantum yield (Figure 3). When fluorescence spectra of 4F AMB-ND were evaluated, however, a very different result was obtained. Trp fluorescence emission was strongly reduced suggesting AMB quenching of 4F Trp fluorescence emission. To characterize the ability of AMB to quench 4F Trp fluorescence aliquots of AMB were added to a solution of empty 4F ND and the effect on 4F Trp fluorescence emission intensity determined. An AMB concentration dependent decrease in Trp fluorescence emission intensity was observed that was maximal at 8 μg AMB per 40 μg 4F ND. A Stern-Volmer plot of the quenching data revealed a Ksv = 7.7 × 104.
Figure 3. Effect of lipid and AMB on 4F Trp fluorescence emission.
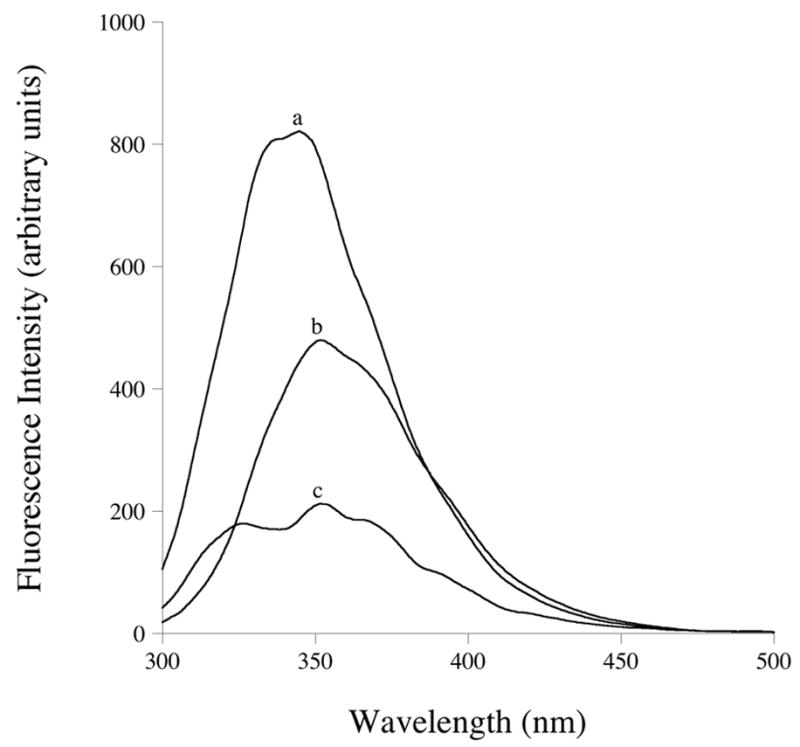
4F peptide in buffer (b); empty 4F ND (a) and 4F AMB-ND (c). Samples were excited at 280 nm and emission monitored from 300 to 500 nm.
3.3 Electron microscopy
To characterize the structure and morphology of ND particles prepared with 4F, negative stain-electron microscopy was performed. 4F AMB-ND were similar in morphology to apoA-I AMB-ND reported earlier [9], displaying a population of particles with diameter of ~12 nm (range 6–24 nm) (Figure 4).
Figure 4. Electron microscopy of ND samples.
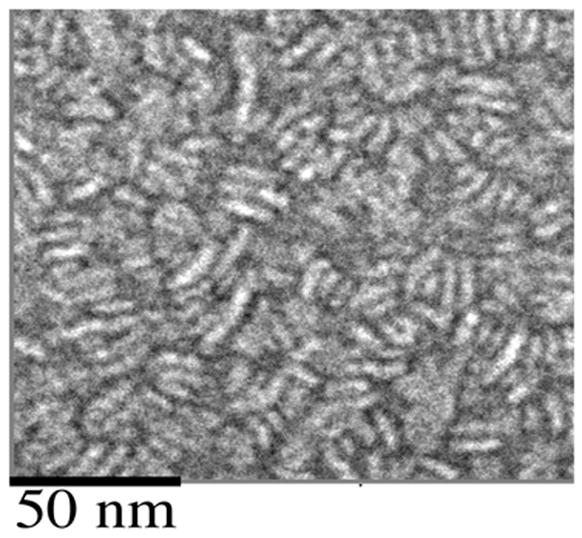
A sample of 4F-AMB-ND, negatively stained with uranyl acetate, was imaged on a JEOL 1230 transmission electron microscope at 120 keV. Magnification = 40 000x.
