Abstract
Upon morphogenesis, the simple neuroepithelium of the optic vesicle gives rise to four basic tissues in the vertebrate optic cup: pigmented epithelium, sensory neural retina, secretory ciliary body and muscular iris. Pigmented epithelium and neural retina are established through interactions with specific environments and signals: periocular mesenchyme/BMP specifies pigmented epithelium and surface ectoderm/FGF specifies neural retina. The anterior portions (iris and ciliary body) are specified through interactions with lens although the molecular mechanisms of induction have not been deciphered. As lens is a source of FGF, we examined whether this factor was involved in inducing ciliary body. We forced the pigmented epithelium of the embryonic chick eye to express FGF4. Infected cells and their immediate neighbors were transformed into neural retina. At a distance from the FGF signal, the tissue transitioned back into pigmented epithelium. Ciliary body tissue was found in the transitioning zone. The ectopic ciliary body was never in contact with lens tissue. In order to assess the contribution of the lens on the specification of normal ciliary body, we created optic cups in which the lens had been removed while still pre-lens ectoderm. Ciliary body tissue was identified in the anterior portion of lens-less optic cups. We propose that the ciliary body may be specified at optic vesicle stages, at the same developmental stage when the neural retina and pigmented epithelium are specified and we present a model as to how this could be accomplished through overlapping BMP and FGF signals.
Keywords: Ciliary body, development, chick, eye, retina, retrovirus, FGF
INTRODUCTION
The ciliary body is a muscular and secretory tissue, found directly behind the lens. Its main role is to produce the aqueous fluid that fills the eye and nourishes the lens and cornea. The continual production of aqueous maintains the eye in a pressurized and inflated state, which is required for the correct alignment of the visual apparatus (Hart, 1992). Accumulation of pressure within the embryonic eye has been shown to be essential for its continued growth and development (Coulombre, 1956; Coulombre and Coulombre, 1957). In addition to fluid production, the ciliary body is also the source for many of the proteins of the inner limiting membrane (ILM), a basal lamina organized by the endfeet of the retinal ganglion cells of the retina. The assembly and presence of this retinal basal lamina is essential for survival of the ganglion cells (Halfter et al., 2005b). Likewise, proteins of the vitreous, such as collagenIX and tenascin-C are synthesized from the ciliary body. The expression of these ciliary specific proteins begins well before overt differentiation of the ciliary body. These expression data are among several lines of evidence implying that the ciliary body may be functional before it is histologically recognizable (Beebe, 1986).
In the chick, the optic cup is formed by embryonic day 2 (e2)/HH stage 14 (Hamburger and Hamilton, 1992). For the next 3 days of incubation, as the eye enlarges, the presumptive ciliary body/iris epithelia at the margin of the optic cup are not remarkable in any way. They can be distinguished by a lack of neurogenesis, as compared to the neural retina. Periocular neural crest adds itself around the double layered epithelium of the optic cup. In the posterior eye, the mesenchyme forms the sclera and choroid. In the front of the eye, the mesenchyme coalesces by e6 on the margin of the optic cup to form the stroma of the ciliary body, and more anteriorly, the iris. The iris becomes deeply pigmented and the ciliary body develops its characteristic folded appearance after e8.
The epithelial layers of the ciliary body are a continuation of the retina of the optic cup; thus, the inner non-pigmented ciliary epithelium is contiguous with the neural retina and the outer pigmented ciliary epithelium is contiguous with the retinal pigmented epithelium. The abrupt transition between sensory neural retina and non-pigmented ciliary body epithelium is seen at the ora serrata in the adult chick eye and becomes noticeable after e7. The bilayered epithelium of the iris is the further anterior extension and finally the tip of the optic cup-derived neuroepithelium. As these widely different cell types in the eye share a common origin, one reasonable explanation for their ontogeny is that the environment that each portion of the optic cup finds itself is instructive. The tissue interactions in the back of the eye are very different from those in the front, with the main difference being the presence of the lens in the front of the eye, in close contact with the lip of the optic cup throughout development. However, two very different types of tissue differentiate from the neuroepithelium of the anterior optic cup. Therefore, there is a question as to how the lens can induce the secretory ciliary body epithelium and the muscular iris epithelium. To date, the molecular mechanism behind the role of the lens in the development of the anterior optic cup has not been elucidated.
In this report, we describe ciliary body tissue that is found at the edges of patches of induced neural retina. Neural retina was induced within the pigmented epithelium by infecting pigmented epithelial cells with an FGF-expressing retrovirus. The pigmented epithelium is readily converted into neural retina after exposure to FGF or even to activated elements of the downstream signaling cascade (Azuma et al., 2005; Galy et al., 2002; Hyer et al., 1998; Vogel-Hopker et al., 2000; Zhao et al., 2002; Zhao et al., 2001). In the optic vesicle the neuroepithelium is specified as neural retina on one end and pigmented epithelium at the other, in a linear fashion (Chen and Cepko, 2000; Nguyen and Arnheiter, 2000). We propose that the introduction of an FGF source into pigmented epithelium recreates the conditions of the optic vesicle, implying that the ciliary body is specified in the optic vesicle. We test this idea by isolating the optic vesicle from the influence of the overlying surface ectoderm once the neural retina has been specified. The optic vesicle continues to develop and forms a lens-less optic cup. A ciliary body domain can be identified in the resulting eye tissue. We present a model to explain these findings and discuss them in the context of what is currently understood about the development of the anterior of the optic cup.
MATERIALS AND METHODS
In situ hybridization
A plasmid containing a 586 bp fragment specific for the long isoform of the chick collagenIXα1 was kindly provided by Drs. David Beebe and Elena Frolova. A plasmid containing a 1.3 kb fragment of the chicken HuD gene was kindly provided by Dr. James Weston. Plasmids containing fragments of chicken nidogen and lamininβ1 were kindly provided by Dr. Willi Halfter. A 330-bp full-length thymosinβ4 probe was generated by RT-PCR using cDNA from e14 chicken embryonic brain as template (SuperScriptTM III First-Strand Synthesis for RT-PCR, Invitrogen). The amplified product was cloned into pCR2.1 (Invitrogen), sequenced, and used for making riboprobes (cRNA). Antisense and control sense cRNA probes were made using digoxygenin-labelled dUTP (Roche) and RNA polymerases (Promega), according to standard labeling protocols (Promega). Paraffin and cryo-sections were prepared at 10 μm, dewaxed (for paraffin), and in situ hybridization was performed according to standard protocols (Nieto et al., 1996; Schaeren-Wiemers and Gerfin-Moser, 1993). Described briefly here: 1-After rehydration/thawing, sections were treated in proteinase K solution, refixed, acetylated and dried slightly. 2-Approximately 1 μg/ml of indicated probe was added to hybridization solution (40% formamide, 5X SSC, with additives) and slides were incubated overnight at 65C. After washing in SSC/formamide solution and treatment with RNAse to remove any non-specific bound probe, the sections were blocked using Blocking Reagent (Roche) and incubated overnight with alkaline phosphatase labeled anti-DIG Fab’ fragments (Roche). After extensive washing, the probe was detected using BCIP/NBT (Roche). Staining was continued until signal could be clearly detected in comparison with the sense control.
Immunohistochemistry
All tissue was excised, freshly frozen in a mixture of OCT: sucrose and sectioned at 10 μm. Upon thawing, all slides were fixed for 5 min in 4% PFA. Monoclonal antibodies against islet-1 protein (clone 40.2D), collagenIX protein (clone 2c2), tenascin-C protein (clone M1-B4), β-gal protein (clone 40-1A) and were used at a concentration of 1:4. These antibodies were obtained through the Developmental Studies Hybridoma Bank, under the auspices of the NICHD and maintained by the University of Iowa. Sheep polyclonal antibody against delta-crystallin was a kind gift from Dr. Joram Piatigorsky and was used at 1:1000. Monoclonal antibodies against Hu (Molecular Probes, Eugene OR) and rabbit polyclonal antibodies against β-gal protein (Cappel) were used at dilutions of 1:500 on 10μm frozen sections. Monoclonal antibody against connexin43 (BD Transduction Labs) was used at a concentration of 1:10,000. Secondary anti-mouse: AlexaFluor 568 (red label) and anti-rabbit: AlexaFluor 488 and anti-mouse: AlexaFluor 488 (green label) antibodies (Molecular Probes) were used at 1:500. Secondary anti-sheep: Cy3 antibodies (Jackson Immunologicals) were used at a concentration of 1:500. Imaging and capture were done on an Olympus AX-70 coupled to a Hamamatsue cooled-LCD camera using OpenLab software.
Retroviral injection
Biological activities of the FGF construct used in this study have been extensively described (Itoh et al., 1996; Mikawa, 1995; Mima et al., 1995). The retrovirus used is a replication-defective variant based on the avian spleen necrosis virus. Retroviral propagation, testing and injection have been described extensively in Hyer et al. (1997). Briefly, retroviral particles are collected from the supernatant of packaging cells and centrifuged at 15,000g, 25C for 1.5 hour. Particles are resuspended at a concentration of 106 virions/ml with 100μg/ml final concentration of polybrene (Hexadimethrine bromide-Sigma). Testing of infectivity and titer is done by infecting a test culture and visualizing with X-gal histochemistry. The retroviral vector called pZid is a new construct based on the Moloney Murine Leukemia Virus, and was developed as a method to create high titers of replication incompetent retroviral vectors that express dominant negative constructs, where it is not possible to create virus producing cell lines with the construct. A complete description of the system is being prepared for publication. To inject retroviruses of either type, a small hole is made in the eggshell and the embryo exposed. A pulled glass needle is filled with viral solution, directed to the desired tissue and pressure injected (Harvard Apparatus Injector model PLI-100). The embryos are sealed with Parafilm (American Can) and re-incubated until the desired age.
