Abstract
Neural stem cells, which exhibit self-renewal and multipotentiality, are generated in early embryonic brains and maintained throughout the lifespan. The mechanisms of their generation and maintenance are largely unknown. Here, we show that neural stem cells are generated independent of RBP-Jκ, a key molecule in Notch signaling, by using RBP-Jκ−/− embryonic stem cells in an embryonic stem cell-derived neurosphere assay. However, Notch pathway molecules are essential for the maintenance of neural stem cells; they are depleted in the early embryonic brains of RBP-Jκ−/− or Notch1−/− mice. Neural stem cells also are depleted in embryonic brains deficient for the presenilin1 (PS1) gene, a key regulator in Notch signaling, and are reduced in PS1+/− adult brains. Both neuronal and glial differentiation in vitro were enhanced by attenuation of Notch signaling and suppressed by expressing an active form of Notch1. These data are consistent with a role for Notch signaling in the maintenance of the neural stem cell, and inconsistent with a role in a neuronal/glial fate switch.
Keywords: Presenilin, RBP-Jκ, embryonic stem cell, self-renewal, multipotentiality, cell cycle time
Neural stem cells, which are considered the ultimate lineage precursors to all neuronal and glial cells in the mammalian nervous system, are present not only in the developing brain but also in the adult brain (Weiss et al. 1996; Gage 2000). Although neural stem cells have a fundamental role in generating cellular diversity in the developing mammalian nervous system and in maintaining normal brain functions in adult brains (Lois and Alvarez-Buylla 1994; Tropepe et al. 1999; Shors et al. 2001), little is known concerning molecular mechanisms regulating the generation and maintenance of neural stem cells. In vitro, single neural stem cells proliferate to form clonally derived floating sphere colonies (neurospheres), which contain cells that, upon dissociation into single cells, give rise to new sphere colonies (self-renewal) and cells that can differentiate into neurons or glia (multipotentiality). Fibroblast growth factor-2 (FGF2)-responsive neural stem cells first appear in vivo at embryonic day (E) 8.5 and a separate and additive population of epidermal growth factor (EGF)-responsive neural stem cells arises from the earlier born FGF2-responsive stem cells by asymmetric division between E11 and E13 (Burrows et al. 1997; Mayer-Proschel et al. 1997; Tropepe et al. 1999). Both FGF2-responsive and EGF-responsive neural stem cells expand their populations and extend their cell cycle times during later embryogenesis (Martens et al. 2000). In the adult forebrain, neural stem cells are present as a relatively quiescent subpopulation in the subependyma, a remnant of the embryonic germinal zone (Morshead et al. 1994). This population persists into senescence, and the number is maintained throughout life (Tropepe et al. 1997). Thus, the generation and the size of the neural stem-cell population are tightly regulated during embryogenesis as well as in the adult. Recent evidence suggests that Notch signaling plays a role in preserving the neural stem-cell population. Mice deficient for Hes1, one of the downstream effectors of Notch signaling, display a decrease in the number of embryonic neural stem cells (Nakamura et al. 2000); however, how Notch activation underlies the existence of neural stem cells and regulates the size of neural stem-cell pools throughout life is unclear.
The Notch-signaling pathway is crucial for many diverse cell-fate decisions during development in vertebrates and in invertebrates (Kimble and Simpson 1997; Artavanis-Tsakonas et al. 1999; Selkoe 2000). After binding the ligands Dll or Jagged in mammals, the intracellular domain of the Notch receptor (NICD) is cleaved and then the signal is transmitted to the nucleus via a mediator, RBP-Jκ in mammals (Schroeter et al. 1998; Qi et al. 1999). Activation of Notch signaling results in altered expression of target genes such as Hes1/5 (Kageyama and Nakanishi 1997). The protease that is responsible for the cleavage of the Notch receptor at the plasma membrane and the generation of NICD has not yet been identified definitively. Recent evidence suggests that presenilins are essential participants in this cleavage event or could themselves be the proteases dubbed as γ-secretase (Selkoe 2000). Thus, the presenilins may be key molecules regulating the activity of the Notch-signaling pathway, and mice deficient for presenilin1 (PS1) have neurodevelopmental abnormalities in the germinal zone of the forebrain (Shen et al. 1997; Wong et al. 1997).
Using mice as well as embryonic stem (ES) cells deficient for presenilins, Notch1, or RBP-Jκ, we asked whether neural stem cells can be generated during embryogenesis and whether they can be maintained without appropriate Notch activation. Multipotent neural stem cells can be formed from RBP-Jκ−/− ES cells, but Notch pathway molecules are essential in neural stem cells both to expand their population size by dividing symmetrically in the developing brain and to maintain the size of the neural stem-cell pool in the adult brain. A gene dosage effect of PS1 also was observed on the cell cycle times of the remaining neural stem cells in the adult brain. Our results suggest that PS1 and subsequent appropriate Notch signaling play an important role in the homeostasis of the mammalian central nervous system.
