Abstract
In wild-type Saccharomyces cerevisiae, replication forks slowed during their passage through telomeric C1–3A/TG1–3 tracts. This slowing was greatly exacerbated in the absence of RRM3, shown here to encode a 5′ to 3′ DNA helicase. Rrm3p-dependent fork progression was seen at a modified Chromosome VII-L telomere, at the natural X-bearing Chromosome III-L telomere, and at Y‘-bearing telomeres. Loss of Rrm3p also resulted in replication fork pausing at specific sites in subtelomeric DNA, such as at inactive replication origins, and at internal tracts of C1–3A/TG1–3 DNA. The ATPase/helicase activity of Rrm3p was required for its role in telomeric and subtelomeric DNA replication. Because Rrm3p was telomere-associated in vivo, it likely has a direct role in telomere replication.
Keywords: Telomere, helicase, telomerase, replication, RRM3, yeast
Telomeres are the natural ends of eukaryotic chromosomes. In most organisms, the very ends of chromosomes consist of simple repeated sequences. For example, Saccharomyces cerevisiae chromosomes end in 350 ± 75 bp of C1–3A/TG1–3 DNA. Middle repetitive DNA elements are often found immediately internal to the simple repeats. Saccharomyces has two types of subtelomeric repeats, the Y‘ element, which is found in up to four tandem copies on about two-thirds of yeast telomeres, and the X element, which is found on virtually all telomeres (Chan and Tye 1983). The telomeric repeats are assembled into a non-nucleosomal DNA protein complex, the telosome (Wright et al. 1992), which contains multiple copies of the C1–3A/TG1–3-binding Rap1 protein (Conrad et al. 1990; Wright et al. 1992), as well as Sir proteins, Rif proteins, the single-strand TG1–3-DNA-binding Cdc13p (Bourns et al. 1998; Tsukamoto et al. 2001), and the heterodimeric Ku complex (Gravel et al. 1998). In contrast, X and Y‘ DNA are assembled into nucleosomes (Wright et al. 1992). However, subtelomeric nucleosomes differ from nucleosomes in most other regions of the genome as they are also bound by the Sir (Hecht et al. 1996; Strahl-Bolsinger et al. 1997) and Ku (Martin et al. 1999) complexes.
Because conventional DNA polymerases cannot replicate the very ends of linear DNA molecules, special mechanisms are required to prevent the loss of terminal DNA. In most eukaryotes, including Saccharomyces, this end-replication problem is solved by telomerase, a reverse transcriptase that uses its RNA component as a template to lengthen the G-rich strand of telomeric DNA. However, in the absence of telomerase, only a few base pairs of telomeric DNA are lost per telomere per S phase (Lundblad and Szostak 1989). Thus, most of the C1–3A/TG1–3 telomeric tract and the entire subtelomeric DNA are duplicated by conventional, semiconservative DNA replication. In Saccharomyces, semiconservative replication of both the subtelomeric repeats (McCarroll and Fangman 1988; Ferguson et al. 1991) and the telomeric C1–3A/TG1–3 tracts (Wellinger et al. 1993a) as well as telomerase elongation of telomeres (Marcand et al. 2000) occur late in the S phase.
The Saccharomyces Pif1p, a 5′ to 3′ DNA helicase (Lahaye et al. 1993), is a negative regulator of the telomerase pathway (Schulz and Zakian 1994; Zhou et al. 2000). Telomere length is inversely proportional to the amount of Pif1p: Overexpression of Pif1p causes telomere shortening, and reduced expression results in lengthening. Both effects on telomere length require the helicase activity of Pif1p (Zhou et al. 2000). In the absence of Pif1p, telomerase-mediated de novo telomere addition at spontaneous and induced chromosome breaks is elevated 200- to 1000-fold (Schulz and Zakian 1994; Mangahas et al. 2001; Myung et al. 2001).
The PIF1 gene is the prototype member of a helicase subfamily that is conserved from yeast to humans (for review, see Bessler et al. 2001). Although Saccharomyces has 134 ORFs that encode helicase-like proteins (Shiratori et al. 1999), only one of these, Rrm3p, has significant similarity to Pif1p by the criterion of a BLAST search (Zhou et al. 2000). RRM3 encodes a 723-amino-acid protein that is 60% similar to Pif1p over a 485-amino-acid region that encompasses the seven helicase motifs (Bessler et al. 2001). RRM3 was first identified because its mutation increases recombination in ribosomal DNA (rDNA; Keil and McWilliams 1993), which results in the accumulation of rDNA circles (Ivessa et al. 2000). However, this recombination is probably a secondary consequence of defects in rDNA replication as, in the absence of Rrm3p, replication forks pause at multiple sites throughout the rDNA. Separation of converged replication forks within the rDNA is especially impaired in rrm3Δ cells. Pif1p also influences rDNA replication, although its effects are relatively modest.
In this paper, we describe the role of Rrm3p at telomeres. Although Rrm3p, like Pif1p, is a 5′ to 3′ DNA helicase, we found that Rrm3p was important for conventional replication of telomeric DNA, rather than for the telomerase pathway. In the absence of Rrm3p, a natural pausing of replication forks within telomeric C1–3A/TG1–3 repeats was greatly increased, and replication forks paused at specific sites within subtelomeric DNA. The ATPase/helicase function of Rrm3p was needed for its role at telomeres. Because Rrm3p was telomere-associated, its effects on telomere replication are likely direct.
Results
Rrm3p is an ATPase and 5′ to 3′ DNA helicase
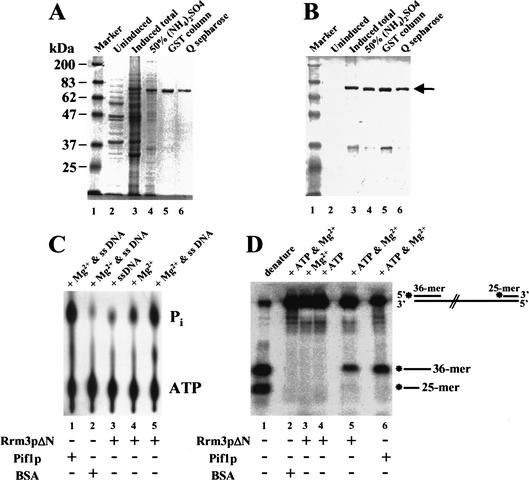
We were unable to purify full-length Rrm3p from baculovirus-infected Sf9 cells, bacteria, or yeast, encountering problems similar to those reported for the full-length Saccharomyces Sgs1p helicase (Bennett et al. 1998). Therefore, we expressed a truncated version of Rrm3p as a GST fusion protein in S. cerevisiae. This polypeptide, hereafter called Rrm3pΔN, contained amino acids 194 to 723 of the 723-amino-acid protein, including all seven helicase motifs as well as 56 amino acids amino-terminal of the first helicase motif (Bessler et al. 2001). Rrm3pΔN was expressed under the control of a galactose-inducible promoter. Only galactose-grown cells contained a polypeptide of the appropriate molecular weight that cross-reacted with anti-Rrm3p antibodies (Fig. 1B, cf. lanes 2 and 3). Rrm3pΔN was purified to near homogeneity (Fig. 1A and B show, respectively, Coomassie blue-stained or immunoblotting of Rrm3pΔN throughout its purification).
Figure 1.
Recombinant Rrm3 protein has ATPase and 5′ to 3′ DNA helicase activity. A truncated form of Rrm3p (Rrm3pΔN) was fused to GST and expressed in Saccharomyces cerevisiae under control of the galactose-inducible GAL1 promoter. Yeast proteins were resolved by 8% SDS-PAGE and detected by (A) Coomassie blue staining or (B) immunoblotting. In A and B, lane 1 is size markers in kilodaltons. (Because covalent coupling of marker proteins to the blue dye used for visualization affects their mobility in SDS-PAGE, the 83-kD marker protein has a slower mobility than the 87-kD Rrm3pΔN.) Lanes 2 and 3 contain a crude extract of proteins from glucose grown (lane 2) or galactose grown (lane 3) cells. Lanes 4–6 contain proteins from galactose-grown cells after sequential purification by 50% ammonium sulfate precipitation (lane 4), fractionation on Glutathione sepharose 4B (lane 5), and fractionation on Q-sepharose (lane 6). (C) The ATPase reaction products were developed in a polyethylimine (PEI) cellulose plate and visualized on a Molecular Dynamics PhosphorImager. Lane 5 contains [γ-P32]ATP, M13 single-stranded DNA, Mg2+, and Rrm3pΔN. The other lanes were the same except lane 1 had Pif1p in place of Rrm3pΔN; lane 2 had BSA in place of Rrm3pΔN; lane 3 had no Mg2+; and lane 4 had no single-stranded DNA. (D) For the helicase assay, the DNA substrate was linear M13 single-stranded DNA to which kinase labeled 36-mer and 25-mer oligonucleotides had been annealed. Lane 1 contains the heat-denatured DNA substrate; lane 5 contains Rrm3pΔN, the DNA substrate, ATP and Mg2+. The other lanes are the same as lane 5 except lane 2 contains BSA in place of Rrm3pΔN; lane 3 has no ATP; lane 4 has no Mg2+; and lane 6 contains Pif1p in place of Rrm3pΔN.