Experimental lens-less optic cups
Microsurgical techniques and the production of lens-less optic cups have been fully described in Hyer et al. 2003. Briefly, the embryos are carefully staged such that they have 16 or 17 somites and no lens placode (HH stage 12+). A solution of 1.5% Nile blue sulfate (Sigma) in water was applied to the ectoderm overlying the optic vesicle. This causes a slight blistering of the ectoderm, and facilitates its removal with glass needles, without damaging the underlying neuroectoderm. The embryos are then resealed and reincubated for the specified number of hours, removed and processed for staining protocols.
RESULTS
The formation of non-neurogenic transition zones
A replication-incompetent retrovirus that co-expresses FGF and beta-galactosidase was targeted to the pigmented epithelium domain of a stage-10 optic vesicle by microinjection (Fig 1A). The infected cells and their progeny can be followed by staining with X-gal. After infections, embryos were resealed and incubated for a further 4 days. All studies were done on infected and normal eyes at embryonic day 5 (e5). Eyes infected with the FGF-expressing retrovirus had areas of depigmentation on the eye, compared with those infected with control retrovirus expressing only β-gal (Fig 1B, control data not shown). X-gal staining revealed that depigmented areas form on the eye, around infected cells (arrow, Fig 1B). Depigmented areas form at all positions on the orb; areas in the most posterior of the eye cannot be seen in whole mount view.
Figure 1.
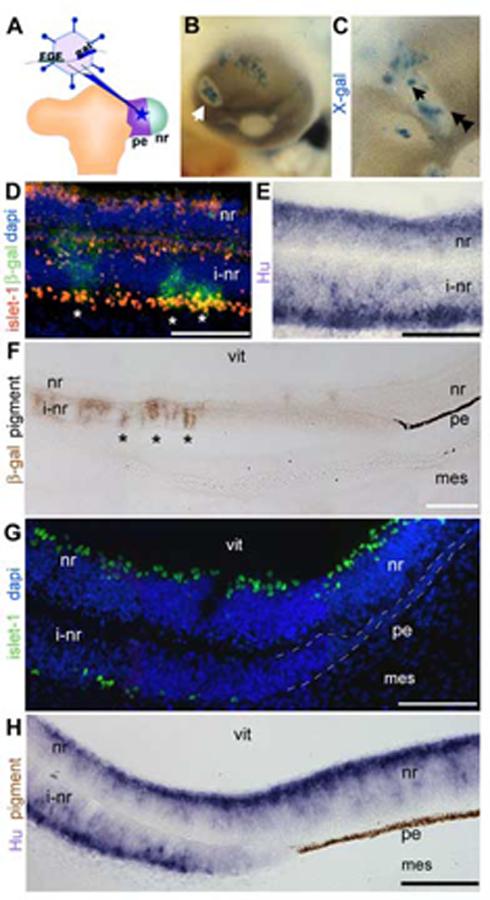
Pigmented epithelium transforms into neural retina and a non-neurogenic, non-pigmented tissue.
(A) Schematic of the operation for introducing FGF-expressing retrovirus into the pigmented epithelium. The retroviral vector co-expresses FGF and β-gal and is introduced into the pigmented epithelium domain of the optic vesicle. (B) Whole mount chick eye at e5 after infection as in A. Infected cells (blue: X-gal) found at the center of an area of depigmentation (arrow). (C) Magnified view of a depigmented patch, single arrow indicates center, double arrowheads indicate transition zone. (D) Immunohistochemistry on sections through center of infected portions of eye as in C (single arrow) with antibodies against islet-1 (red) and β-gal (green). (E) In situ hybridization on sections as in D were examined using cRNA directed against Hu (purple). (F) Immunohistochemistry on sections through the edges of infected portions of the eye as in C (double arrowhead), with antibody against β-gal (brown) to indicate foci of infection. At a distance from the infected cells, the induced neural retina transitions back into pigmented epithelium. (G) Transition zone as in F probed with an antibody against islet-1 (green) and DAPI nuclear stain. (H) Transition zone as in F expressing Hu (purple). Brown is endogenous pigmentation. pe: pigmented epithelium, nr: neural retina, vit: vitreal surface, i-nr: induced neural retina, mes: periocular mesenchyme. Scale: in D-G bar=50 μm; in H, I bar=100 μm.
Sections generated through the center of depigmented areas (single arrowhead, Fig 1C) were analyzed for expression of islet-1 and Hu. Islet-1 recognizes differentiating retinal ganglion cells while Hu expression identifies newly committed neurogenic cells of the neural retina, and therefore is expressed more robustly at e5. In the eye, Hu is specific for the retina. These stains revealed that the depigmented regions were areas where the pigmented epithelium has been converted into neural retina, as expected (Fig 1D,E).
Examination of the edges of the depigmented regions (double arrowheads, Fig 1C) in section revealed that at a distance from the FGF source/infected cells, visualized with immunohistochemistry against β-gal, the induced neural retina transitioned back into a pigmented epithelium (Fig 1F). In the intervening region, between induced neural retina and pigmented epithelium, was a transition zone that was not neurogenic, as shown by lack of islet-1 (Fig 1H) or Hu expression (Fig 1I). As shown clearly in Fig 1F and H, the transition zone was not pigmented.
Non-neurogenic transition zones express collagenIX, a ciliary body marker
The non-pigmented, non-neurogenic transition zones, created at the edges of induced neural retina patches, were reminiscent of the non-pigmented, non-neurogenic epithelium at the lip of the optic cup. The anterior of the e5 chick optic cup is not anatomically distinguishable from the rest of the retina. However, it is already expressing collagenIX, a vitreal protein that is synthesized and secreted from the ciliary body throughout development (Halfter et al., 2005a). The collagenIX expressing anterior does not overlap with the forming retina, as identified with islet-1 (Fig 2A). Although collagenIX is found within the vitreous and in the forming sclera, the only neuroepithelium tissue to express collagenIX is the optic cup margin.
Figure 2.
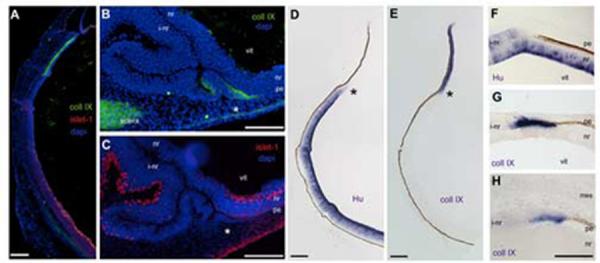
CollagenIX is expressed at the transition from pigmented to neural tissue.
Immunohistochemistry and in situ hybridization on e5 eye tissue. (A) Antibodies against collagenIX (green) and islet-1 (red) are expressed in unique domains. Blue is DAPI staining. (B) CollagenIX is ectopically expressed in the transition zone (asterisk). (C) Section adjacent to B, the transition zone in B is contiguous with an induced neural retina, identified by islet-1 (red). (D, E) HuD and CollagenIX expression on adjacent sections of normal eye, asterisks indicate mutually exclusive expression of HuD and collagenIX (F) Transition zone, HuD expression. (G) CollagenIX expression, adjacent section to F. (H) CollagenIX expression in a transition zone at the extreme posterior of the optic cup. L: lens, vit: vitreal surface, pe: pigmented epithelium, nr: neural retina, vit: vitreal surface, i-nr: induced neural retina, mes: periocular mesenchyme. Scale: in A-E bar=150μm; in F-H bar =50μm.
We next examined adjacent sections through transition zones at the edges of FGF-induced neural retina patches with these markers. CollagenIX is expressed in the thickened non-pigmented tissue immediately adjacent to the pigmented epithelium tissue. At a distance from the transition zone, the contiguous layer could be recognized as an islet-1 expressing induced neural retina (Fig 2C).
Islet-1 recognizes neurogenic cells only once they have differentiated. We wondered if the induced collagenIX expression in the transition zones was a unique expression domain, or a subset of a neural retina domain. We examined e5 eye and transition zones for the expression of Hu and for the expression of a ciliary body-specific isoform of collagenIX (formally referred to as collagenIX α1-chain long isoform). Hu expression in the endogenous neural retina was robust and this was reduced as the tissue continued anteriorly (Fig 2D). CollagenIX expression, in contrast, showed a strong and unique expression in the anterior optic cup/future ciliary body, and this expression did not overlap with Hu expression (Fig 2E). The expression of Hu and collagenIX in the optic neuroepithelium appear to be mutually exclusive.
Using the same pair of markers, we examined adjacent sections through transition zones at the edges of FGF-induced neural retina patches. We examined patches that formed in the front half of the orb and patches that formed in the back half, surrounded by the peri-ocular mesenchyme. In the front of the eye, the induced neural retina does not have strong Hu expression, but it is still considerably thickened compared to the endogenous neural retina underneath it (Fig 2F). CollagenIX is expressed in the tissue immediately after it loses pigmentation, and that expression ceases once the tissue can be identified as retina, by Hu expression (Fig 2G). This discrete expression of collagenIX in the transition zone was also seen when the transition zone formed in the back of the eye, at a point removed from any lens influence (Fig 2H).
Additional markers of the presumptive ciliary body are expressed in transition zones
To determine if the collagenIX expression data was indicating that the transition zone was ciliary body tissue, we examined several other ciliary body markers on FGF-induced transition zones.
Laminin1 is a component of the inner limiting membrane, a retina specific basal lamina. It has been demonstrated that the mRNAs for the 3 distinct protein subunits that make up the laminin1 trimer (alpha1, beta1 and gamma1) are each expressed in ciliary body, and not in the sensory neural retina (Dong and Chung, 1991; Dong et al., 2002; Sarthy and Fu, 1990). Using a probe directed against lamininβ1, we saw high levels of expression in the ciliary margin, compared with adjacent neural retina (Fig 3A). When we examined the transition zone between pigmented epithelium and induced neural retina, we saw that expression of lamininβ1 mRNA was induced in the non-pigmented tissue immediately adjacent to the contiguous pigmented epithelium (Fig 3B,C).
Figure 3.