3.4 Biological activity of 4F AMB-ND
To evaluate if substitution of 4F for apoA-I in AMB-ND affects its biological activity, growth inhibition assays were performed with the yeast, S. cerevisiae. As shown in Figure 5, 4F AMB-ND and apoA-I AMB-ND were equivalent in terms of their ability to inhibit S. cerevisiae growth. Fifty % growth inhibition was achieved at 0.09 μg/ml and 0.12 μg/ml for 4F AMB-ND and apoA-I AMB-ND, respectively. In control incubations, empty ND, 4F peptide alone or apoA-I alone had no inhibitory activity at concentrations 50x the maximum concentration shown in Fig. 5 (data not shown). As shown in Table 1, both 4F AMB-ND and apoA-I AMB-ND were effective inhibitors of C. albicans, A. fumigatus and C. neoformans growth. Empty 4F ND or empty apoA-I ND did not inhibit fungal growth.
Figure 5. Effect of apoA-I and 4F AMB-ND on growth of Saccharomyces cerevisiae.
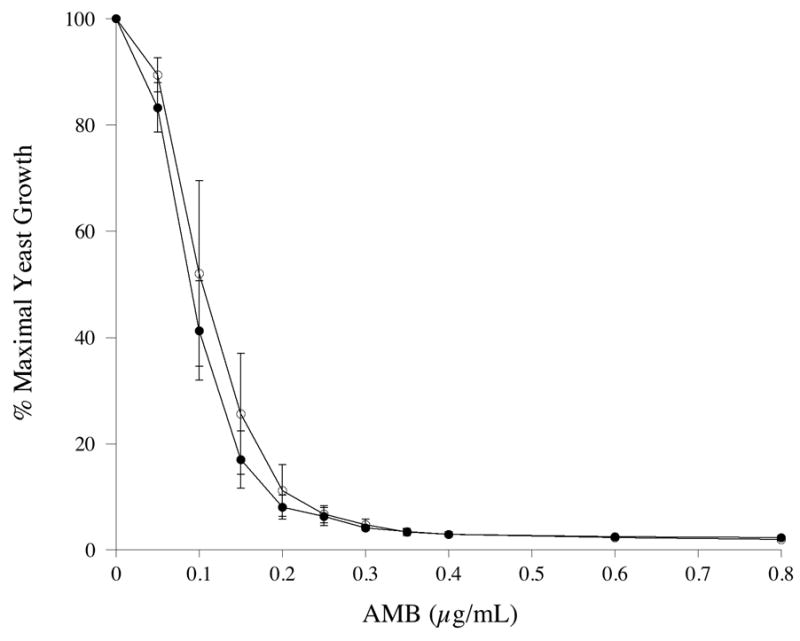
Twenty μl of a saturated overnight culture of S. cerevisiae grown in YEPD media was used to inoculate 5 ml YEPD media in the presence of indicated amounts of specified AMB-ND. Cultures were incubated at 30°C for 16 h at which time the extent of culture growth was determined spectrophotometrically. apoA-I AMB-ND (○); 4F AMB-ND (●). Values reported are the mean ± S.D. (n= 3).
Table 1.
Effect of ND on pathogenic fungal growth.
| MIC (μg/ml)1 | ||||
|---|---|---|---|---|
| Fungal Species2 | 4F AMB-ND | ApoA-I AMB-ND | empty 4F ND | empty apoA-I ND |
| A. fumigatus | 0.06 | 0.10 ± .04 | NI3 | NI |
| C. albicans | 0.02 | 0.02 ± .01 | NI | NI |
| C. neoformans | 0.02 | 0.02 | NI | NI |
MIC = minimum inhibitory concentration. All values reported are the mean ± S.D. (n = 3). When not shown, standard deviation was zero.
Fungi were cultured and growth determined as described in Materials and Methods.
NI = non-inhibitory.