The purified Rrm3pΔN had Mg2+-dependent, single-stranded DNA stimulated ATPase activity (Fig. 1C). To determine if Rrm3pΔN had helicase activity, we used a partially duplex DNA substrate in which a 36-mer oligonucleotide and a 25-mer oligonucleotide were annealed at, respectively, the 5′ and 3′ ends of linear, single-stranded M13 DNA (Fig. 1D). DNA helicases will load onto the single-stranded region between the two oligonucleotides. A 5′ to 3′ DNA helicase will move 5′ to 3′ on the single-stranded segment and displace the 36-mer oligonucleotide, whereas a 3′ to 5′ DNA helicase will move 3′ to 5′ and displace the 25-mer oligonucleotide. Because Rrm3pΔN (Fig. 1D, lane 5), like Pif1p (Fig. 1D, lane 6), displaced only the 36-mer oligonucleotide, we conclude that Rrm3pΔN is a 5′ to 3′ DNA helicase. This helicase activity was ATP-dependent (Fig. 1D, lane 3) and Mg2+-dependent (Fig. 1D, lane 4).
Rrm3p affects telomere length and the ability of Pif1p to inhibit de novo telomere addition
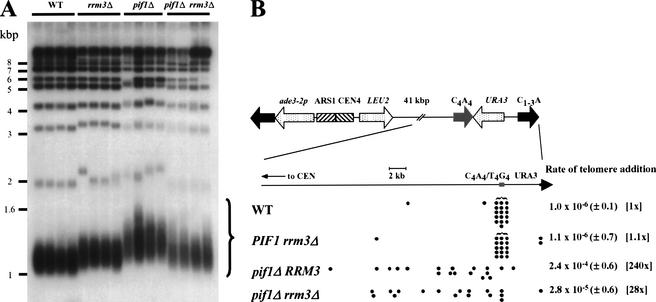
Previous work showed that loss of Pif1p increases telomere length and de novo telomere addition (Schulz and Zakian 1994). Therefore, we examined telomere length and de novo telomere addition in rrm3Δ cells. DNA from wild-type and mutant cells was digested with XhoI and examined by Southern hybridization using a C1–3A/TG1–3 telomeric probe. Loss of Pif1p resulted in an ∼160-bp increase in telomere length (Fig. 2A). Deletion of RRM3 resulted in a more modest (∼75-bp) lengthening. If Rrm3p, like Pif1p, inhibits telomerase, telomeres would be even longer in a pif1Δ rrm3Δ strain than in either singly mutant strain. Alternatively, if Pif1p is a more effective telomerase inhibitor than Rrm3p, telomeres might be the same length in the doubly mutant strain as they are in a pif1Δ strain. In contrast to both expectations, telomeres in pif1Δ rrm3Δ cells were similar in length to rrm3Δ telomeres (Fig. 2A).
Figure 2.
Rrm3p affects telomere length and de novo telomere addition but not in the same ways as Pif1p. (A) DNA was prepared from four individual colonies from each strain: PIF1 RRM3, PIF1 rrm3Δ, pif1Δ RRM3, and pif1Δ rrm3Δ cells. The DNA was digested with XhoI, separated by electrophoresis in a 0.7% agarose gel, prepared for Southern analysis, and probed with a C1–3A/TG1–3 telomeric probe. The large curly brace indicates the terminal XhoI fragments from Y‘-bearing telomeres. Molecular weight markers are in kilobase pairs. (B) The rate of de novo telomere formation on a YAC was determined using 10 plate fluctuation tests (Lea and Coulson 1949), conducted 2–4 times per strain, as described in Schulz and Zakian (1994). Because Leu+ FOAR cells can also be generated by point mutations in URA3, the sites of telomere addition in multiple independent Leu+ FOAR clones were mapped to determine the fraction of these events that were due to de novo telomere addition. Each filled circle marks the physical end of a YAC in one such colony. Leu+ FOAR colonies that contained a YAC of unaltered length were presumed to arise from point mutations in URA3. Numbers in parentheses indicate the range of values seen in independent experiments. Numbers in brackets are the fold difference relative to the wild-type strain.
To determine rates of telomere addition, we used a yeast artificial chromosome (YAC) that had LEU2 on one arm, URA3 near the telomere of the other arm, and a tract of Oxytricha C4A4/T4G4 telomeric DNA internal to the URA3 gene (Fig. 2B; Schulz and Zakian 1994). The Oxytricha sequence is used as a substrate for telomere addition in yeast (Pluta et al. 1984). Cells that express Ura3p die on medium containing 5-fluoroorotic acid (FOA). If de novo telomere addition occurs within the right arm of the YAC, the distal portion of its right arm, including the URA3 gene, will be lost, generating a Leu+ FOA-resistant (FOAR) colony. Consistent with previous results (Schulz and Zakian 1994), wild-type cells had a low rate of de novo telomere addition (10−6), and most addition events (16/18) occurred within the C4A4/T4G4 tract (Fig. 2B), whereas telomere addition was increased 240-fold in the pif1Δ strain, and most (17/18) of these events did not occur at the C4A4/T4G4 tract (Fig. 2B). The rrm3Δ strain had the same rate of de novo telomere addition as wild type (10−6), and most of these events (15/16) occurred at the C4A4/T4G4 tract. The rate of de novo telomere addition in the pif1Δ rrm3Δ strain was 28-fold higher than in wild type. Thus, deletion of RRM3 alone did not affect de novo telomere addition, and its deletion in a pif1Δ strain partially suppressed the elevated telomere addition phenotype of pif1Δ cells (Fig. 2B). Taken together, the telomere length and telomere addition data indicate that although Rrm3p affects telomeres, it acts otherwise than Pif1p.
Replication forks slow as they move through the Chromosome VII-L telomere, and this slowing is exacerbated in the absence of Rrm3p
To determine if Rrm3p affects conventional replication of telomeres, we used two-dimensional (2D) gel electrophoresis (Brewer and Fangman 1987). To examine replication of the left telomere of Chromosome VII, we inserted URA3 within the ADH4 gene, the most distal gene on Chromosome VII-L, in such a way that it deleted the subtelomeric repeats (Fig. 3A). Replication of the end of Chromosome VII-L occurs from an origin of replication (ARS) that lies internal to the terminal ClaI fragment (Fig. 3A), such that the VII-L terminal restriction fragment is replicated by leftward-moving forks. In 2D gels, forked replication intermediates emanate from the position of nonreplicating restriction fragments (Fig. 3A, labeled 1N), become increasingly nonlinear until the replication fork reaches the middle of the fragment, and then become increasingly more linear until they reenter the arc of simple linear molecules with a mass of 2N (Brewer and Fangman 1987).
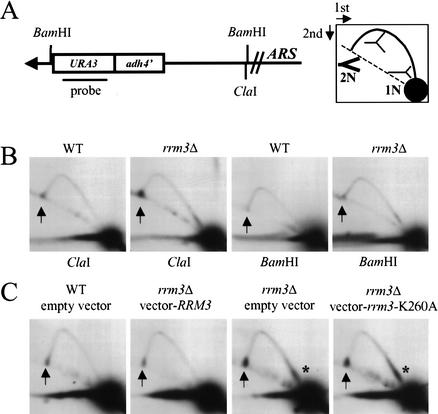
Figure 3.
Replication of telomere VII-L is impaired in the absence of Rrm3p. (A) Structure of the left telomere of Chromosome VII after insertion of URA3 in a strain in which URA3 was deleted from its normal location. The URA3 probe indicated in A was used in B and C as well as in Figure 4. Digestion with ClaI generates a 3.8-kb fragment containing the VII-L telomere. A has a cartoon of the expected pattern of replication intermediates for simple forks moving leftward toward the telomere after their separation in 2D gels. The arc of linear molecules is denoted by the dotted line, 1N marks the position of an unreplicated restriction fragment, and 2N is the same molecule immediately before its replication is complete. The 2N intermediate is drawn in thick lines to emphasize that it was more abundant than other forked replication intermediates. (B) DNA from wild-type (WT) or rrm3Δ cultures was digested with either ClaI (left two panels) or BamHI (right two panels). The arrows in this and subsequent gels denote the position of the 2N spot. (C) DNA was prepared from cultures of wild-type or rrm3Δ strains carrying the plasmid YCplac111, YCplac111 containing RRM3, or YCplac111 containing the rrm3 K260A allele. DNA was digested with ClaI and analyzed by 2D gels. Asterisks mark the region downstream of ADH4 where replication forks slow in the absence of Rrm3p.
When DNA from asynchronous wild-type cells was digested with ClaI and examined by 2D gels, an arc of forked replication intermediates was detected (Fig. 3B). The pattern for rrm3Δ cells was similar except that there was intense hybridization at the 2N position on the arc of linear DNA molecules (hereafter called the 2N spot; Fig. 3B, arrow). Although the 2N spot was also evident in wild-type DNA, it was more intense in rrm3Δ DNA. To estimate the amount of telomeric DNA in the 2N spot, we measured the signal in both the 2N spot and in unreplicated DNA (1N spot) and determined the 2N/1N ratio. This ratio was 10.1 ± 2.7-fold higher in rrm3Δ DNA than in wild-type DNA (average ± standard error; based on five independent experiments).