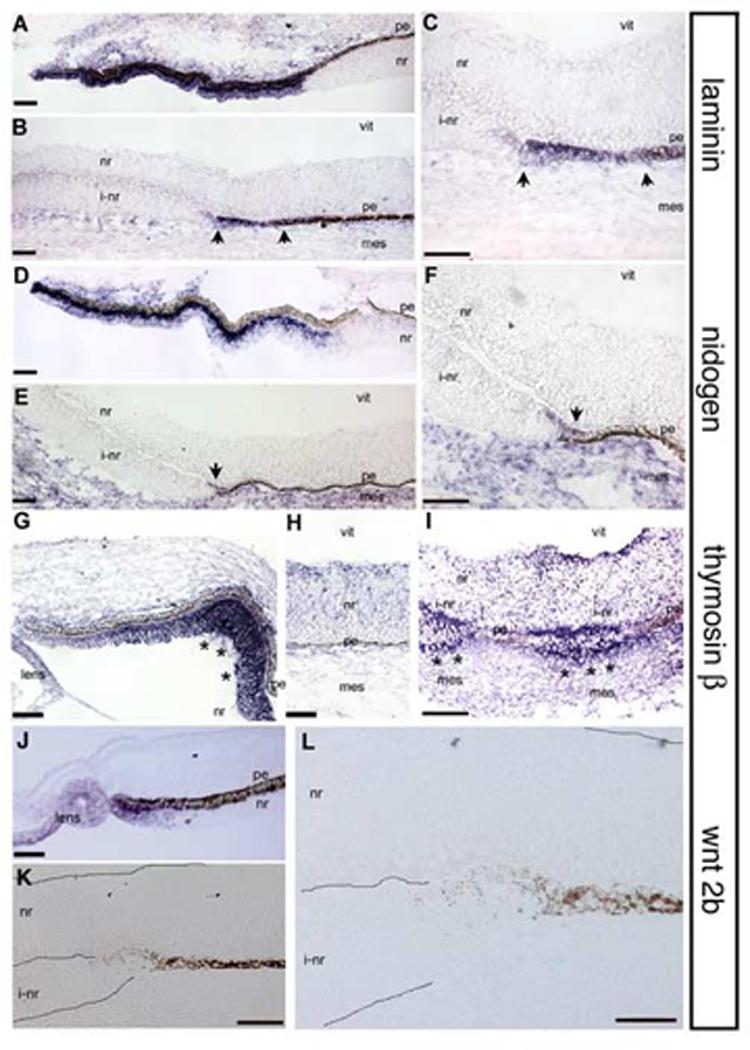
Expression of ciliary body genes in transition zones.
In situ hybridization on e5 tissue. (A) Lamininβ1 is expressed in the presumptive ciliary body and not in the neural retina. (B,C) Lamininβ1 is expressed in the transition zone between induced neural retina and pigmented epithelium (arrows). (D) Nidogen is expressed in the presumptive ciliary body and not in the neural retina. (E,F) Nidogen expression is also seen in induced transition zones (arrow). (G,H) Thymosinβ4 is expressed throughout the optic tissue but in the ciliary body, expression is upregulated (asterisks). (I) Thymosinβ4 is upregulated in the transition zones compared to the endogenous neural retina (asterisks). (J) Wnt2b is specifically expressed at the tip of the optic cup and in the lens epithelium. (K,L) Induced transition zone in the same tissue section as J; wnt2b expression can not be identified. Abbreviations as in Fig 1. Scale: bar=50μm.
We next looked at the expression of nidogen in an e5 ciliary margin and transition zone. Like laminin, nidogen is a component of the inner limiting membrane, and is synthesized and secreted from the ciliary body (Halfter et al., 2000). Although there is expression of nidogen in the adjacent peri-ocular mesenchyme, the message is not detected in the neural retina (Halfter et al., 2000), Fig 3D). In the transition zone, we saw slight but defined expression of nidogen mRNA (arrows, Fig 3E,F).
We also examined the expression of thymosinβ4, an actin binding protein. We find that thymosinβ4 mRNA is expressed ubiquitously at low levels throughout the neural retina, lens and surrounding mesenchyme in the eye. This is not surprising, given that its expected role is in cytoskeletal rearrangement and the neural retina at this stage of development is actively organizing. However, there is a striking upregulation of thymosinβ4 message in the presumptive ciliary body, when compared to the neural retina expression (Fig 3G,H). When we looked for thymosinβ4 expression in transition zones, we saw upregulation of thymosinβ4 mRNA, clearly over the endogenous levels of the juxtaposed neural retina. In a unique example in which the sections revealed two small transition zones separated by pigmented epithelium, we saw upregulation of thymosinβ4 distinctly in each of the transition zones (Fig 3I). This section did not capture the continuation of the transition zone into induced neural retina (at approximately 120 μm more posterior).
Connexin43 was selected as an additional marker because it is the predominant gap junction protein in the adult ciliary body in both mammals (Coca-Prados et al., 1992; Coffey et al., 2002), and chicken (Kubota et al., 2004). One hallmark of the double layered epithelium of the ciliary body are the many junctional complexes that are elaborated (Raviola, 1971). In the developing mouse eye, connexin43 is expressed in the pigmented epithelium, but expression of mRNA and protein in the non-pigmented layers is only seen in the cup margin; connexin43 is not expressed in the neural retina portion of the eye (Ruangvoravat and Lo, 1992; Yancey et al., 1992). We also find a similar expression pattern in the e5 chick eye (Fig 4A,B). Connexin43 protein was highly expressed in the presumptive ciliary body compared to the low expression levels in the posterior of the eye (Fig 4B). When we examined transition zones, we found high levels of connexin43 expressed in the transformed non-pigmented tissue, compared to its neighboring pigmented epithelium (Fig 4C,D).
Figure 4.
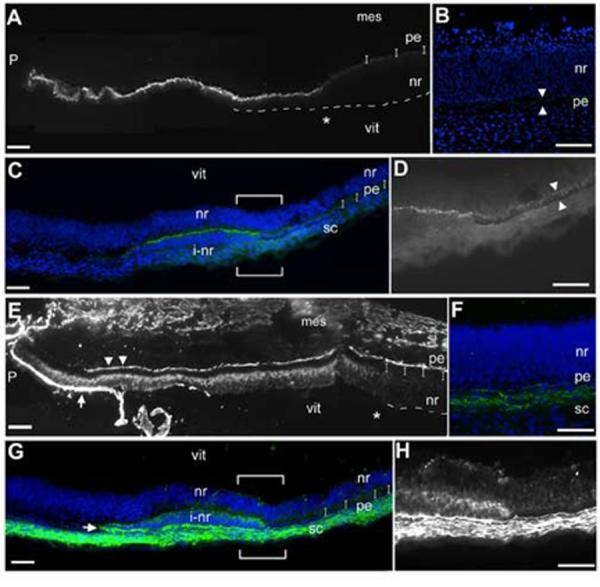
Expression of ciliary body proteins in transition zones.(A) Connexin43 immunohistochemistry shows that the protein is highly expressed at the margins of the optic cup and that expression drops off sharply at the future ora serrata (asterisk). Dashes outline the extent of neural retina and small brackets indicate where the pigmented epithelium is. (B) View of the posterior retina of the stained section in A; In comparison to the anterior margin, the pigmented epithelium expresses low levels of connexin43 (arrowheads) and the neural retina is completely negative. (C) In a small transition zone, connexin43 is highly expressed. Large brackets indicate the area shown in D. (D) Higher magnification of the border between pigmented and non-pigmented tissue in the transition zone shows that high connexin43 expression and a pigmented phenotype are mutually exclusive; pigmented epithelium expresses low levels of connexin (arrowheads). (E) Tenascin-C immunohistochemistry shows that the protein is expressed in the non-pigmented epithelium of the ciliary body. Bright staining on the basal surfaces of the pigmented epithelium (arrowheads) and at the margin (arrow) indicates sites of secretion. Expression drops off at the future ora serrata (asterisk). (F) A view of the posterior retina of the stained section in E: the neural retina is completely negative, while the sclera is positive. (G) In a small transition zone, adjacent to the section shown in C, tenascin-C is expressed within the non-pigmented tissue of the transition zone. Arrow indicates a staining that resembles secreted protein, as in E, arrow. Large brackets indicate the area shown in H. (H) Higher magnification of the border between pigmented epithelium and non-pigmented tissue. All tissue examined at e5. P: pupil opening, vit: vitreal opening, sc: forming sclera, other abbreviations as in Fig 1. Scale: bar=50μm.
Finally, we examined the expression of tenascin-C. Tenascin-C is a component of the vitreous, in addition to being widely expressed throughout the nervous system (Perez and Halfter, 1993; Tucker, 1991). During chick eye development, the protein is first detectable in the margins of the optic cup (Perez and Halfter, 1994). We found that the e5 ciliary margin was strongly positive for tenascin-C protein (Fig 4E), while the neural retina was negative (Fig 4F). The forming sclera also expressed high levels of tenascin-C, and a line of tenascin-C was seen on the mesenchymal interface with the pigmented epithelium. The ciliary body synthesizes and secretes tenacin-C, thus a bright band of immunopositive material was usually seen in the vitreous adjacent to the ciliary body. In transition zones, tenascin-C was expressed within the transformed tissue (Fig 4G,H), similar to the expression seen in the non-pigmented epithelium of the ciliary body (Fig 4E). Interestingly, the transition zones sometimes had an associated bright band of staining (Fig 4G, arrowhead).