Results
Neural stem cells are scarce in the early embryonic brains of Notch1−/− and RBP-Jκ−/− mouse
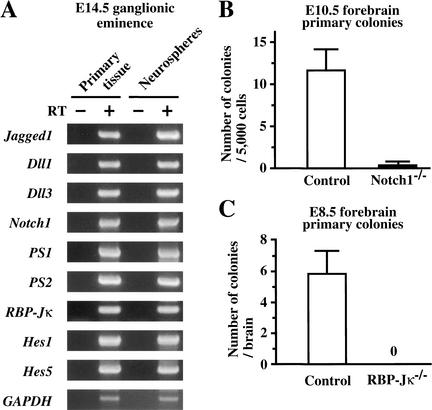
Neural stem-cell behavior can be operationally defined (and empirically tested) as the ability to proliferate and produce progenitor cells, self-renewal capacity, and neural multilineage potential. By use of an in vitro, colony-forming (neurosphere) assay, neurospheres were generated from single neural stem cells of embryonic mouse brains, and were examined for the expression of genes involved in the Notch-signaling pathway. The neurospheres derived from E14.5 CD1 mouse brains expressed all of the Notch pathway genes examined by RT–PCR (Fig. 1A); Jagged1 and Dll1/3, Notch ligands; presenilins, which cleave Notch and regulate Notch activation; Notch1, one of Notch receptors; RBP-Jκ, a transcription factor that is essential for downstream activation of target genes; and Hes1/5, target genes of Notch signaling (Artavanis-Tsakonas et al. 1999). Neurospheres derived from adult mouse subependyma showed the same gene expression profiles (data not shown).
Figure 1.
Notch signaling is critical for neural stem cells. (A) RT–PCR analysis of Notch-signaling pathway gene expression. Neurospheres as well as primary tissues from E14.5 ganglionic eminence of CD1 mouse embryos expressed all of the genes examined; Jagged1, Dll1/3, PS1/2, Notch1, RBP-Jκ, and Hes1/5. GAPDH was used for standardization of the samples. RT (+ and −) indicate that the RNA samples were treated with or without reverse transcriptase. (B) Neural stem cells were depleted almost entirely in E10.5 Notch1−/− forebrains (n = 3), whereas they were detected in the control (Notch1+/− and wild-type littermates) forebrains (n = 7; P < 0.05). (C) Neural stem cells were absent in E8.5 RBP-Jκ−/− forebrains (n = 11), whereas they were present in the control (RBP-Jκ+/− and wild-type littermates) forebrains (n = 25; P < 0.05). The graphed results are shown as means ± S.E.M.
We then used the neurosphere assay to examine the numbers of neural stem cells in embryonic brains deficient for the Notch1 or RBP-Jκ genes. Neural stem cells were depleted almost entirely in the E10.5 Notch1−/− brains (P < 0.05, Fig. 1B) and absent in the E8.5 RBP-Jκ−/− brains (P < 0.05, Fig. 1C) compared with their littermate controls, suggesting the pivotal role of Notch signaling in the existence of neural stem cells in the embryonic brain. However, the early death of Notch1−/− (between E10.5 and E11.5; Conlon et al. 1995) and RBP-Jκ−/− (soon after E8.5; Oka et al. 1995) embryos, and the scarcity of neural stem cells in these embryos made it difficult to further analyze the roles of Notch signaling. Therefore, we utilized PS1-deficient embryos, which can survive until birth (Shen et al. 1997; Wong et al. 1997).
E14.5 neural stem cells are depleted in the PS1−/− brain, and those that are present show less self-renewal capacity and give rise to more neuronal and astroglial progeny
The ganglionic eminences from E14.5 PS1−/− embryos and their littermate controls were dissociated into single cells to generate neurospheres. Samples of the acutely dissociated cells were analyzed for the expression of Nestin (undifferentiated cells), βIII tubulin (immature neurons), MAP2 (neurons), and GFAP (astrocytes). Comparable percentages of Nestin+ cells were found in the cells from PS1−/− (76.8 ± 3.2%, n = 2) and control (79.9 ± 1.9%, n = 6) brains. A slight, but nonsignificant, increase in βIII tubulin+ cells was observed in these acutely dissociated cells from PS1−/− brains (8.9 ± 0.5% vs. control 5.6 ± 1.7%). Neither MAP2+ nor GFAP+ cells were detected in either group.