Because 2N spot DNA has a mass of nearly 2N and a nearly linear structure, it behaves as if the Chromosome VII sister chromatids are almost fully replicated (see cartoon in Fig. 3A). Given that the telomere comprises <10% of the ClaI restriction fragment (Fig. 3A), this behavior suggests that the sister chromatids were held together within the telomere itself. The modified VII-L telomere has a BamHI site 6 bp away from the start of the C1–3A/TG1–3 telomeric tract. To determine if 2N-spot DNA is held together within the telomere, the same DNA preparations that had been examined after ClaI digestion were instead digested with BamHI (Fig. 3B). Because the 2N spot was nearly gone after BamHI digestion (Fig. 3B, right panels), it was caused largely by sister chromatids held together within the C1–3A/TG1–3 tract.
The catalytic activity of Rrm3p is needed for telomere replication
DNA helicases can be multifunctional, having activities in addition to the unwinding of duplex DNA (see, e.g., Sung et al. 1988; Formosa and Nittis 1999). To determine if the ATPase/helicase activity of Rrm3p is needed for replication of telomeric DNA, we used an RRM3 allele in which the invariant lysine in the ATP-binding pocket was mutated to alanine (K260A; Ivessa et al. 2000). Converting this invariant lysine to an alanine eliminates the helicase activity of all helicases that have been thus modified, including Pif1p (Zhou et al. 2000). A centromere plasmid bearing either the K260A rrm3 or wild-type RRM3 genes was introduced into rrm3Δ and wild-type strains. Western analysis showed that the K260A mutant allele produces close to wild-type levels of Rrm3p (Ivessa et al. 2000). DNA from each strain was digested with ClaI, and analyzed by 2D gels (Fig. 3C). The plasmid-borne RRM3 suppressed the telomere replication defects of the rrm3Δ strain (Fig. 3C, second panel), showing that these defects were owing to the absence of Rrm3p. Telomere replication was equally impaired in the strain expressing the K260A Rrm3p (Fig. 3C, fourth panel) as in the strain lacking Rrm3p (Fig. 3C, third panel). Thus, the ATPase/helicase activity of Rrm3p is needed for telomere replication.
In addition to the 2N spot, DNA from rrm3Δ cells showed increased hybridization near the proximal end of the 3.8-kb ClaI fragment (Fig. 3C, asterisk). This pause, which was not detected in DNA from wild-type cells, mapped to a position downstream of ADH4.
The appearance of the 2N spot is cell cycle regulated
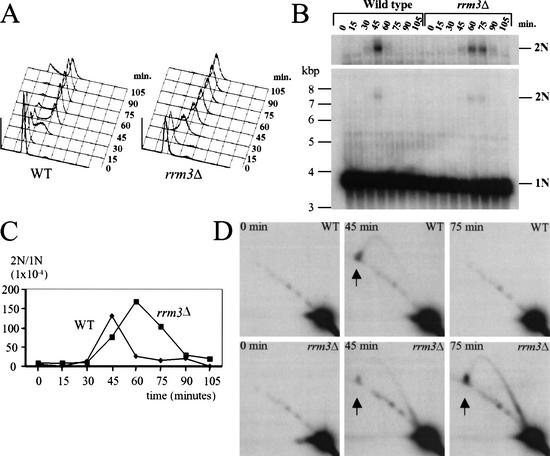
If the 2N spot is an intermediate in telomere replication, it should be absent in DNA from G1 cells and enriched during replication of telomeric DNA, which occurs in the second half of the S phase (McCarroll and Fangman 1988; Wellinger et al. 1993a). To test these predictions, cultures were arrested in late G1 phase by incubation with α factor. After arrest, cells were removed from α factor (zero time point) and allowed to progress through the cell cycle. Samples were harvested at 15-min intervals for analysis by fluorescent activated cell sorting (FACs; Fig. 4A) and by conventional (Fig. 4B) and 2D (Fig. 4D) gel electrophoresis. The α-factor arrest and subsequent release were done at 22°C because a slower growth rate makes it easier to detect replication intermediates. At 22°C, the rrm3Δ strain grew somewhat slower (120-min doubling time) than otherwise isogenic wild-type cells (105-min doubling time).
Figure 4.
The 2N spot is cell cycle regulated, appearing at the time of telomere replication. Wild-type or rrm3Δ cells were G1 arrested with α factor and then removed from the α factor and allowed to progress through the cell cycle. Samples were prepared at the time of removal from α factor (0 min) and then at 15-min intervals. (A) Cells from each time point were analyzed by fluorescent activated cell sorting (FACs). (B) DNA samples from each time point were digested with ClaI, separated by conventional agarose gel electrophoresis, and hybridized with the URA3 probe (Fig. 3A). In a conventional gel, 2N-spot DNA migrates as a 7.6-kb linear fragment. The top panel shows a longer exposure of the 2N spot region of the gel. (C) The data in panel B were quantitated; the 2N to 1N ratio is shown for each time point. (D) Each ClaI-digested DNA sample was also analyzed by 2D gel analysis; representative time points from both strains are shown.
By the criterion of FACs analysis, the wild-type and rrm3Δ strains proceeded similarly through S phase (Fig. 4A). At 30 min after α-factor release, the majority of the cells in both strains were in S phase. In both strains, ∼80% and ∼90% of the cells had a 2C DNA content at, respectively, 60 and 75 min after α-factor release. The major difference in the FACs profiles of the two strains was that at 105 min, 24% of the wild-type cells had a 1C DNA content, indicating movement into the G1 phase of the next cell cycle, whereas at 105 min, there were very few 1C cells in the rrm3Δ culture, suggesting that rrm3Δ cells took longer to traverse from late S phase into the next cell cycle.
DNA from each time point was digested with ClaI and examined by conventional agarose gel electrophoresis (Fig. 4B). The 2N spot generates a 7.6-kb ClaI fragment. The fraction of telomeric DNA in the 2N spot as a function of position within the cell cycle is quantitated in Figure 4C. In both strains, the 2N spot was transitory (Fig. 4B–D). In three independent synchrony experiments, the 2N spot was only detected in the 45-min sample in wild-type DNA. In rrm3Δ cells, the 2N spot peaked in the 60-min sample but was also detected in the 45- and 75-min samples. Although FACs analysis indicated that the progress of chromosomal DNA replication was similar in the two strains (Fig. 4A), the 2N spot peaked later and persisted for a longer period of time in rrm3Δ cells (Fig. 4C). DNA from each time point was also examined by 2D gels (Fig. 4D; data not shown). The 2N spot and forked replication intermediates were only present in time points that contained the 7.6-kb ClaI fragment.
Rrm3p is needed for timely replication of the telomere and subtelomeric DNA on Chromosome III-L
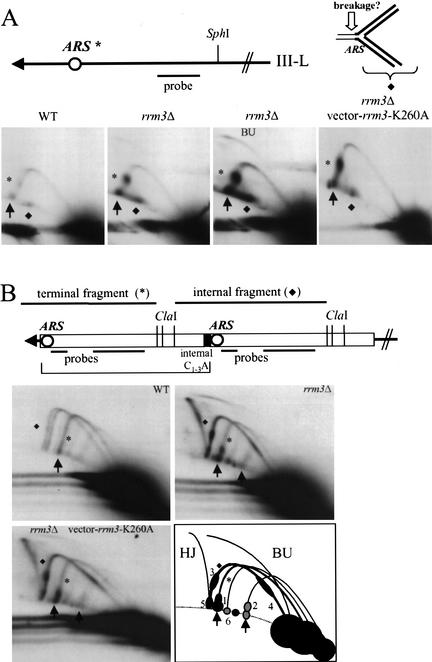
To determine if Rrm3p plays a role at natural telomeres, we used 2D gels to examine replication of the left telomere of Chromosome III in SphI-digested DNA from asynchronous cells (Fig. 5A). The left telomere of Chromosome III has X but no Y‘ DNA (Button and Astell 1986). Although the X has an ARS, the ARS is inactive in wild-type cells (Newlon et al. 1993), such that replication of the terminal SphI fragment occurs by leftward-moving forks (ARS is marked with an asterisk in Fig. 5A). The 2D gels revealed the expected arc of forked replication intermediates in both wild-type (Fig. 5A, left panel) and rrm3Δ DNA (Fig. 5A, center and right panels). Although the 2N spot was visible in DNA from both strains (Fig. 5A, filled arrows), the 2N/1N ratio was 9.4 ± 3.7-fold higher in rrm3Δ than in wild-type DNA (average ± standard error; four independent experiments). There was also intense hybridization on the arc of forked intermediates at the position of the inactive ARS (Fig. 5A, asterisk). Again, replication pausing at this ARS was seen in both wild-type and mutant cells but was 9.9 ± 5.5-fold more intense in rrm3Δ cells (value is ratio of signal in pause divided by 1N spot for rrm3Δ divided by wild type ratio; average ± standard error; four independent experiments). Intense hybridization was also detected at the position of ∼6-kb linear fragments (Fig. 5A, marked by diamond). This structure was present in both mutant and wild-type DNA but was more abundant in rrm3Δ DNA. Because this spot was not present in DNA from G1-arrested cells (data not shown), it was not caused by cross-hybridization to a restriction fragment from another locus. If replication forks that are paused at the ARS break immediately ahead of the fork, they would generate an almost linear fragment of 6 kb (Fig. 5A, open arrowhead indicates the point of breakage). Because the replication intermediates for telomere III-L were the same in cells expressing the mutant K260A Rrm3p as in cells lacking Rrm3p, the ATPase/helicase activity of Rrm3p was needed for its role in replication of the III-L telomere (Fig. 5A).
Figure 5.