The ciliary body is specified in the lens-less optic cup
We were able to show that the addition of FGF as an isolated factor was involved in inducing ciliary body tissue. Our results indicate that a comparatively low concentration of FGF was required, since the induction always occurred at a distance from the infection/source. FGF is one of several growth factors produced in the lens (Lovicu and McAvoy, 2005). We wanted to assess whether the FGF contribution of the lens was sufficient for ciliary body specificity. Using techniques established in the lab (Hyer et al., 2003) we removed the pre-lens ectoderm at stage 12+ prior to formation of the lens placode, in order to create lens-less optic cups (Fig 5A). We had found that the early removal of the ectoderm precludes lens development, yet the optic vesicle invaginates normal. The embryos were resealed and allowed to incubate for a further 24 hours/stage 16 (n=10). When they were examined, it was clear that the operated eye had invaginated but no lens was seen in the whole mount view (Fig 5B,C). Sections were generated through the eye region and demonstrated that the operated eye had formed a cup, but without forming a lens (Fig 5D). Sections through the entire eye region were immunostained for delta-crystallin, the major avian crystallin, expressed from the point of lens placode formation through development of a mature lens (Kamachi et al., 2001). No delta-crystallin was seen at any point in the operated eye, confirming that no lens tissue had formed; the contralateral control eye served as an internal positive control (Fig 5E,F). We looked for islet1 and collagenIX expression, but no signals could be detected, and we concluded that the proteins expression was insufficiently robust at HH stage 16.
Figure 5.
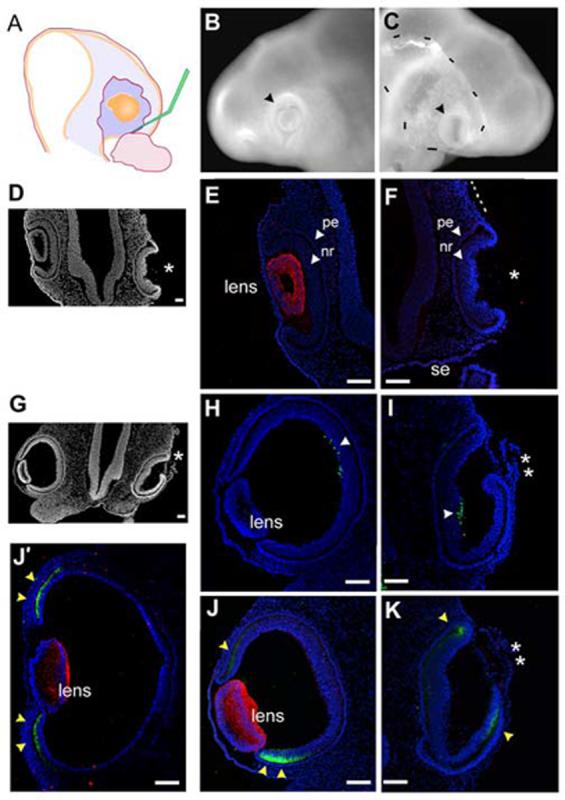
The optic cup can form without concomitant lens formation.
(A) Stage 12+ pre-lens ectoderm is surgically removed from over the optic vesicle. (B) Contralateral control eye, 24 hr after surgery (stage 16). Optic vesicle has formed a cup (arrowhead) with a centrally located lens. (C) Operated side, the vesicle has invaginated and no lens is apparent (arrowhead). Dashes indicate the edge of surface ectoderm, as it has not reformed over the optic tissue. (D) Section (coronal) through the eye region of embryo in B,C. Operated side formed an optic cup, no lens is seen (asterisk). (E) Delta-crystallin expression identifies lens tissue, optic cup morphology is seen with DAPI stained nuclei. (F) Delta-crystallin expression is not seen in the lens-less optic cup. The surface ectoderm is not present over the optic cup or the more dorsal portion of the head (dashed line). (G) Low magnification view of section through the eye region of an embryo removed 40 hr (stage 19) after pre-lens ectoderm removal at stage 12+. (H,I) Islet-1 expression (arrowheads) confirms that the lens-less optic cup has been specified correctly into pigment epithelium and neural retina. (J,K) Delta-crystallin (red) and collagenIX (green) expression on a section adjacent to G. Delta-crystallin is seen in the contralateral control eye (J), but the lens-less optic cup does not react (K); the tissue in front of the cup is not lens tissue (double asterisk). CollagenIX is more robust in the ventral than the dorsal domain at this stage (yellow arrowheads), but by stage 21 both dorsal and ventral domains are equal (J’). In the lens-less optic cup (K) collagenIX is also expressed, and in a dorsoventral pattern similar to that of the control eye. Scale: in A, D-E bar=100μm; in B,C bar=100μm.
Therefore, we performed the same experiment, letting the embryos reincubate for further 40 hours until stage 19 (n=15). Sections through the eye region revealed that there was no obvious lens present (Fig 5G). Islet1 staining demonstrated that the lens-less optic cup had correctly organized a neural retina domain and cells were beginning to differentiate at the back of the retina (Fig 5H, I). We next looked at the expression of delta-crystallin and collagenIX. This staining revealed that the tissue in front of the lens-less optic cup was not lens tissue, as it was completely negative for delta-crystallin throughout all sections of the operated eye (Fig J, K). Surprisingly, collagenIX staining revealed that the lens-less optic cup was expressing this protein at the lips of the optic cup, where the ciliary body usually forms. The expression pattern in the lens-less optic cup was identical to that of the contralateral control eye; that is, the ventral domain of expression was more robust than the dorsal domain. This is a stage specific expression pattern that resolves into equal domains by stage 21 (Fig J’).
We examined the collagenIX staining in several lens-less optic cups. All examples were stained with delta-crystallin antibody to confirm that no lens tissue was present, and all negative staining results were controlled by positive staining in the contralateral control eye. In the majority of lens-less optic cups, the lips of the optic cups seemed to form normally, with a thinner external “pigmented epithelial” layer, a “hinge region” where the epithelium turns back on itself to create the bilayer, and a thicker internal “neural retina” layer (Fig 6A). CollagenIX staining was found in the inner layer and excluded from the outer layer (Fig B, C). However, there were examples, in which the collagenIX expression was found in the outer layer, which itself appeared abnormally thickened (Fig 6D, E). Additional examples were seen of invaginations that gave more complex looking tissue, but in which the expression of collagenIX was fairly normal (Fig 6E,F). Still, in all cases, collagenIX expression was seen in the vicinity of the optic cup lip and was never seen in the posterior neural retina.
Figure 6.
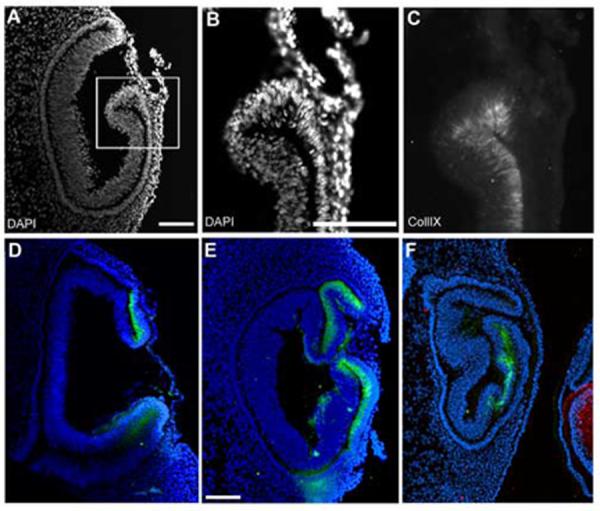
The ciliary body marker collagenIX is expressed in lens-less optic cups.
(A) DAPI stained nuclei reveal the morphology of a lens-less optic cup. (B,C) higher magnification of blocked area in A, with DAPI (B) and collagenIX (C) signal illustrates that collagenIX staining is specific for the inner layer of the optic cup, even in the absence of lens tissue. (D-F) Three additional examples of lens-less optic cups, all internally controlled with delta-crystallin staining in the contralateral eye, not shown, except for F, the control lens of an adjacent embryo. Scale: bar=100μm.
DISCUSSION
Ciliary body tissue is identified at a distance from an FGF source
We found that ciliary body tissue could be induced independently of the lens. We used several unambiguous markers of the ciliary body to confirm the cell fate of the induced ciliary body. Laminin and nidogen are proteins of the inner limiting membrane of the eye. Detailed studies have determined that although the proteins are found throughout the eye, they are both synthesized in and secreted from the ciliary body and are essential components for the formation of the membrane and subsequent development of the eye (Halfter et al., 2005a; Libby et al., 2000). These genes are not normally expressed in the neural retina, or in the pigmented epithelium. Expression of lamininβ1 commences at e3.5 in chick and continues into adulthood; nidogen begins to be expressed in the optic cup margin at e2.5 and is localized in the ciliary body until at least post hatch day 10 (Dong and Chung, 1991; Halfter et al., 2005a). We localized message for the beta subunit of laminin1, and the gamma and alpha subunit are expressed similarly (Libby et al., 2000). Laminin is thought to be the first protein recognized by the endfeet of the retinal ganglion cells, and so establishes the basal lamina. This basal lamina is mandatory for normal retinal development; when it is disrupted, the ganglion cells undergo apoptosis (Halfter et al., 2005b). We found these mRNAs expressed ectopically in the induced transition zones, indicating that the transition zones are unambiguously ciliary body tissue.
We also localized two specific proteins of the vitreous, collagenIX and tenascin-C, to the transition zones. These proteins are normally expressed by the ciliary body and secreted into the vitreous (Halfter et al., 2005a). Within the chick optic cup, collagenIX is a specific marker, expressed only the ciliary body, from e3/HH stage 20 until after hatching (Halfter et al., 2005b; Kubota et al., 2004; Swiderski and Solursh, 1992b). CollagenIX protein is also found in the forming cornea (Linsenmayer et al., 1990), but that did not interfere with our studies. The in situ probe we used was directed against the ciliary body-specific long isoform of the alpha subunit, and its expression confirmed all our immunohistochemical data (Hyer, 2004, Kubota, 2004). Tenascin-C has a slightly more dynamic expression pattern, and by e9 is expressed within the central retina (Perez and Halfter, 1993), but we restricted ourselves to examining stages where in the optic cup epithelium it was clearly specific for the ciliary body. In transition zones we found very robust and clear expression of both collagen IX and tenascin-C.