4. Discussion
Studies have shown that rationally designed amphipathic peptides can serve as excellent models for apolipoproteins. In the case of ND drug delivery vehicles, these particles are formed by taking advantage of the ability of amphipathic apolipoproteins to solubilize certain phospholipid vesicle substrates, transforming them into a relatively homogeneous population of disk-shaped bilayers whose perimeter is circumscribed by apolipoprotein molecules. Characterization studies have shown that the apolipoprotein component orients with the long axis of their alpha helices perpendicular to the fatty acyl chains of phospholipid molecules around the edge of the disk [3]. In this manner, the hydrophobic face of individual helix segments interacts with the acyl chains while the polar face is directed toward the aqueous milieu. Given this molecular organization, it is conceivable that amphipathic helical peptides could substitute for the protein component of ND.
Our results indicate that 4F and apoA-I form ND particles of similar size and efficiently solubilize significant amounts of the water insoluble polyene antibiotic, AMB. Indeed, in terms of phospholipid vesicle solubilization activity, 4F appears to be superior to apoA-I. Similar to results reported earlier, 4F but not apoA-I, can solubilize egg PC [2]. In studies of 4F fluorescence properties upon association with DMPC and AMB, we found that AMB is a highly effective quencher of Trp fluorescence emission, suggesting AMB has associated with the ND particles. These findings were verified and extended by electron microscopy. Micrographs of AMB ND prepared using 4F or apoA-I displayed similar morphology, comprised of a discoidal shape with a diameter in the range of 12.5 nm. Since the biological activity of AMB-ND prepared with 4F were equivalent to that of AMB-ND prepared with apoA-I, these results suggest that 4F may offer a suitable substitute for apoA-I for formulation of AMB-ND.
The advantages of 4F versus apoA-I as a component of ND structure include the much smaller size of the peptide (18 amino acids versus 243 in the case of apoA-I). Given this large difference in molecular size and the fact that the product ND are of similar diameter, it is evident that multiple copies of peptide must align around the perimeter of the ND particle. Previous characterization studies revealed that apoA-I ND possess two copies of apoA-I, with the protein aligned in a belt-like manner around the periphery of the disk particle. Considering that 4F ND have a similar diameter, it is reasonable to speculate that 4F ND contain 20 or more peptide molecules per disc, presumably aligned perpendicular to the fatty acyl chains of ND phospholipids oriented with the hydrophobic face of the alpha helix directed toward the particle surface.
Whereas 4F AMB-ND displayed equivalent biological activity to apoA-I AMB-ND with respect to yeast and pathogenic fungi growth inhibition properties, it remains to be determined if these results can be extended to an in vivo setting. At the same time, it is worth noting that 4F and related peptides are known to function as anti-atherosclerotic agents in vivo [6, 7]. Thus, it is likely that 4F itself will not manifest in vivo toxicity when presented as a component of ND.
Another key advantage of peptides versus protein as a component of ND particle structure relates to versatility. Given the utility of solid phase peptide synthesis versus bacterial expression of recombinant apolipoprotein using GMP methods, the cost of producing AMB-ND may be significantly reduced. Furthermore, this approach eliminates the possibility that bacterial toxins such as lipopolysaccharide may contaminate a given ND preparation. The ease of amino acid substitution also simplifies optimization studies since large numbers of amino acid substitutions can be introduced and rapidly evaluated.
Acknowledgments