Rrm3p affects replication of natural, X-, and Y‘-bearing telomeres. (A) Structure of the left telomere of Chromosome III. An inactive ARS in the subtelomeric X element, 1 kb from the chromosome end, is indicated by an asterisk. Genomic DNA was digested with SphI, which generates a 4-kb terminal fragment from Chromosome III-L, and analyzed by 2D gels using the probe shown in A. Pausing at the inactive ARS is denoted by an asterisk. A diamond marks a molecule on the arc of simple linears that has a mass of ∼6 kb. A 6-kb, almost linear fragment is generated by breakage in front of the fork stalled at the ARS (see cartoon). The third panel is a longer exposure of the second panel that makes it easier to see replication bubbles (BU). The fourth panel is the pattern in an rrm3Δ strain that carries the rrm3-K260A allele on plasmid YCplac111 (as in Fig. 3C). (B) Structure of Y‘ elements. Up to four tandem Y‘ elements are found at a given telomere: the bracket indicates the position of the most terminal Y‘ element on a hypothetical telomere containing two Y‘ repeats. Genomic DNA from asynchronous cells was digested with ClaI and analyzed by 2D gels using the combination of probes shown in the cartoon. The pattern of replication intermediates for three strains is shown: RRM3 (WT), rrm3Δ, and rrm3Δ carrying the rrm3-K260A allele on plasmid YCplac111. The arc labeled with an asterisk is the 5.3-kb ClaI fragment from terminal Y‘ long elements; the arc labeled with a diamond is the 6.2-kb ClaI fragment from internal Y‘ long elements (two size variants of this fragment are visible in wild-type DNA). The rightmost arrow in the rrm3Δ gel is at the position for the 2N spot for Y‘ short telomeres. In the schematic of the 2D gel for rrm3Δ Y‘ DNA, arrows point to the 2N spot for Y‘ long (leftmost arrow) and Y‘ short (rightmost arrow) telomeres. Pauses in rrm3Δ DNA are labeled 1–6 (see text). (HJ) Holliday junctions between internal Y‘ long elements; (BU) bubble-containing replication intermediates for internal Y‘ long elements.
If the X ARS on Chromosome III-L were used as an origin of DNA replication, replication of the SphI fragment would generate bubble-shaped replication intermediates. In wild-type cells, even longer exposure of the gel in Figure 5A (left panel) did not reveal bubble-shaped intermediates, consistent with the finding that this ARS is not active in wild-type cells (Newlon et al. 1993). However, bubble-shaped intermediates were visible in rrm3Δ DNA (Fig. 5A, second panel; longer exposure of same gel is shown in Fig. 5A, third panel; BU, bubble-shaped intermediates). Although the absence of Rrm3p enabled this normally inactive ARS to fire, even in rrm3Δ cells, the X ARS was not active at most of the Chromosome III-L telomeres as most of the III-L replication intermediates were simple forked molecules.
Replication and recombination of Y‘ DNA is affected by Rrm3p
About two-thirds of yeast telomeres bear one to four tandem copies of Y‘ (Fig. 5B; Chan and Tye 1983). Some Y‘ elements are right next to a telomere (terminal Y‘; Fig. 5B, asterisk) and some are not (internal Y‘; Fig. 5B, diamond). Short tracts of C1–3A/TG1–3 DNA are often found between tandem Y‘ elements (Walmsley et al. 1984). Y‘ has an ARS that is active in at least some strains (Ferguson et al. 1991). When Y‘ ARSs are inactive, ClaI fragments from internal (and terminal) Y‘ elements are replicated by forks moving toward the telomere. If the Y‘ ARS on an internal Y‘ element is active, its replication will begin in the center of the ClaI fragment and expand bidirectionally, producing bubble-shaped replication intermediates. The pattern of Y‘ replication intermediates is complicated by the fact that Y‘ comes in two sizes, Y‘ long (6.7 kb) and Y‘ short (5.2 kb; Louis and Haber 1990), and the size of both varies by the number of 36-bp repeats they contain (Horowitz and Haber 1984). In the strain used here for 2D gel analysis, most Y‘ elements were Y‘ longs (Fig. 5B).
To examine replication of Y‘ telomeres, DNA was prepared from an asynchronous culture, digested with ClaI, and analyzed by 2D gels using Y‘ probes (Fig. 5B). Digestion with ClaI releases a 5.3-kb fragment from terminal Y‘ long elements (Fig. 5B, asterisk) and a 6.2-kb fragment from internal Y‘ long elements (Fig. 5B, diamond). In Y‘ long telomeres, the 2N spot was visible in both rrm3Δ and wild-type DNA, but the 2N/1N ratio was 2.9 ± 0.32-fold higher in rrm3Δ DNA (average ± standard error; four independent experiments; Fig. 5B, 2N spot denoted by arrows). In rrm3Δ DNA, the 2N spot was also visible at Y‘ short telomeres (Fig. 5B, right panel, arrows). The threefold increase in the fraction of DNA in the 2N spot for Y‘-bearing telomeres in rrm3Δ cells was smaller than the ∼10-fold increase at the left telomeres of Chromosome VII or III. However, as detailed below, there were many sites of replication pausing within Y‘ DNA in addition to the pause within the telomere itself.
The pattern of Y‘ intermediates in rrm3Δ DNA was complicated. There were several structures that were detected only in rrm3Δ DNA, such as Holliday junctions between internal Y‘ elements (HJ; Fig. 5B, cartoon). The presence of Holliday junctions indicates that Y‘–Y‘ recombination was elevated in rrm3Δ cells. An intermediate that behaved like an internal Y‘ with an active origin was also detected only in rrm3Δ DNA (BU, bubble; Fig. 5E, cartoon). Although the absence of Rrm3p increased the frequency of firing of the internal Y‘ ARS, the Y‘ ARS was active in only a fraction of internal Y‘ elements, as most internal Y‘ elements were in simple forked intermediates, even in the rrm3Δ strain (Fig. 5B, cartoon, diamond). There were additional sites of replication fork pausing in rrm3Δ DNA (Fig. 5B, cartoon, 1–6). Sites 1 and 2 mapped near the 2N spot on Y‘ long and Y‘ short telomeres. Site 3 mapped to the position of the Y‘ ARS and the internal C1–3A/TG1–3 tract for internal Y‘ long elements replicated by forks moving toward the telomere, as would be expected if all of the Y‘ ARSs on the telomere were inactive. Site 4 mapped to the same region in internal Y‘ long elements replicated by forks moving away from the telomere, as would be expected if the ARS in a distal Y‘ on the same telomere were active. Pausing was detected near the end of the ClaI fragments from internal Y‘ long (site 5) and internal Y‘ short (site 6) elements. The replication intermediates for cells expressing the mutant K260A Rrm3p were the same as in cells lacking Rrm3p, indicating that Rrm3p acts catalytically during Y‘ replication.
Replication forks pause as they pass through internal tracts of C1–3A/TG1–3 DNA, and this pausing is exacerbated in the absence of Rrm3p
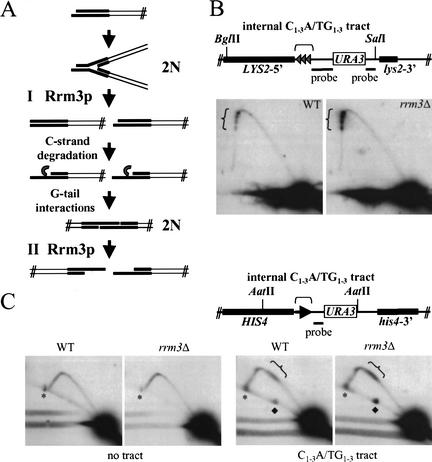
Two models can explain the formation of the 2N spot. First, the 2N spot might arise from sister chromatids that are held together by unreplicated telomeric DNA. In this model, Rrm3p affects conventional, semiconservative replication of telomeric DNA (Fig. 6A, Model I). Alternatively, the 2N spot could form after semiconservative replication of telomeres (Fig. 6A, Model II). Conventional replication of telomeres is followed by C-strand degradation, a process that generates single-strand TG1–3 tails on both ends of linear chromosomes (Wellinger et al. 1993a,b, 1996). In vitro, linear plasmids with TG1–3 tails can interact to form stable telomere–telomere associations (Fig. 6A, G-tail interaction step). If G-tail interactions formed between the ends of sister chromatids in vivo, they would generate intermediates that behaved like the 2N spot in 2D gels. In this model, Rrm3p would be important for unwinding base-paired G-tails (Fig. 6A, Model II).
Figure 6.