Finally, we detailed a ciliary body specific upregulation of connexin43 and thymosinβ4. Under our experimental conditions, it was possible to discriminate between the low level connexin43 expression of the pigmented epithelium and the high level of expression of the ciliary body. We saw the same upregulation in transition zones, particularly striking when the entire edge of a transition was captured, as in Fig 4C. Connexin43 expression in the ciliary body is well documented (Coca-Prados et al., 1992; Coffey et al., 2002), and it is thought the elaboration of gap junctions between the two epithelial layers of the ciliary body is essential for aqueous humor production (Civan and Macknight, 2004). In at least two studies reporting ciliary body specific differential gene expression, connexin43 was identified (Das et al., 2005; Kubota et al., 2004). Thymosinβ4 expression is also upregulated in the chick ciliary body, but has not been ascribed a particular ciliary body function. It is widely expressed throughout the nervous system, and is thought to have a role in those tissues undergoing dynamic rearrangements and growth. Thymosinβ4 has been identified in several studies as being upregulated in the optic cup margin (Kubo et al., 2003; Thut et al., 2001). We find that thymosinβ4 is expressed throughout the retinal tissue, but that it is highly expressed in the ciliary body. We also find it upregulated in transition zones, which coordinates the results presented in this study with other reported studies.
The expression of these proteins and message for proteins in the e5 retina indicates that although the margin of the optic cup is not histologically identifiable as ciliary body tissue, it is already producing essential proteins for continued eye development.
Using these markers, ectopic ciliary body tissue was found in various positions in the optic cup, and most pointedly in the posterior of the cup. However, it is has been demonstrated that ectopic lenses also induce ectopic ciliary body tissue, identified either histologically (Beebe, 1986; Genis-Galvez, 1966; Giroud, 1957; Stroeva, 1967), or molecularly (Thut et al., 2001). In order to interpret our finding with these demonstrated results, we consider that the newly formed chick lens is a source of FGFs, specifically FGF1, FGF2, FGF 19 [called FGF15 in mouse] (Lovicu and McAvoy, 2005). In fact, lens tissue has been used in classical experiments as a source of FGF (Lopashov, 1983). FGF is a potent inducer of neural retinal cell fate in the optic tissue, and almost any member of the family can produce this result: FGF1 and 2 (Hyer et al., 1998; Park and Hollenberg, 1989; Pittack et al., 1991; Shimogori et al., 2004), FGF8 (Vogel-Hopker et al., 2000), and FGF9 (Zhao et al., 2001). This study used a retrovirus vector to create ectopic sources of FGF and thus ectopic patches of neural retinal tissue. It may be that experiments using implanted lenses also created ectopic ciliary body by first inducing ectopic regions of neural retina.
The specification of the ciliary body in the absence of the lens (in vivo)
Our previous work on eye development detailed various subtle stages in optic vesicle and surface ectoderm development (Hyer et al., 2003). From this work, we know that if the surface/pre-lens ectoderm is removed at HH stage 12+/16-17 somites, it is at a stage when the neural retina has already been specified, as visualized by Chx-10 expression. The optic vesicle will still be able to undergo morphogenesis into an optic cup without the concomitant formation of the lens. We used this information to design an experiment to test if the lens was required to specify the ciliary body tissue. As we knew that the neural retina domain had been specified, the removal of pre-lens ectoderm served to isolate the optic tissue from any further interactions with factors coming from the ectoderm of forming lens. We saw that the optic vesicles treated in this way did form cups, in isolation from lens tissue, and that the cups did have ciliary body tissue. Our marker of ciliary body tissue was expressed in a stage appropriate manner. Thus, it could not be detected in either normal or lens-less optic cup at stage 16, but was expressed in both normal and experimental cups at stage 19. Analysis with additional markers for older ciliary body tissue could not be carried out, as the lens-less optic cups do not continue to maintain a cup like appearance. This is due to continued growth of the neural retina tissue without growth of the eye as a whole, upon which the entire organization of a cup is lost. Therefore, we must base our interpretation on just one marker. However, as collagenIX is expressed only in the ciliary body of all the neuroepithelial derivatives, we can reasonably conclude that the margins of the lens-less optic cups are correctly specified.
An interesting observation from the lens-less optic cups is that the ciliary body tissue and the lip of the cup, do not always precisely match up, as they do in the normal eye. For example, in Fig 6D and F, the collagenIX expression domain is incorrectly placed in the outer layer in a portion of the optic cup margin. This raises interesting possibilities about the specification of where the lip will form, and when that event occurs. In all of our FGF created transition zones, we never observed areas of ectopic invagination, so we would hypothesize that there is a separate pathway for the establishment of the hinge.
A model for specifying the ciliary body domain
In this study, we first identified that FGF is the probable component of the lens in those classical experiments in which induced lens tissue induced ectopic ciliary body. However, we also found that lens tissue itself did not need to be present in order to specify ciliary body tissue, based on the expression of the ciliary body marker collagenIX. From these two disparate findings, we propose a model in which the ciliary body is established at optic vesicle stages, perhaps using the same signaling environment that is in place for the establishment of the pigmented epithelium and neural retina domains.
The pigmented epithelium cell fate is induced from uncommitted optic vesicle tissue through exposure to either Activin or BMP. The source of this signal may be the surrounding mesenchyme, or the future pigmented epithelium, depending on the temporal window examined (Dudley and Robertson, 1997; Fuhrmann et al., 2000; Vogel-Hopker et al., 2000). Indeed, high levels of ectopic BMP have been shown to induce ectopic pigmented epithelium, and are proposed to be required for that cell fate (Hyer et al., 2003; Okubo and Hogan, 2004).
In contrast, FGF is known to induce neural retinal cell fate. The neuroepithelium of the distal optic expresses receptors for FGF (Ohuchi et al., 1994; Wilke et al., 1997). FGF1 and FGF2 are expressed in the overlying surface ectoderm, where the distal tip of the optic vesicle will eventually contact (de Iongh and McAvoy, 1993; Pittack et al., 1991). It has been shown that FGF1 and FGF2 are not essential for eye development (Miller et al., 2000). However, there is overwhelming evidence from many groups that a signal transduced through the FGF receptor induces neural retinal tissue. Although it is not known how their expression is established, FGF8, FGF19 (called FGF 15 in mouse) are both expressed in the distal tip of the chick optic vesicle and remain candidates for the instructive signal (Francisco-Morcillo et al., 2005; Kurose et al., 2004; Vogel-Hopker et al., 2000).
We hypothesize that the ciliary body is specified through a combination of both FGF and BMP, which has been shown for other cell fates during development (Barron et al., 2000; Dudley and Robertson, 1997; Lough et al., 1996). What we have shown in support of this idea is that introduction of FGF into a BMP-expressing tissue somehow creates both neural retina, and ciliary body tissue, and these two cell types are formed in response to what we imagine as a concentration gradient of FGF (Fig 7A). It has already been demonstrated that different concentrations of FGF have different effects on lens tissue (Lovicu and Overbeek, 1998) and different levels of BMP signal have recently been shown to have different results for optic cup patterning (Murali et al., 2005). In addition, it is known that FGF and BMP have antagonistic effects on important downstream targets during eye specification, namely Mitf and Chx-10 (Horsford et al., 2005; Martinez-Morales et al., 2004).
Figure 7.
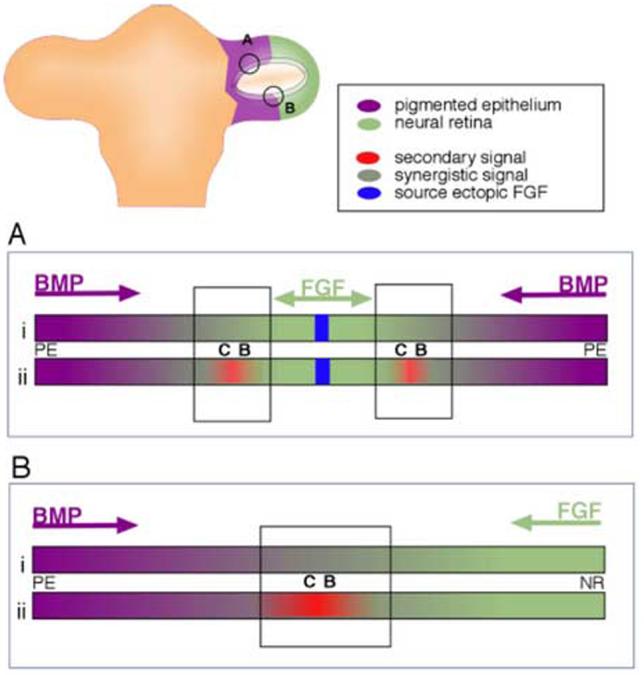
Model for establishment of ciliary body tissue during optic vesicle stages. (A) Ciliary body tissue is created at the edges of ectopic patches of neural retina, as demonstrated in this study. Hypothetically, this might occur either directly (i) through overlapping BMP and FGF signals, or indirectly (ii), through the creation of a second signaling center (red), that itself is created through overlapping BMP and FGF signals. (B) Extrapolation of experimental result to the establishment of the ciliary body at the border between the neural retina and the pigmented epithelial domains in the optic vesicle.
Alternatively, we can consider that FGF and BMP signaling establishes the ciliary body indirectly, through a second and distinct signaling center, created at the overlap of FGF and Activin/BMP signals (Fig 7B). A possible candidate for this signal might be Wnt2b (also called Wnt13). This Wnt family member, and its receptor are expressed in the pigmented epithelium and in the extreme edge of the optic cup shortly after the optic cup forms (stage 20 in chick: Jasoni et al., 1999). As development proceeds, expression continues in the future ciliary body tissue, in both mouse and chick, into adulthood (Jasoni et al., 1999; Kubo et al., 2003; Liu et al., 2003). In considering our model, we find certain similarities between the developing eye and the developing cerebral cortex, where antagonism between BMP and FGF signals organizes a wnt2b expression domain (Shimogori et al., 2004). Wnt2b overexpression in the developing chick eye induced ectopic expression of collagenIX (Kubo et al., 2003; Cho and Cepko, 2006); When examined in vitro, Wnt2b overexpression did not induce ciliary body in vitro, as the explants proliferated extensively, which is not a hallmark of the ciliary body tissue (Kubo et al., 2005). When the Wnt pathway was overactivated in vivo (using an activated β-catenin retrovirus), the posterior neural retina expressed markers of the ciliary body and iris (Cho and Cepko, 2006). There are several lines of evidence suggesting that Wnt signal induces peripheral cell fated in the optic cup, although interfering with Wnt signal led, in the cited report, to a loss of iris tissue, and did not affect ciliary body tissue that severely. To date we have not been able to detect ectopic Wnt2b expression in the transition zones described here (Fig 3K,L), and this illustrates that the role of Wnt signal in the anterior cell fates is more complex than supposed.