This work was supported by grants from the National Institutes of Health (AI-162541 and AI-061354). The authors would like to thank James Murray for his aid in preparing the helical wheel diagram as well as Jennifer Beckstead for assistance with graphics. J.B.P., R.L.B. and P.D.H. acknowledge support from the Livermore Laboratory Science and Technology Office under 06-SI-003. We also acknowledge microscopy facility access from Dr. Holland Cheng, Department of Molecular and Cellular Biology, University of California at Davis. This work was performed under the auspices of the United States Department of Energy by the University of California, Lawrence Livermore National Laboratory under contract number W-7405-ENG-48.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bolard J, Legrand P, Heitz F, Cybulska B. One-sided action of amphotericin B on cholesterol-containing membranes is determined by its self-association in the medium. Biochemistry. 1991;30:5707–15. doi: 10.1021/bi00237a011. [DOI] [PubMed] [Google Scholar]
- 2.Datta G, Chaddha M, Hama S, Navab M, Fogelman AM, Garber DW, et al. Effects of increasing hydrophobicity on the physical-chemical and biological properties of a class A amphipathic helical peptide. J Lipid Res. 2001;42:1096–104. [PubMed] [Google Scholar]
- 3.Davidson WS, Silva RA. Apolipoprotein structural organization in high density lipoproteins: belts, bundles, hinges and hairpins. Curr Opin Lipidol. 2005;16:295–300. doi: 10.1097/01.mol.0000169349.38321.ad. [DOI] [PubMed] [Google Scholar]
- 4.Hristova K, Wimley WC, Mishra VK, Anantharamiah GM, Segrest JP, White SH. An amphipathic alpha-helix at a membrane interface: a structural study using a novel X-ray diffraction method. J Mol Biol. 1999;290:99–117. doi: 10.1006/jmbi.1999.2840. [DOI] [PubMed] [Google Scholar]
- 5.Mishra VK, Anantharamaiah GM, Segrest JP, Palgunachari MN, Chaddha M, Sham SW, et al. Association of a model class A (apolipoprotein) amphipathic alpha helical peptide with lipid: high resolution NMR studies of peptide.lipid discoidal complexes. J Biol Chem. 2006;281:6511–9. doi: 10.1074/jbc.M511475200. [DOI] [PubMed] [Google Scholar]
- 6.Navab M, Anantharamaiah GM, Reddy ST, Hama S, Hough G, Grijalva VR, et al. Apolipoprotein A-I mimetic peptides. Arterioscler Thromb Vasc Biol. 2005;25:1325–31. doi: 10.1161/01.ATV.0000165694.39518.95. [DOI] [PubMed] [Google Scholar]
- 7.Navab M, Anantharamaiah G, Reddy ST, Van Lenten BJ, Datta G, Garber D, et al. Potential clinical utility of high-density lipoprotein-mimetic peptides. Curr Opin Lipidol. 2006;17:440–4. doi: 10.1097/01.mol.0000236371.27508.d4. [DOI] [PubMed] [Google Scholar]
- 8.Nelson KG, Bishop J, Ryan RO, Titus R. Nanodisk-associated amphotericin B fully clears Leishmania major infection in susceptible BALB/c mice. Antimicrob Agents Chemother. 2005;50:1238–44. doi: 10.1128/AAC.50.4.1238-1244.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oda MN, Hargreaves P, Beckstead JA, Redmond KA, Van Antwerpen R, Ryan RO. Reconstituted high-density lipoprotein enriched with the polyene antibiotic, amphotericin B. J Lipid Res. 2005;47:260–7. doi: 10.1194/jlr.D500033-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Pfaller MA, Barry AL. Evaluation of a novel colorimetric broth microdilution method for antifungal susceptibility testing of yeast isolates. J Clin Microbiol. 1994;32:1992–96. doi: 10.1128/jcm.32.8.1992-1996.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pownall HJ, Massey JB, Kusserow SK, Gotto AM. Kinetics of lipid-protein interactions: interaction of apolipoprotein A-I from human plasma high density lipoproteins with phosphatidylcholines. Biochemistry. 1978;17:1183–88. doi: 10.1021/bi00600a008. [DOI] [PubMed] [Google Scholar]
- 12.Ryan RO, Forte TM, Oda MN. Optimized bacterial expression of human apolipoprotein AI. Protein Exp Purif. 2003;27:98–103. doi: 10.1016/s1046-5928(02)00568-5. [DOI] [PubMed] [Google Scholar]
- 13.Segrest JP, Garber DW, Brouillette CG, Harvey SC, Anantharamaiah GM. The amphipathic alpha helix: a multifunctional structural motif in plasma apolipoproteins. Adv Protein Chem. 1994;45:303–69. doi: 10.1016/s0065-3233(08)60643-9. [DOI] [PubMed] [Google Scholar]
- 14.Tall AR, Small DM, Deckelbaum RJ, Shipley GG. Structure and thermodynamic properties of high density lipoprotein recombinants. J Biol Chem. 1977;252:4701–11. [PubMed] [Google Scholar]
- 15.Tiballi RN, He X, Zarins LT, Revankar SG, Kauffman CA. Use of a colorimetric system for yeast susceptibility testing. J Clin Microbiol. 1995;33:915–7. doi: 10.1128/jcm.33.4.915-917.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weers PMM, Narayanaswami V, Ryan RO. Modulation of the lipid binding properties of the N-terminal domain of human apolipoprotein E3. Eur J Biochem. 2001;268:3728–35. doi: 10.1046/j.1432-1327.2001.02282.x. [DOI] [PubMed] [Google Scholar]