Internal tracts of C1–3A/TG1–3 cause replication fork pausing, and this pausing is increased in an rrm3Δ strain. (A) The cartoon shows replication of a yeast telomere. The telomeric C1–3A/TG1–3 tract is in bold. There are two models that can explain the appearance of the 2N spot. In Model I, the 2N spot is formed by replication forks pausing before or within the C1–3A/TG1–3 tract. In this model, Rrm3p promotes semiconservative replication of telomeres. After DNA replication, the C strand of telomeric DNA is degraded to form long single-strand TG1–3 tails (Wellinger et al. 1993a,b, 1996). If the TG1–3 tails on sister chromatids interacted by stable G–G base pairing, they would generate DNA that behaves like 2N-spot DNA. In Model II, Rrm3p would promote dissociation of G-base-paired telomeres. Only the first model predicts that Rrm3p should promote replication through internal C1–3A/TG1–3 tracts. (B) Three 276-bp tracts of C1–3A/TG1–3 DNA, each separated from the adjacent tract by 19 bp of polylinker DNA, were inserted within the LYS2 locus (Stavenhagen and Zakian 1994). DNA from wild-type and rrm3Δ cells containing the C1–3A/TG1–3 tract was digested with BglII and SalI, and examined by 2D gels. The probe detects a 7-kb fragment. (C) DNA from wild-type or rrm3Δ cells with or without (no tract) a 500-bp C1–3A/TG1–3 tract inserted within HIS4 was digested with AatII and analyzed by 2D gels, using the indicated probe. The probe detects a 3.6-kb AatII fragment in the no-tract control strains and a 4.1-kb AatII fragment in strains with the C1–3A/TG1–3 tract. The asterisks mark an ∼8-kb AatII fragment that cross-reacts with the hybridization probe. This cross-reacting band falls fortuitously near the position of 2N-spot DNA in the strains with the C1–3A/TG1–3 tract. The linear fragment of ∼5.5 kb that is marked by a diamond is seen only in the presence of the 0.5-kb C1–3A/TG1–3 tract. Because its abundance is proportional to the amount of pausing at the C1–3A/TG1–3 tract, it is probably caused by breakage of stalled replication intermediates immediately ahead of the replication fork as in Figure 5A. In panels B and C, brackets indicate the position of the C1–3A/TG1–3 tracts.
The two models cannot be distinguished readily by physical analysis of 2N-spot DNA. For example, brief digestion with mung bean nuclease degrades both replication forks and G-tail-associated telomeres (Wellinger et al. 1993b). However, if the first model were correct, Rrm3p might also affect the rate of fork movement through internal tracts of C1–3A/TG1–3 DNA. To test this possibility, we used a strain having three 276-bp tracts of C1–3A/TG1–3 DNA inserted at the LYS2 locus, a site far from a telomere (Stavenhagen and Zakian 1994). The “triple tract” strain was constructed in such a way that there are 19 bp of polylinker DNA between each C1–3A/TG1–3 tract. This ∼800-bp C1–3A/TG1–3 tract represses transcription of a nearby URA3 gene, albeit inefficiently compared with telomeres (Stavenhagen and Zakian 1994). Because silencing by internal C1–3A/TG1–3 tracts requires Rap1p and the Sir complex (Stavenhagen and Zakian 1994), these tracts must bind many of the proteins found at telomeres, at least in some cells (see also Bourns et al. 1998).
DNA was prepared from wild-type and rrm3Δ cells bearing the ∼800-bp C1–3A/TG1–3 tract within LYS2 (Fig. 6B). Pausing was seen at the C1–3A/TG1–3 tracts in both strains but in two independent experiments was about twofold higher in rrm3Δ cells (ratio of the fraction of hybridization in the tracts over the signal in the 1N spot for rrm3Δ compared with wild type was 2.0 and 2.1). There were four discrete sites of pausing in both strains, suggesting that forks pause before and after passage through a C1–3A/TG1–3 tract. A 500-bp tract consisting solely of C1–3A/TG1–3 DNA and inserted within HIS4, ∼65 kb from the left telomere of Chromosome III, also slowed replication fork progression (Fig. 6C). In two independent experiments, this pausing was increased about twofold (ratios of 1.7 and 1.9) in the rrm3Δ strain. We speculate that Rrm3p-dependent pausing was less dramatic at internal tracts of C1–3A/TG1–3 DNA than at telomeres because internal tracts are less likely to assemble into a non-nucleosomal chromatin structure as inferred from their reduced ability to repress transcription of a nearby gene (Stavenhagen and Zakian 1994). In the absence of the C1–3A/TG1–3 tract, there was no Rrm3p-dependent pausing in the 3.6-kb restriction fragment that contains HIS4 (Fig. 6C, no tract controls). Because Rrm3p is needed for fork progression through internal C1–3A/TG1–3 tracts, the most parsimonious explanation is that Rrm3p also promotes fork progression through duplex telomeric DNA (Fig. 6, Model I). However, we cannot exclude the possibility that Rrm3p also acts after conventional DNA replication to promote separation of G-tail–G-tail interactions (Fig. 6, Model II).
Rrm3p is associated with telomeric DNA in vivo
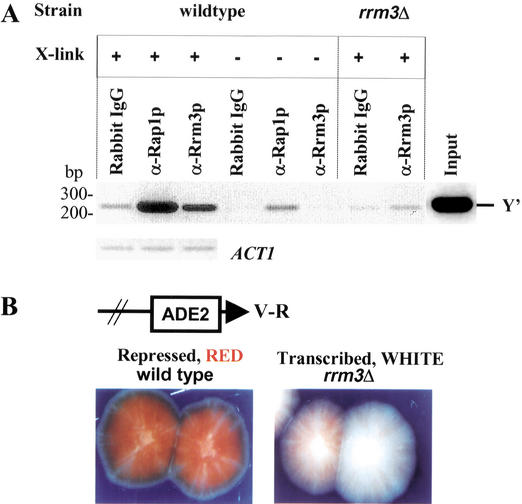
Because helicases affect multiple processes, Rrm3p could affect telomere replication directly or indirectly. If Rrm3p affects telomere replication directly, it should be telomere-associated in vivo. To address this possibility, we used chromatin immunoprecipitation (Fig. 7A). Chromatin was cross-linked in vivo with formaldehyde, sheared, and precipitated with either protein-A-purified preimmune antibodies (rabbit IgG) or antigen-affinity-purified anti-Rrm3p antibodies (α-Rrm3p; Fig. 7A). As a positive control, chromatin was also precipitated with an anti-Rap1p serum (α-Rap1p), as Rap1p is the major telomere-binding protein in yeast (Conrad et al. 1990; Wright and Zakian 1995). The cross-links in the immunoprecipitates were reversed, DNA was purified, and then amplified by the polymerase chain reaction (PCR) using primers for a 233-bp portion of the subtelomeric Y‘ element that is 30 bp upstream of the C1–3A/TG1–3 tracts. As a control, primers that amplify a 131-bp fragment from the ACT1 gene were used. Immunoprecipitation with either anti-Rap1p or anti-Rrm3p, but not with preimmune antibodies, precipitated Y‘ telomeric DNA in wild-type cells that had been cross-linked with formaldehyde in vivo. However, anti-Rrm3p antibodies did not precipitate telomeric DNA from rrm3Δ cells or from wild-type cells in the absence of in vivo cross-linking. Serial dilutions of the PCR-amplified DNA revealed that Y‘ telomeric DNA was enriched 5 ± 1.2-fold (average ± S.D.) compared with the amount of Y‘ DNA after precipitation with preimmune antibodies (data not shown). In contrast, ACT1 DNA was present at similar levels in the preimmune and immune precipitates (Fig. 7A). As precipitation of Y‘ DNA with anti-Rrm3 antibodies did not occur in the absence of cross-linking, the association of Rrm3p with telomeric DNA occurred in vivo.
Figure 7.
Rrm3p is associated with telomeric DNA in vivo and has a modest effect on TPE. (A) Chromatin was prepared from otherwise isogenic wild-type or rrm3Δ cells that had been cross-linked (+ cross-link) or not (− cross-link) with formaldehyde in vivo. Immunoprecipitation was carried out using either protein-A-purified preimmune IgG (rabbit IgG), a polyclonal Rap1p antiserum (α-Rap1p; Conrad et al. 1990), or affinity-purified anti-Rrm3p polyclonal antibodies (Ivessa et al. 2000). The DNA in the precipitate was PCR-amplified for 28 cycles using Y‘ primers that detect a 233-bp portion of the subtelomeric Y‘ element that begins 30 bp upstream of the terminal C1–3A/TG1–3 tracts or for 31 cycles using ACT1 primers. The PCR products were separated in a 2.3% agarose gel and visualized by staining with ethidium bromide. PCR amplification of the input DNA with telomeric primers is also shown (Input). Although Rrm3p association with telomeric DNA was eliminated in the absence of in vivo cross-linking, some Rap1p association with telomeric DNA was detected in the no cross-linking control. (B) TPE was measured in a strain with URA3 next to the left telomere of Chromosome VII (Gottschling et al. 1990) and ADE2 next to the right telomere of Chromosome V (Wiley and Zakian 1995). Wild-type or rrm3Δ cells were plated on media containing low amounts of adenine, and the color of the resulting colonies was examined.
Rrm3p is needed for wild-type levels of telomeric silencing
Telomere position effect (TPE) refers to the transcriptional silencing of genes near telomeres (Gottschling et al. 1990). To determine if Rrm3p affects TPE, we used a strain with ADE2 next to the V-R telomere and URA3 next to the VII-L telomere. Many of the colonies produced by wild-type cells were red with white sectors (Fig. 7B, left panel), reflecting repression of ADE2 transcription. In contrast, many of the rrm3Δ cells produced mostly white colonies with many red sectors (Fig. 7B, right panel), suggesting a reduction in TPE. Consistent with this result, the fraction of FOAR cells, those in which the telomeric URA3 gene was repressed, was 3.6-fold lower in rrm3Δ cells (average fraction of FOAR cells ± S.D. was 0.54 ± 0.12 in wild-type cells and 0.15 ± 0.047 in rrm3Δ cells). Therefore, Rrm3p is required for wild-type levels of TPE, but its effects on TPE are modest.