The hypothesis presented in Figure 7 implies that both FGF and BMP are essential signals for ciliary body cell fate. In order to begin to test this model, we have attempted to inhibit or reduce signaling through the FGFR1, the main receptor for the eye tissue (Ohuchi et al, 1995), using dominant negative FGFR1 constructs delivered by replication incompetent retroviral vectors to optic vesicle tissue. Retroviral delivery of exogenous genes works very well for this system, as the markers used in this study are expressed after 96 hours of additional development, while electroporated plasmidial constructs are not expressed strongly after 48 hours in chick. Perhaps not surprisingly, these attempts did not provide easily interpretable results. The dominant negative FGFR1 construct caused infected cells to withdraw from proliferation in neural retinal tissue (Fig 8,9) resulting in a majority of infected clones that only contained one or two cells, particularly in the nasal hemisphere of the eye. Control viruses, either a full-length version of the FGFR1 (Fig 8C) or constructs expressing LacZ alone (Fig 8B, Fig 9C), gave rise to a majority of clones containing significantly more cells, and exhibiting a well defined pattern of radial expansion (Turner and Cepko, 1990). A withdrawal from proliferation has not been previously observed in retinas overexpressing DN-FGFR1 constructs (Deng et al., 1997; Celli et al., 1998; McFarlane et al., 1998; Zhang et al., 2003), although these studies did not use retroviral constructs, which are expected to more robustly express the construct throughout development: Overexpression approximating 100-fold is needed to see an effect with dominant negative constructs (Ueno et al., 1992). Other studies utilizing retroviruses to overexpress dominant negative FGFR constructs in muscle tissue also observed reduced proliferation in infected tissues (Itoh et al., 1996; Flanagan-Street et al., 2000). However, expression of a DN-FGFR1 in lens tissue had no effect on clone size (Huang et al., 2003), indicating that the effect may be specific to tissue type. Further work on testing the hypothesis should target signaling through TGFβ family members.
Figure 8.
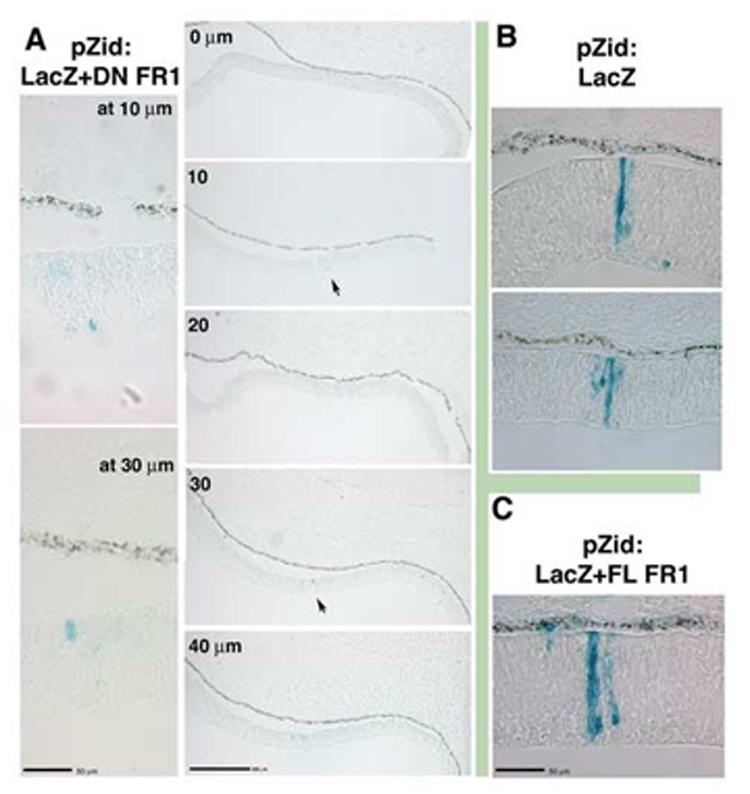
Overexpression of truncated FGFR1 causes reduced proliferation in neural retinal cells by embryonic day 4.(A) Infection at stage 11 with a retrovirus overexpressing a kinase domain truncation of FGFR1 and co-expressing LacZ (pZid: LacZ+DN FR1). Infected cells (blue:Xgal) in the nasal compartment were found as isolated cells or as groups of two (arrows), as seen by examining isolated clones in several adjacent sections (sections are 10μm each, higher magnification views revealed that at 10μm there was a single blue cell and at 30 μm there were a pair of blue cells). (B) In cells infected with control virus overexpressing only the LacZ gene (pZid: LacZ), infected cells give rise to progeny that demonstrated well-characterized clonal growth pattern in the nasal compartment. (C) Likewise, control virus overexpressing the LacZ gene and a full length version of the FGFR1 (pZid: LacZ+FL FR1) gave rise to infected cells that proliferate and extend radially.
Figure 9.
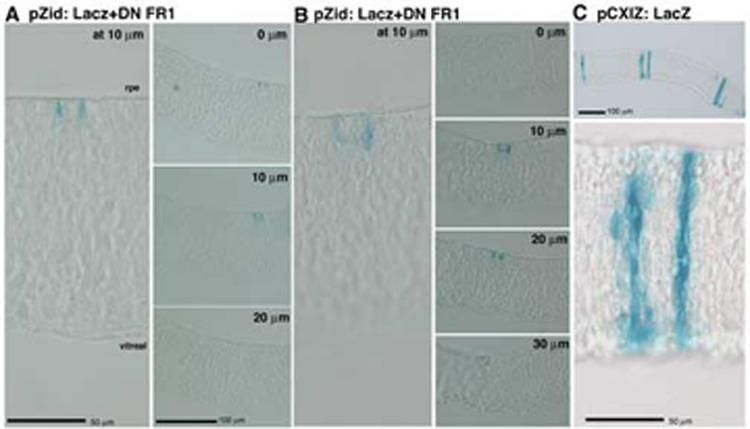
Overexpression of truncated FGFR1 causes reduced proliferation in neural retinal cell by embryonic day 7.(A, B) Infection at stage 11 and examination of clones arising in the temporal domain demonstrates that infected cells were mainly found as isolated cells or pairs. Again, adjacent 10μm sections were examined to determine size of clones. (C) Control retroviral infection gaves rise to large clones of cells by e7 that extended radially throughout the neuroepithelium.
What we have been able to demonstrate here is that lens tissue cannot have a unique signal for the specification of ciliary body, although we should not rule out that there might be a lens-derived inducer of iris tissue. Also, we cannot rule out a role for the lens in the further development and maintenance of the ciliary body, as this may depend on the incorporation of neural crest derived to the epithelium. The lens has been shown to play a central role in organizing the migration and development of the neural crest in the front of the eye, particularly those of the developing cornea (Beebe and Coats, 2000). The studies presented here examined the effect of lens removal prior to corneal formation, and therefore did not assess the maturation of the ciliary body. In the future, the maturation of the ciliary body in a lens-less model can be examined, using techniques similar to those presented by Beebe and Coates (2000). As for further ciliary body development, we suspect that synergistic FGF and BMP signaling remains crucial: It has been shown that loss of BMP signaling (through noggin overexpression) leads to a loss of the ciliary body (Zhao et al., 2002). Likewise, FGF9 null mice show an embryonic mis-specification of the ciliary margin (Zhao et al., 2001). These reports describe effects that are occurring well after we have documented that the ciliary body is established (stage 19). It should be noted that the mature ciliary body consists of both a non-pigmented and a pigmented epithelium. This work, because of the markers employed, describes the establishment of the non-pigmented portion; the establishment of the pigmented portion remains to be elucidated.