Discussion
Rrm3p is a 5′ to 3′ DNA helicase (Fig. 1) that affects progression of replication forks through telomeric and subtelomeric DNA (Figs. 3–6) as well as telomere length (Fig. 2A), the ability of Pif1p to inhibit telomerase-mediated telomere addition (Fig. 2B), and telomere position effect (Fig. 7B). Rrm3p was telomere-associated in vivo (Fig. 7A), arguing that its effects on telomeres are likely direct. Point mutations in the ATP-binding Walker A box that eliminate the activity of other helicases had the same effects on telomere replication as null alleles of RRM3 (Figs. 3C, 5). Therefore, Rrm3p acts catalytically during replication of telomeric DNA.
A new intermediate in telomere replication, the 2N spot, is described in this study. The 2N spot was seen at the modified VII-L telomere (Figs. 3, 4) as well as at natural X- (Fig. 5A) and Y‘-bearing telomeres (Fig. 5B). The 2N spot had the mass and structure expected for sister chromatids held together within the telomeric C1–3A/TG1–3 tract. This interpretation was confirmed by showing that the 2N spot at the Chromosome VII-L telomere was lost upon BamHI digestion (Fig. 3B). As expected for a replication intermediate, the 2N spot was cell cycle regulated, appearing and disappearing at specific times in the cell cycle (Fig. 4). In asynchronous cells, the 2N spot in rrm3Δ cells was 10 times more abundant than in wild-type cells. In synchronous cells, it persisted for a longer amount of time in rrm3Δ than in wild-type cells (Fig. 4C). These data are consistent with a model in which the 2N spot is a normal intermediate in telomere replication whose half-life is increased in the absence of Rrm3p, suggesting that the Rrm3p helicase plays a role in its processing.
In addition to its effects on fork progression through telomeric C1–3A/TG1–3 tracts, loss of Rrm3p exacerbated the natural pausing at the silent ARS within the subtelomeric X on Chromosome III-L (Fig. 5A) and revealed new replication pauses within the subtelomeric region of Chromosome VII-L (Fig. 3C, asterisk) and at multiple sites within Y‘ DNA, including two that mapped to a region that contains both an inactive ARS and an internal C1–3A/TG1–3 tract (Fig. 5B). Cells lacking Rrm3p also had an increased abundance of Holliday junctions between tandem Y‘ elements (Fig. 5B) and DNA breakage at specific sites (Figs. 5A, 6C). Similarly, loss of Rrm3p results in replication pausing at specific sites within rDNA, including at silent replication origins and regulatory regions for transcription of the 35S and 5S rRNAs. Recombination and DNA breakage are also elevated in rDNA in rrm3Δ cells (Ivessa et al. 2000). Likewise, DNA breakage accompanies replication fork stalling in bacteria (see, e.g., Seigneur et al. 1998). The increased breakage and recombination seen in the absence of Rrm3p are likely secondary consequences of faulty DNA replication.
Although Rrm3p affects fork progression in rDNA (Ivessa et al. 2000) and telomeres (this paper), there are many regions that are unaffected by loss of Rrm3p. As assayed by 2D gels, replication of a 4.5-kb region that accounts for half of the rDNA repeat unit (Ivessa et al. 2000), a 7-kb region containing LYS2, and a 3.6-kb region containing HIS4 (Fig. 6) are unaffected in a rrm3Δ strain. Nonetheless, Rrm3p activity is not limited to the rDNA and telomeres. For example, Rrm3p also affected fork progression at internal tracts of C1–3A/TG1–3 DNA, even when these tracts were far from a telomere (Fig. 6).
In addition to defects in telomere replication, Rrm3p affected telomere length (Fig. 2), TPE (Fig. 7B), and the frequency of firing of subtelomeric X and Y‘ replication origins (Fig. 5). Because these effects were modest, they may be secondary to the delay in conventional telomere replication. For example, stalling of replication forks within telomeric DNA might cause replication fork slippage, which, in turn, could bring about telomere lengthening. Firing of latent replication origins in X and Y‘ DNA (Fig. 5) probably results from the delayed arrival of replication forks to subtelomeric regions.
The data in this paper have implications for helicase subfamilies. As expected from their high level of sequence similarity, Rrm3p and Pif1p are both 5′ to 3′ DNA helicases (Fig. 1D). Although both helicases act at telomeres, they affect different steps in telomere replication. Rrm3p promoted conventional, semiconservative replication of telomeric DNA (Figs. 3–6), whereas Pif1p inhibits telomerase (Zhou et al. 2000; Myung et al. 2001). By itself, Rrm3p did not affect the ability of telomerase to add telomeres to double strand breaks, and its deletion partially suppressed Pif1p’s inhibition of these events (Fig. 2B). Pif1p had no effect on conventional replication of telomeres as assayed by 2D gels (data not shown). Thus, the sequence similarity that defines the Pif1 subfamily of DNA helicases most likely reflects their ability to recognize or be recruited to common DNA substrates.
Rrm3p, a 5′ to 3′ DNA helicase, acts catalytically to promote fork progression at rDNA (Ivessa et al. 2000) and telomeres (this paper). Several models are consistent with this behavior. First, Rrm3p might be a regional-specific replicative helicase that acts at both telomeres and rDNA. However, replication of rDNA and telomeres is slowed but not prevented in its absence, and fork progression throughout the rDNA also requires the MCM complex (J. Bessler and V.A. Zakian, unpubl.). Thus, Rrm3p is unlikely to be the sole replicative helicase, at least for rDNA. Alternatively, Rrm3p might facilitate passage of replication forks through large protein–DNA complexes. This model is consistent with the fact that sites that display Rrm3p-dependent pauses, including telomeres (Figs. 3–5), internal tracts of C1–3A/TG1–3 DNA (Fig. 6), the replication fork barrier (RFB) in the rDNA (Ivessa et al. 2000), highly transcribed promoters (Ivessa et al. 2000), and silent replication origins (Fig. 5; Ivessa et al. 2000), are assembled into large protein–DNA complexes (Wright et al. 1992; Santocanale and Diffley 1996; Bourns et al. 1998). For example, Rrm3p might be part of a chromatin-remodeling machinery that makes such regions accessible to the conventional DNA replication machinery, or Rrm3p might be required to restart forks that collapse as they attempt to pass through these regions.
Replication fork collapse is a common occurrence in both prokaryotes and eukaryotes (for review, see Cox et al. 2000; Marians 2000; Rothstein et al. 2000). For example, in Escherichia coli, both DNA damage and DNA–protein complexes are thought to trigger fork collapse, resulting in the demise of ∼30% of the replication forks emanating from oriC. Two bacterial DNA helicases, PriA and Rep, appear to have roles in replication fork restart. Although eukaryotic proteins with roles in fork restart have not been identified, the Rrm3p DNA helicase has many of the characteristics expected for such an activity.
Materials and methods
Expression, purification, and activities of Rrm3pΔN
To make a truncated version of Rrm3p, a 1587-bp fragment of RRM3 that began at amino acid 194 and went to the stop codon was PCR-amplified from a clone containing full-length RRM3 and inserted at the BamHI site of pEG(KT) (Mitchell et al. 1993) to generate pEG(KT)-Rrm3ΔN. In pEG(KT), proteins are expressed as carboxy-terminal fusions to GST and are expressed under the control of the galactose-inducible GAL1 promoter. pEG(KT)-Rrm3ΔN was transformed into the protease-deficient S. cerevisiae BCY123 strain (Bennett et al. 1998). Expression was carried out using minor modifications of the methods described in Bennett et al. (1998). Cells were harvested, washed, and resuspended in 8-vol of lysis buffer (50 mM Tris at pH 7.8, 500 mM NaCl, 4 mM MgCl2, 40 μg/mL DNase I, 10 mM dithiothreitol, 0.1% Triton X-100, 0.004% 1-octanol) and a mixture of protease inhibitors. The resuspended cells were disrupted by passing them twice through a homogenizer. After centrifugation, the supernatant was brought to 50% saturation with ammonium sulfate. The precipitate was collected by centrifugation, dissolved in 20 mL of PBS supplemented with 5 mM dithiothreitol, 0.5% Triton X-100, 0.001% 1-octanol, and the mixture of protease inhibitors. The soluble fraction recovered by centrifugation was loaded onto a Glutathione sepharose 4B (Pharmacia) column equilibrated with PBS. Protein was eluted with 20 mL of elution buffer (50 mM Tris at pH 8.8, 30 mM reduced glutathione, 50 mM NaCl, 10 mM dithiothreitol, 0.1% Triton X-100, 0.001% 1-octanol). The elution was loaded onto a 10-mL Q-sepharose column (Pharmacia) equilibrated with 50 mM Tris-HCl buffer at pH 7.8 (50 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol, 0.002% Triton X-100) at a flow rate of 30 mL/h. The column was washed with the equilibration buffer, and eluted with a linear NaCl gradient. During its purification, Rrm3pΔN was followed by immunoblot analysis using the anti-Rrm3p serum described in Ivessa et al. (2000). Rrm3pΔN eluted from the Q-sepharose column between 150 and 200 mM NaCl.
The ATPase and helicase assays were 20-μL reactions containing 200 ng of recombinant Rrm3pΔN or 100 ng of recombinant Pif1p (purified as described in Zhou et al. 2000). The ATPase assays were in ATPase buffer (25 mM HEPES at pH 7.6, 5 mM MgCl2, 2 mM ATP, 1 mM dithiothreitol, 100 μg/mL BSA) at 37°C for 30 min. Each reaction contained 0.5 μCi of [γ-32P]ATP and 0.2 μg/μL M13mp18 single-stranded DNA. Reactions were stopped by the addition of 1 μL of 0.5 M EDTA, and 0.5 μL of each reaction was spotted on polyethylimine (PEI) cellulose plate (Baker). The plate was developed in 0.8 M LiCl, and dried with hot air. For helicase assays, the 5′ 25-mer oligonucleotide was 5′-GTTGTAAAACGACGGCCAGTGAATT-3′ and the 36-mer oligonucleotide was 5′-CGTAATCATGGT CATAGCTGTTTCCTGTGTGAAATT-3′. For the helicase assay, 2.5 pmole of both the 32P-end-labeled 25-mer and 36-mer oligonucleotides were annealed in a 75-μL reaction mixture with 2.5 pmole of single-stranded M13mp7 DNA that had been linearized by digestion with EcoRI. The annealed substrates were purified with the Chroma Spin-1000 column (Clontech). The 20-μL helicase activity assays had 30 fmole of DNA substrate in 20 mM HEPES (pH 7.6), 5 mM MgAc2, 4 mM ATP, 100 μg/mL BSA, 5% glycerol, 1 mM DTT, and were carried out at 37°C for 10 min. Reactions were stopped by addition of 5 μL of a stop buffer (50 mM Tris at pH 7.8, 100 mM EDTA, 2% SDS). Products were analyzed by electrophoresis in a 10% polyacrylamide gel (89 mM Tris borate at pH 8.3, 2 mM EDTA).
Strains, DNA preparation, and gel electrophoresis
The two rrm3Δ alleles used in this paper were both precise deletions of the RRM3 ORF that were made by PCR, insertion of HIS3 (TPE experiments), or TRP1 (all other experiments) and introduced into diploid cells by integrative transformation. Haploid Trp+ or His+ segregants were identified after sporulation and tetrad dissections. The rrm3-K260A allele was described in Ivessa et al. (2000) and the pif1Δ allele in Schulz and Zakian (1994). The rrm3Δ pif1Δ strain was made by mating singly mutant strains and sporulating the resulting diploid. Telomere length analysis was carried out in both the VPS106 (Schulz and Zakian 1994) and YPH499 (Sikorski and Hieter 1989) backgrounds with similar results. Replication intermediates were analyzed in the VPS106 background. The left telomere of Chromosome VII was modified as in Gottschling et al. (1990). The strain with the triple tract of C1–3A/TG1–3 is YJS-TTL-URA (Stavenhagen and Zakian 1994). The strain containing the C1–3A/TG1–3 tract at HIS4 was generously provided by S.-C. Teng (Princeton University, NJ). It was made using a HpaI-linearized version of pRS306 that had a portion of HIS4 (bp 1401–2400) inserted at the KpnI site and an ∼500-bp C1–3A/TG1–3 tract, cloned from a recombinationally lengthened natural telomere inserted at the EcoRI site.
For most experiments, cells were grown to a density of 1–2 × 107 cells/mL at 30°C. Cell growth was stopped by putting cells on ice in the presence of 0.1% NaN3. For the synchrony experiment, cells were arrested with 5 mg/L α factor (Sigma) at 22°C for ∼3 h. Cells were released from the G1 arrest by adding pronase to 125 μg/mL, then were grown at 22°C with samples taken at 15-min intervals. Cells were processed for FACs analysis using 1 μM SYTOX Green (Molecular Probes) after fixation in 70% ethanol and digestion with 0.25 mg/mL RNase A and 1 mg/mL proteinase K (Sigma). DNA was isolated from yeast nuclei obtained by a glass bead procedure (Huberman et al. 1987). To observe replication intermediates, minor modification of the neutral/neutral 2D gel electrophoresis method (Brewer and Fangman 1987) was used. Typically, the first dimension was 0.35% (w/v) agarose in 1× TBE (Tris borate–EDTA) at 0.6–0.7 V/cm at room temperature for 45–48 h. The second dimension was 0.9% (w/v) 1× TBE agarose gels plus 0.3 μg/mL ethidium bromide at 2.6–3 V/cm at 4°C for 18–24 h. To examine Y‘ intermediates (Fig. 5D), the first-dimensional gel was run for 60 h and the second-dimensional gel for 48 h. Quantification of autoradiograms was performed by storage phosphor imaging using the Molecular Dynamics 400A PhosphorImager. Image analysis was performed using the ImageQuant software. As a background correction, the intrinsic parameter LocalMedian was applied.
Other assays
The rate of forming new telomeres was determined in the VPS106 background using the assay described in Schulz and Zakian (1994) except that a slightly different YAC, YAC-VS9, was used. Chromatin immunoprecipitations were carried out with minor modifications of the methods described in Hecht et al. (1996) in the YPH499 background (Sikorski and Hieter 1989). Sequences for primers are available upon request. The Y‘ primers are 240 bp and 33 bp away from the telomere. A 20-μL aliquot of the 50-μL PCR products was resolved in a 2.3% agarose gel containing 200 μg/L of ethidium bromide. A twofold dilution series of the PCR products was loaded in the same gel to estimate the fold enrichment of telomeric Y‘ DNA in the immunoprecipitates.
TPE values were determined in the YPH499 background (Sikorski and Hieter 1989) in a strain with URA3 next to the left telomere of Chromosome VII (Gottschling et al. 1990), and ADE2 at the right telomere of Chromosome V (Wiley and Zakian 1995). To determine the percentage of FOAR cells, single colonies growing on YC plates were cored out and resuspended in water, and 10-fold serial dilutions were plated on YC-URA, YC, and FOA plates.
Acknowledgments
We thank J. Bessler for constructing YCplac111-RRM3 and YCplac111-rrm3K260A and S.-C. Teng for constructing the strain with the C1–3A/TG1–3 tract at HIS4. We thank A. Taggart for help with the synchrony experiments. We also thank J. Bessler, B. Lenzmeier, M. Mateyak, J. Torres, and L. Vega for comments on the manuscript. We thank the National Institutes of Health (R37 GM26938), the Austrian NSF, and the Leukemia and Lymphoma Society (A.S.I.), the American Cancer Society (V.P.S.), and the National Institutes of Health (E.K.M.) for financial support.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL vzakian@molbio.princeton.edu; FAX (609) 258-1701.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.982902.
References
- Bennett RJ, Sharp JA, Wang JC. Purification and characterization of the Sgs1 DNA helicase activity of Saccharomyces cerevisiae. J Biol Chem. 1998;273:9644–9650. doi: 10.1074/jbc.273.16.9644. [DOI] [PubMed] [Google Scholar]
- Bessler JB, Torres JZ, Zakian VA. The Pif1p subfamily of helicases: Region specific DNA helicases. Trends Cell Biol. 2001;11:60–65. doi: 10.1016/s0962-8924(00)01877-8. [DOI] [PubMed] [Google Scholar]
- Bourns BD, Alexander MK, Smith AM, Zakian VA. Sir proteins, Rif proteins and Cdc13p bind Saccharomyces telomeres in vivo. Mol Cell Biol. 1998;18:5600–5608. doi: 10.1128/mcb.18.9.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer BJ, Fangman WL. The localization of replication origins on ARS plasmids in S. cerevisiae. Cell. 1987;51:463–471. doi: 10.1016/0092-8674(87)90642-8. [DOI] [PubMed] [Google Scholar]
- Button LL, Astell CR. The Saccharomyces cerevisiae chromosome III left telomere has a type X, but not a type Y‘, ARS region. Mol Cell Biol. 1986;6:1352–1356. doi: 10.1128/mcb.6.4.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CSM, Tye B-K. Organization of DNA sequences and replication origins at yeast telomeres. Cell. 1983;33:563–573. doi: 10.1016/0092-8674(83)90437-3. [DOI] [PubMed] [Google Scholar]
- Conrad MN, Wright JH, Wolf AJ, Zakian VA. RAP1 protein interacts with yeast telomeres in vivo: Overproduction alters telomere structure and decreases chromosome stability. Cell. 1990;63:739–750. doi: 10.1016/0092-8674(90)90140-a. [DOI] [PubMed] [Google Scholar]
- Cox MM, Goodman MF, Kreuzer KN, Sherratt DJ, Sandler SJ, Marians KJ. The importance of repairing stalled replication forks. Nature. 2000;404:37–41. doi: 10.1038/35003501. [DOI] [PubMed] [Google Scholar]
- Ferguson BM, Brewer BJ, Reynolds AE, Fangman WL. A yeast origin of replication is activated late in S phase. Cell. 1991;65:507–515. doi: 10.1016/0092-8674(91)90468-e. [DOI] [PubMed] [Google Scholar]