Acknowledgments
The authors gratefully acknowledge Mark Galdo for masterful technical assistance, and members of the Alvarez-Buylla Lab, for generously sharing equipment. Anita Lal, Tene Cage and Sharon Liu are thanked for invaluable support and discussions. This work was supported through funding provided by the National Glaucoma Research, a program of the American Health Assistance Foundation and from a grant from the National Eye Institute (NEI) through grant EY015429.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azuma N, Tadokoro K, Asaka A, Yamada M, Yamaguchi Y, Handa H, Matsushima S, Watanabe T, Kida Y, Ogura T, et al. Transdifferentiation of the retinal pigment epithelia to the neural retina by transfer of the Pax6 transcriptional factor. Hum Mol Genet. 2005;14:1059–68. doi: 10.1093/hmg/ddi098. [DOI] [PubMed] [Google Scholar]
- Barron M, Gao M, Lough J. Requirement for BMP and FGF signaling during cardiogenic induction in non-precardiac mesoderm is specific, transient, and cooperative. Dev Dyn. 2000;218:383–93. doi: 10.1002/(SICI)1097-0177(200006)218:2<383::AID-DVDY11>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Beebe DC, Coats JM. The lens organizes the anterior segment: Specification of neural crest cell differentiation in the avian eye. Dev Biol. 2000;220:424–431. doi: 10.1006/dbio.2000.9638. [DOI] [PubMed] [Google Scholar]
- Beebe DC. Development of the ciliary body: a brief review. Trans Ophthalmol Soc U K. 1986;105(Pt 2):123–30. [PubMed] [Google Scholar]
- Celli G, LaRochelle WJ, Mackm S, Sharp R, Merlino G. Soluble dominant-negative receptor uncovers essential roles for fibroblast growth factors in multi-organ induction and patterning. EMBO. 1998;17:1642–1655. doi: 10.1093/emboj/17.6.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CM, Cepko CL. Expression of Chx10 and Chx10-1 in the developing chicken retina. Mech Dev. 2000;90:293–7. doi: 10.1016/s0925-4773(99)00251-8. [DOI] [PubMed] [Google Scholar]
- Cho S-H, Cepko CL. Wnt2b/b-catenin-mediated canonical Wnt signaling determines the peripheral fates of the chick eye. Development. 2006;133:3167–3177. doi: 10.1242/dev.02474. [DOI] [PubMed] [Google Scholar]
- Civan MM, Macknight AD. The ins and outs of aqueous humour secretion. Exp Eye Res. 2004;78:625–31. doi: 10.1016/j.exer.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Coca-Prados M, Ghosh S, Gilula NB, Kumar NM. Expression and cellular distribution of the alpha 1 gap junction gene product in the ocular pigmented epithelium. Curr Eye Res. 1992;11:113–22. doi: 10.3109/02713689209000061. [DOI] [PubMed] [Google Scholar]
- Coffey KL, Krushinsky A, Green CR, Donaldson PJ. Molecular profiling and cellular localization of connexin isoforms in the rat ciliary epithelium. Exp Eye Res. 2002;75:9–21. doi: 10.1006/exer.2002.1187. [DOI] [PubMed] [Google Scholar]
- Coulombre AJ. The role of intraocular pressure in the development of the chick eye. I. Control of eye size. J Exptl. Zool. 1956;133:211–225. [Google Scholar]
- Coulombre AJ, Coulombre JL. The role of intraocular pressure in the development of the chick eye: III Ciliary body. Am. J Ophth. 1957;44:85–93. doi: 10.1016/0002-9394(57)90435-x. [DOI] [PubMed] [Google Scholar]
- Coulombre AJ, Coulombre JL. Regeneration of neural retina from the pigmented epitheliulm in the chick embryo. Dev Biol. 1965;12:79–92. doi: 10.1016/0012-1606(65)90022-9. [DOI] [PubMed] [Google Scholar]
- Das AV, James J, Rahnenfuhrer J, Thoreson WB, Bhattacharya S, Zhao X, Ahmad I. Retinal properties and potential of the adult mammalian ciliary epithelium stem cells. Vision Res. 2005;45:1653–66. doi: 10.1016/j.visres.2004.12.017. [DOI] [PubMed] [Google Scholar]
- de Iongh R, McAvoy JW. Spatio-temporal distribution of acidic and basic FGF indicates a role for FGF in rat lens morphogenesis. Dev Dyn. 1993;198:190–202. doi: 10.1002/aja.1001980305. [DOI] [PubMed] [Google Scholar]
- Deng C, Bedford M, Li C, Xu X, Yang X, Dunmore J, Leder P. Fibroblast growth factor receptor-1 is essential for norml neural tube and limb development. Dev Biol. 1997;185:42–54. doi: 10.1006/dbio.1997.8553. [DOI] [PubMed] [Google Scholar]
- Dong LJ, Chung AE. The expression of the genes for entactin, laminin A, laminin B1 and laminin B2 in murine lens morphogenesis and eye development. Differentiation. 1991;48:157–72. doi: 10.1111/j.1432-0436.1991.tb00254.x. [DOI] [PubMed] [Google Scholar]
- Dong S, Landfair J, Balasubramani M, Bier ME, Cole G, Halfter W. Expression of basal lamina protein mRNAs in the early embryonic chick eye. J Comp Neurol. 2002;447:261–73. doi: 10.1002/cne.10245. [DOI] [PubMed] [Google Scholar]
- Dudley AT, Robertson EJ. Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Dev Dyn. 1997;208:349–62. doi: 10.1002/(SICI)1097-0177(199703)208:3<349::AID-AJA6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Flanagan-Street H, Hannon K, McAvoy MJ, Hullinger R, Olwin BB. Loss of FGF receptor 1 signaling reduces skeletal muscle mass and disrupts myofiber organization in the developing limb. Dev Biol. 2000;218:21–37. doi: 10.1006/dbio.1999.9535. [DOI] [PubMed] [Google Scholar]
- Francisco-Morcillo J, Sanchez-Calderon H, Kawakami Y, Belmonte JC, Hidalgo-Sanchez M, Martin-Partido G. Expression of Fgf19 in the developing chick eye. Brain Res Dev Brain Res. 2005;156:104–9. doi: 10.1016/j.devbrainres.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Fuhrmann S, Levine EM, Reh TA. Extraocular mesenchyme patterns the optic vesicle during early eye development in the embryonic chick. Dev. 2000;127:4599–4609. doi: 10.1242/dev.127.21.4599. [DOI] [PubMed] [Google Scholar]
- Galy A, Neron B, Planque N, Saule S, Eychene A. Activated MAPK/ERK kinase (MEK-1) induces transdifferentiation of pigmented epithelium into neural retina. Dev Biol. 2002;248:251–264. doi: 10.1006/dbio.2002.0736. [DOI] [PubMed] [Google Scholar]
- Genis-Galvez JM. Role of lens in the morphogenesis of the iris and cornea. Nature. 1966;210:209–210. doi: 10.1038/210209a0. [DOI] [PubMed] [Google Scholar]
- Giroud A. Phenomenes d’induction et leurs perturbations chez les mammiferes. Acta Anat. 1957;30:297–306. [PubMed] [Google Scholar]
- Halfter W, Dong S, Schurer B, Osanger A, Schneider W, Ruegg M, Cole GJ. Composition, synthesis, and assembly of the embryonic chick retinal basal lamina. Dev Biol. 2000;220:111–28. doi: 10.1006/dbio.2000.9649. [DOI] [PubMed] [Google Scholar]
- Halfter W, Dong S, Schurer B, Ring C, Cole GJ, Eller A. Embryonic synthesis of the inner limiting membrane and vitreous body. IOVS. 2005a;46:2202–9. doi: 10.1167/iovs.04-1419. [DOI] [PubMed] [Google Scholar]
- Halfter W, Willem M, Mayer U. Basement membrane-dependent survival of retinal ganglion cells. IOVS. 2005b;46:1000–9. doi: 10.1167/iovs.04-1185. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Dev Dyn. 1992;195:231–72. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Hart WM. Intraocular Pressure in Adler’s Physiology of the Eye. Mosby Year Book; St. Louis: 1992. [Google Scholar]
- Horsford DJ, Nguyen MT, Sellar GC, Kothary R, Arnheiter H, McInnes RR. Chx10 repression of Mitf is required for the maintenance of mammalian neuroretinal identity. Development. 2005;132:177–87. doi: 10.1242/dev.01571. [DOI] [PubMed] [Google Scholar]
- Huang J-X, Feldmeier M, Shui Y-B, Beebe DC. Evaluation of firboblast growth factor signaling during lens fiber differentiation. IOVS. 2003;44:680–690. doi: 10.1167/iovs.01-1177. [DOI] [PubMed] [Google Scholar]
- Hyer J. Looking at an oft-overlooked part of the eye: A new perspective on ciliary body development in chick. Dev Neuroscience. 2004;26:456–65. doi: 10.1159/000082287. [DOI] [PubMed] [Google Scholar]
- Hyer J, Kuhlman J, Afif E, Mikawa T. Optic cup morphogenesis requires pre-lens ectoderm but not lens development. Dev Biol. 2003;259:351–363. doi: 10.1016/s0012-1606(03)00205-7. [DOI] [PubMed] [Google Scholar]
- Hyer J, Mima T, Mikawa T. FGF1 patterns the optic vesicle by directing the placement of the neural retina domain. Development. 1998;125:869–77. doi: 10.1242/dev.125.5.869. [DOI] [PubMed] [Google Scholar]
- Hyer J, Mikawa T. Retroviral techniques for studying organogenesis with a focus on heart development. Mol Cell Biochem. 1997;172:23–35. [PubMed] [Google Scholar]
- Itoh N, Mima T, Mikawa T. Loss of fibroblast growth factor receptors is necessary for terminal differentiation of embryonic limb muscle. Development. 1996;122:291–300. doi: 10.1242/dev.122.1.291. [DOI] [PubMed] [Google Scholar]
- Jasoni C, Hendrickson A, Roelink H. Analysis of chicken Wnt-13 expression demonstrates coincidence with cell division in the developing eye and is consistent with a role in induction. Dev Dyn. 1999;215:215–24. doi: 10.1002/(SICI)1097-0177(199907)215:3<215::AID-AJA4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Kamachi Y, Uchikawa M, Tanouchi A, Sekido R, Kondoh H. Pax6 and SOX2 form a co-DNA-binding partner complex that regulates initiation of lens development. Genes Dev. 2001;15:1272–86. doi: 10.1101/gad.887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo F, Takeichi M, Nakagawa S. Wnt2b controls retinal cell differentiation at the ciliary marginal zone. Development. 2003;130:587–98. doi: 10.1242/dev.00244. [DOI] [PubMed] [Google Scholar]
- Kubo F, Takeichi M, Nakagawa S. Wnt2b inhibits differentiation of retinal progenitor cells in the absence of Notch activity by downregulating the expression of proneural genes. Development. 2005;132:2759–70. doi: 10.1242/dev.01856. [DOI] [PubMed] [Google Scholar]
- Kubota R, McGuire C, Dierks B, Reh TA. Identification of ciliary epithelial-specific genes using subtractive libraries and cDNA arrays in the avian eye. Dev Dyn. 2004;229:529–40. doi: 10.1002/dvdy.20000. [DOI] [PubMed] [Google Scholar]
- Kurose H, Bito T, Adachi T, Shimizu M, Noji S, Ohuchi H. Expression of Fibroblast growth factor 19 (Fgf19) during chicken embryogenesis and eye development, compared with Fgf15 expression in the mouse. Gene Expr Patterns. 2004;4:687–93. doi: 10.1016/j.modgep.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Libby RT, Champliaud MF, Claudepierre T, Xu Y, Gibbons EP, Koch M, Burgeson RE, Hunter DD, Brunken WJ. Laminin expression in adult and developing retinae: evidence of two novel CNS laminins. J Neurosci. 2000;20:6517–28. doi: 10.1523/JNEUROSCI.20-17-06517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmayer TF, Gibney E, Gordon MK, Marchant JK, Hayashi M, Fitch JM. Extracellular matrices of the developing chick retina and cornea. Localization of mRNAs for collagen types II and IX by in situ hybridization. IOVS. 1990;31:1271–6. [PubMed] [Google Scholar]
- Liu H, Mohamed O, Dufort D, Wallace VA. Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev Dyn. 2003;227:323–34. doi: 10.1002/dvdy.10315. [DOI] [PubMed] [Google Scholar]
- Lopashov GV. Transdifferentiation of pigmented epithelium induced by the influence of lens epithelium in frogs. Differentiation. 1983;24:27–32. doi: 10.1111/j.1432-0436.1983.tb01298.x. [DOI] [PubMed] [Google Scholar]
- Lough J, Barron M, Brogley M, Sugi Y, Bolender DL, Zhu X. Combined BMP-2 and FGF-4, but neither factor alone, induces cardiogenesis in non-precardiac embryonic mesoderm. Dev Biol. 1996;178:198–202. doi: 10.1006/dbio.1996.0211. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, Overbeek PA. Overlapping effects of different members of the FGF family on lens fiber differentiation in transgenic mice. Development. 1998;125:3365–77. doi: 10.1242/dev.125.17.3365. [DOI] [PubMed] [Google Scholar]
- Martinez-Morales JR, Rodrigo I, Bovolenta P. Eye development: a view from the retina pigmented epithelium. Bioessays. 2004;26:766–77. doi: 10.1002/bies.20064. [DOI] [PubMed] [Google Scholar]
- McFarlane S, Zuber ME, Holt CE. A role for the fibroblast growth factor in cell fate decisions in the developing vetebrate retina. Development. 1998;125:3967–3975. doi: 10.1242/dev.125.20.3967. [DOI] [PubMed] [Google Scholar]
- Mikawa T. Retroviral targeting of FGF and FGFR in cardiomyocytes and coronary vascular cells during heart development. Ann N Y Acad Sci. 1995;752:506–16. doi: 10.1111/j.1749-6632.1995.tb17459.x. [DOI] [PubMed] [Google Scholar]
- Miller DL, Ortega S, Bashayan O, Basch R, Basilico C. Compensation by Fibroblast Growth Factor 1 (FGF1) does not account for the mild phenotypic defects observed in FGF2 null mice. Mol Cell Biol. 2000;20:2260–2268. doi: 10.1128/mcb.20.6.2260-2268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima T, Ohuchi H, Noji S, Mikawa T. FGF can induce outgrowth of somatic mesoderm both inside and outside of limb-forming regions. Dev Biol. 1995;167:617–20. doi: 10.1006/dbio.1995.1053. [DOI] [PubMed] [Google Scholar]
- Murali D, Yoshikawa S, Corrigan RR, Plas DJ, Crair MC, Oliver G, Lyons KM, Mishina Y, Furuta Y. Distinct developmental programs require different levels of Bmp signaling during mouse retinal development. Development. 2005;132:913–23. doi: 10.1242/dev.01673. [DOI] [PubMed] [Google Scholar]
- Nguyen M-TT, Arnheiter H. Signaling and transcriptional regulation in early mammalian eye development: a link between FGF and MITF. Dev. 2000;127:3581–3591. doi: 10.1242/dev.127.16.3581. [DOI] [PubMed] [Google Scholar]
- Nieto MA, Patel K, Wilkinson DG. In situ hybridization analysis of chick embryos in whole mount and tissue sections. Methods Cell Biol. 1996;51:219–35. doi: 10.1016/s0091-679x(08)60630-5. [DOI] [PubMed] [Google Scholar]
- Ohuchi H, Koyama E, Myokai F, Nohno T, Shiraga F, Matsuo T, Matsuo N, Taniguchi S, Noji S. Expression patterns of two fibroblast growth factor receptor genes during early chick eye development. Exp Eye Res. 1994;58:649–58. doi: 10.1006/exer.1994.1062. [DOI] [PubMed] [Google Scholar]
- Okubo T, Hogan BL. Hyperactive Wnt signaling changes the developmental potential of embryonic lung endoderm. J Biol. 2004;3:11. doi: 10.1186/jbiol3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CM, Hollenberg MJ. Basic fibroblast growth factor induces retinal regeneration in vivo. Dev Biol. 1989;134:201–5. doi: 10.1016/0012-1606(89)90089-4. [DOI] [PubMed] [Google Scholar]
- Perez RG, Halfter W. Tenascin in the developing chick visual system: distribution and potential role as a modulator of retinal axon growth. Dev Biol. 1993;156:278–92. doi: 10.1006/dbio.1993.1076. [DOI] [PubMed] [Google Scholar]
- Perez RG, Halfter W. Tenascin protein and mRNA in the avian visual system: distribution and potential contribution to retinotectal development. Perspectives Dev Neurobiol. 1994;2:75–87. [PubMed] [Google Scholar]
- Pittack C, Jones M, Reh TA. Basic fibroblast growth factor induces retinal pigment epithelium to generate neural retina in vitro. Development. 1991;113:577–88. doi: 10.1242/dev.113.2.577. [DOI] [PubMed] [Google Scholar]
- Raviola G. The fine structure of the ciliary zonule and ciliary epithelium. With special regard to the organization and insertion of the zonular fibrils. Invest Ophthalmol. 1971;10:851–69. [PubMed] [Google Scholar]
- Ruangvoravat CP, Lo CW. Connexin 43 expression in the mouse embryo: localization of transcripts within developmentally significant domains. Dev Dyn. 1992;194:261–81. doi: 10.1002/aja.1001940403. [DOI] [PubMed] [Google Scholar]
- Sarthy PV, Fu M. Localization of laminin β1 mRNA in retinal ganglion cells by in situ hybridization. J Cell Biol. 1990;110:2099–108. doi: 10.1083/jcb.110.6.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeren-Wiemers N, Gerfin-Moser A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry. 1993;100:431–40. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
- Shimogori T, Banuchi V, Ng HY, Strauss JB, Grove EA. Embryonic signaling centers expressing BMP, WNT and FGF proteins interact to pattern the cerebral cortex. Development. 2004;131:5639–47. doi: 10.1242/dev.01428. [DOI] [PubMed] [Google Scholar]
- Stroeva OG. The correlation of the process of proliferation and determination in the morphogenesis of iris and ciliary body in rats. J. Embryol. exp. Morph. 1967;18:269–87. [PubMed] [Google Scholar]
- Swiderski RE, Solursh M. Differential co-expression of the long and short form of type IX collagen transcripts during avian limb chondrogenesis in ovo. Development. 1992b;115:169–179. doi: 10.1242/dev.115.1.169. [DOI] [PubMed] [Google Scholar]
- Thut CJ, Rountree RB, Hwa M, Kingsley DM. A large-scale in situ screen provides molecular evidence for the induction of eye anterior segment structures by the developing lens. Dev Biol. 2001;231:63–76. doi: 10.1006/dbio.2000.0140. [DOI] [PubMed] [Google Scholar]
- Tucker RP. The distribution of J1/tenascin and its transcript during the development of the avian cornea. Differentiation. 1991;48:59–66. doi: 10.1111/j.1432-0436.1991.tb00243.x. [DOI] [PubMed] [Google Scholar]
- Turner DL, Snyder EY, Cepko CL. Lineage-independent determination of cell type in the embryonic mouse retina. Neuron. 1990;4:833–845. doi: 10.1016/0896-6273(90)90136-4. [DOI] [PubMed] [Google Scholar]
- Ueno H, Gunn M, Dell K, Tseng A, Jr., Williams LA. A truncated form of fibroblast growth factor receptor 1 inhibits signal transduction by multiple types of fibroblast growth factor receptors. J Biol Chem. 1992;267:1470–1476. [PubMed] [Google Scholar]
- Vogel-Hopker A, Momose T, Rohrer H, Yasuda K, Ishihara L, Rapaport DH. Multiple functions of fibroblast growth factor-8 (FGF-8) in chick eye development. Mech Dev. 2000;94:25–36. doi: 10.1016/s0925-4773(00)00320-8. [DOI] [PubMed] [Google Scholar]
- Wilke TA, Gubbels S, Schwartz J, Richman JM. Expression of fibroblast growth factor receptors (FGFR1, FGFR2, FGFR3) in the developing head and face. Dev Dyn. 1997;210:41–52. doi: 10.1002/(SICI)1097-0177(199709)210:1<41::AID-AJA5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Yancey SB, Biswal S, Revel JP. Spatial and temporal patterns of distribution of the gap junction protein connexin43 during mouse gastrulation and organogenesis. Development. 1992;114:203–12. doi: 10.1242/dev.114.1.203. [DOI] [PubMed] [Google Scholar]
- Zhang L, El-Hodiri HM, Ma HF, Zhang X, Servetnick M, Wensel TG, Jamrich M. Targeted expression of the dominant-negative FGFR4a in the eye using Xrx1A regulatory sequences interferes with normal retinal development. Development. 2003;130:4177–4186. doi: 10.1242/dev.00626. [DOI] [PubMed] [Google Scholar]
- Zhao S, Chen Q, Hung FC, Overbeek PA. BMP signaling is required for development of the ciliary body. Development. 2002;129:4435–4442. doi: 10.1242/dev.129.19.4435. [DOI] [PubMed] [Google Scholar]
- Zhao S, Hung FC, Colvin JS, White A, Dai W, Lovicu FJ, Ornitz DM, Overbeek PA. Patterning the optic neuroepithelium by FGF signaling and Ras activation. Development. 2001;128:5051–5060. doi: 10.1242/dev.128.24.5051. [DOI] [PubMed] [Google Scholar]