- Formosa T, Nittis T. Dna2 mutants reveal interactions with DNA polymerase α and Ctf4, a Pol α accessory factor, and show that full Dna2 helicase activity is not essential for growth. Genetics. 1999;151:1459–1470. doi: 10.1093/genetics/151.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschling DE, Aparicio OM, Billington BL, Zakian VA. Position effect at S. cerevisiae telomeres: Reversible repression of Pol II transcription. Cell. 1990;63:751–762. doi: 10.1016/0092-8674(90)90141-z. [DOI] [PubMed] [Google Scholar]
- Gravel S, Larrivee M, Labrecque P, Wellinger RJ. Yeast Ku as a regulator of chromosomal DNA end structure. Science. 1998;280:741–744. doi: 10.1126/science.280.5364.741. [DOI] [PubMed] [Google Scholar]
- Hecht A, Strahl-Bolsinger S, Grunstein M. Spreading of transcriptional repressor SIR3 from telomeric heterochromatin. Nature. 1996;383:92–96. doi: 10.1038/383092a0. [DOI] [PubMed] [Google Scholar]
- Horowitz H, Haber JE. Subtelomeric regions of yeast chromosomes contain a 36 base-pair tandemly repeated sequence. Nucleic Acids Res. 1984;12:7105–7121. doi: 10.1093/nar/12.18.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman JA, Spotila LD, Nawotka KA, El-Assouli SM, Davis LR. The in vivo replication origin of the yeast 2μm plasmid. Cell. 1987;51:473–481. doi: 10.1016/0092-8674(87)90643-x. [DOI] [PubMed] [Google Scholar]
- Ivessa AS, Zhou J-Q, Zakian VA. The Saccharomyces Pif1p DNA helicase and the highly related Rrm3p have opposite effects on replication fork progression in ribosomal DNA. Cell. 2000;100:479–489. doi: 10.1016/s0092-8674(00)80683-2. [DOI] [PubMed] [Google Scholar]
- Keil RL, McWilliams AD. A gene with specific and global effects on recombination of sequences from tandemly repeated genes in Saccharomyces cerevisiae. Genetics. 1993;135:711–718. doi: 10.1093/genetics/135.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahaye A, Leterme S, Foury F. PIF1 DNA helicase from Saccharomyces cerevisiae. Biochemical characterization of the enzyme. J Biol Chem. 1993;268:26155–26161. [PubMed] [Google Scholar]
- Lea DE, Coulson CA. The distribution of the numbers of mutants in bacterial populations. J Genet. 1949;49:264–285. doi: 10.1007/BF02986080. [DOI] [PubMed] [Google Scholar]
- Louis EJ, Haber JE. Mitotic recombination among subtelomeric Y‘ repeats in Saccharomyces cerevisiae. Genetics. 1990;124:547–559. doi: 10.1093/genetics/124.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad V, Szostak JW. A mutant with a defect in telomere elongation leads to senescence in yeast. Cell. 1989;57:633–643. doi: 10.1016/0092-8674(89)90132-3. [DOI] [PubMed] [Google Scholar]
- Mangahas JL, Alexander MK, Sandell LL, Zakian VA. Repair of chromosome ends after telomere loss in Saccharomyces. Mol Biol Cell. 2001;12:4078–4089. doi: 10.1091/mbc.12.12.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcand S, Brevet V, Mann C, Gilson E. Cell cycle restriction of telomere elongation. Curr Biol. 2000;10:487–490. doi: 10.1016/s0960-9822(00)00450-4. [DOI] [PubMed] [Google Scholar]
- Marians KJ. PriA-directed replication fork restart in Escherichia coli. Trends Biochem Sci. 2000;25:185–189. doi: 10.1016/s0968-0004(00)01565-6. [DOI] [PubMed] [Google Scholar]
- Martin SG, Laroche T, Suka N, Grunstein M, Gasser SM. Relocalization of telomeric Ku and SIR proteins in response to DNA strand breaks in yeast. Cell. 1999;97:621–633. doi: 10.1016/s0092-8674(00)80773-4. [DOI] [PubMed] [Google Scholar]
- McCarroll RM, Fangman WL. Time of replication of yeast centromeres and telomeres. Cell. 1988;54:505–513. doi: 10.1016/0092-8674(88)90072-4. [DOI] [PubMed] [Google Scholar]
- Mitchell DA, Marshall TK, Deschenes RJ. Vectors for the inducible overexpression of glutathione S-transferase fusion proteins in yeast. Yeast. 1993;9:715–723. doi: 10.1002/yea.320090705. [DOI] [PubMed] [Google Scholar]
- Myung K, Chen C, Kolodner RD. Multiple pathways cooperate in the suppression of genome instability in Saccharomyces cerevisiae. Nature. 2001;411:1073–1076. doi: 10.1038/35082608. [DOI] [PubMed] [Google Scholar]
- Newlon CS, Collins I, Dershowitz A, Deshpande AM, Greenfeder SA, Ong LY, Theis JF. Analysis of replication origin function on Chromosome III of Saccharomyces cerevisiae. Cold Spring Harbor Symp Quant Biol. 1993;58:415–423. doi: 10.1101/sqb.1993.058.01.048. [DOI] [PubMed] [Google Scholar]
- Pluta AF, Dani GM, Spear BB, Zakian VA. Elaboration of telomeres in yeast: Recognition and modification of termini from Oxytricha macronuclear DNA. Proc Natl Acad Sci. 1984;81:1475–1479. doi: 10.1073/pnas.81.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R, Michel B, Gangloff S. Replication fork pausing and recombination or “gimme a break.”. Genes & Dev. 2000;14:1–10. [PubMed] [Google Scholar]
- Santocanale C, Diffley JF. ORC- and Cdc6-dependent complexes at active and inactive chromosomal replication origins in Saccharomyces cerevisiae. EMBO J. 1996;15:6671–6679. [PMC free article] [PubMed] [Google Scholar]
- Schulz VP, Zakian VA. The Saccharomyces PIF1 DNA helicase inhibits telomere elongation and de novo telomere formation. Cell. 1994;76:145–155. doi: 10.1016/0092-8674(94)90179-1. [DOI] [PubMed] [Google Scholar]
- Seigneur M, Bidnenko V, Ehrlich SD, Michel B. RuvAB acts at arrested replication forks. Cell. 1998;95:419–430. doi: 10.1016/s0092-8674(00)81772-9. [DOI] [PubMed] [Google Scholar]
- Shiratori A, Shibata T, Arisawa M, Hanaoka F, Murakami Y, Eki T. Systematic identification, classification, and characterization of the open reading frames which encode novel helicase-related proteins in Saccharomyces cerevisiae by gene disruption and Northern analysis. Yeast. 1999;15:219–253. doi: 10.1002/(SICI)1097-0061(199902)15:3<219::AID-YEA349>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavenhagen JB, Zakian VA. Internal tracts of telomeric DNA act as silencers in Saccharomyces cerevisiae. Genes & Dev. 1994;8:1411–1422. doi: 10.1101/gad.8.12.1411. [DOI] [PubMed] [Google Scholar]
- Strahl-Bolsinger S, Hecht A, Luo K, Grunstein M. SIR2 and SIR4 interactions differ in core and extended telomeric heterochromatin in yeast. Genes & Dev. 1997;11:83–93. doi: 10.1101/gad.11.1.83. [DOI] [PubMed] [Google Scholar]
- Sung P, Higgins D, Prakash L, Prakash S. Mutation of lysine-48 to arginine in the yeast RAD3 protein abolishes its ATPase and DNA helicase activities but not the ability to bind ATP. EMBO J. 1988;7:3263–3269. doi: 10.1002/j.1460-2075.1988.tb03193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y, Taggart AKP, Zakian VA. The role of the Mre11–Rad50–Xrs2 complex in telomerase-mediated lengthening of Saccharomyces cerevisiae telomeres. Curr Biol. 2001;11:1328–1335. doi: 10.1016/s0960-9822(01)00372-4. [DOI] [PubMed] [Google Scholar]
- Walmsley RM, Chan CSM, Tye B-K, Petes TD. Unusual DNA sequences associated with the ends of yeast chromosomes. Nature. 1984;310:157–160. doi: 10.1038/310157a0. [DOI] [PubMed] [Google Scholar]
- Wellinger RJ, Wolf AJ, Zakian VA. Origin activation and formation of single-strand TG1–3 tails occur sequentially in late S phase on a yeast linear plasmid. Mol Cell Biol. 1993a;13:4057–4065. doi: 10.1128/mcb.13.7.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Saccharomyces telomeres acquire single-strand TG1–3 tails late in S phase. Cell. 1993b;72:51–60. doi: 10.1016/0092-8674(93)90049-v. [DOI] [PubMed] [Google Scholar]
- Wellinger RJ, Ethier K, Labrecque P, Zakian VA. Evidence for a new step in telomere maintenance. Cell. 1996;85:423–433. doi: 10.1016/s0092-8674(00)81120-4. [DOI] [PubMed] [Google Scholar]
- Wiley E, Zakian VA. Extra telomeres, but not internal tracts of telomeric DNA, reduce transcriptional repression at Saccharomyces telomeres. Genetics. 1995;139:67–79. doi: 10.1093/genetics/139.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JH, Zakian VA. Protein–DNA interactions in soluble telosomes from Saccharomyces cerevisiae. Nucleic Acids Res. 1995;23:1454–1460. doi: 10.1093/nar/23.9.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JH, Gottschling DE, Zakian VA. Saccharomyces telomeres assume a non-nucleosomal chromatin structure. Genes & Dev. 1992;6:197–210. doi: 10.1101/gad.6.2.197. [DOI] [PubMed] [Google Scholar]
- Zhou J-Q, Monson EM, Teng S-C, Schulz VP, Zakian VA. The Pif1p helicase, a catalytic inhibitor of telomerase lengthening of yeast telomeres. Science. 2000;289:771–774. doi: 10.1126/science.289.5480.771. [DOI] [PubMed] [Google Scholar]