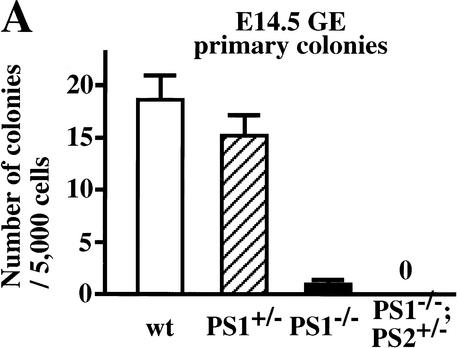
Using the neurosphere assay, we examined the number of neural stem cells in the presenilin-deficient embryonic brain. The number of neural stem cells isolated from the E14.5 PS1−/− brains was decreased by 93% compared with their wild-type littermate controls (P < 0.05, Fig. 2A). We have shown previously that separate, but coexisting populations of FGF2-responsive and EGF-responsive neural stem cells are present at E14.5 (Tropepe et al. 1999). EGF-responsive neural stem cells were also depleted in the PS1−/− brains (data not shown), excluding the possibility that the FGF-responsive neural stem cells are selectively affected by the PS1 mutation. The cerebral hemorrhages frequently observed in the PS1−/− brain at this stage might influence the survival of the neural stem cells. However, this possibility seems unlikely because the decrease in the number of neural stem cells was found as well in PS1−/− brains without any hemorrhage (2 of 12 brains). The disruption of the PS2 gene alone has little effect on the normal development of mice, but it enhances the phenotype in PS1−/− embryos (Donoviel et al. 1999; Herreman et al. 1999). Consistent with this, no neurosphere colonies were found in the cultures of ganglionic eminence cells from E14.5 PS1−/−;PS2+/− embryos.
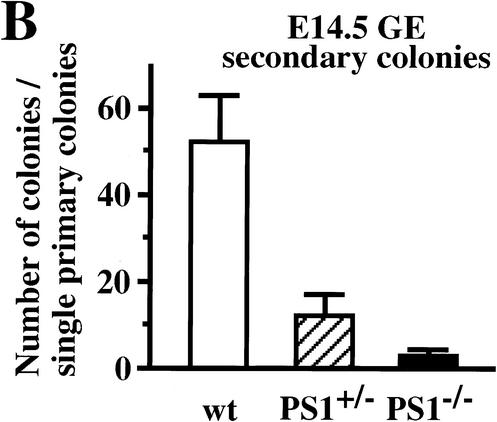
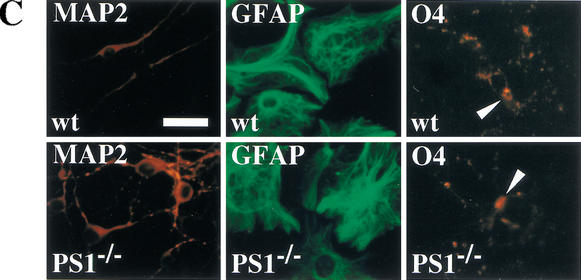
Figure 2.
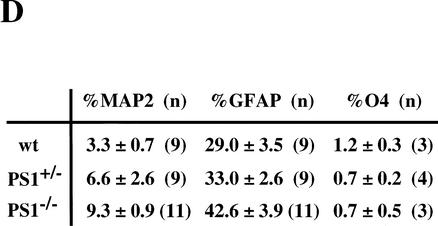
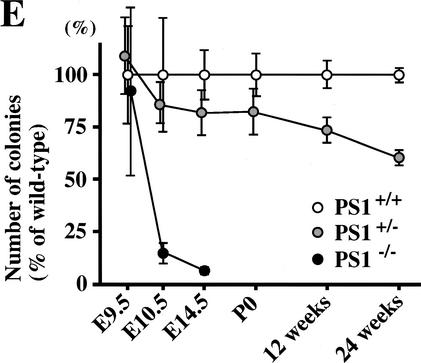
Neural stem cells are depleted in E14.5 PS1−/− brains. (A) The neural stem cells isolated from the ganglionic eminence (GE) of E14.5 wild-type (n = 13), PS1+/− (n = 12), PS1−/− (n = 12), and PS1−/−;PS2+/− (n = 2) brains are shown. (B) To assess self-renewal capacity of the primary sphere-forming neural stem cells, single primary neurosphere colonies of similar size (∼0.2 mm in diameter) were manually dissociated and recultured. Fewer secondary spheres were formed from each primary PS1−/− or PS1+/− sphere than from each primary wild-type sphere (wild type, n = 15; PS1+/−, n = 9; PS1−/−, n = 8; P < 0.05 vs. wild type). (C,D) Single primary sphere colonies from E14.5 wild-type, PS1+/−, and PS1−/− brains were induced to differentiate and immunolabeled for neurons (MAP2), astrocytes (GFAP), and oligodendrocytes (O4, arrowheads). Bar, 25 μm. (D) Greater percentages of neurons expressing MAP2 and astrocytes expressing GFAP were observed differentiating from PS1−/− spheres compared with wild-type control spheres (P < 0.05). (E) The relative numbers of neural stem cells in PS1 mutant and wild-type brains are plotted across development. The PS1 mutant mice have normal numbers of neural stem cells early in development, but then they are lost over developmental time in a gene dosage-sensitive manner. The graphed and tabled results are shown as means ± S.E.M.
Single primary neurosphere colonies were capable of producing new secondary neurosphere colonies after 7 d in vitro. The number of descendant secondary neurospheres generated from the subcloning of a single primary neurosphere can be considered as an estimate of the extent to which the initial primary neurosphere-forming stem cell underwent symmetric (expansionary) divisions (Reynolds and Weiss 1996). The numbers of secondary neurosphere colonies from the E14.5 PS1+/− and PS1−/− brains were decreased by 77% and 95%, respectively, compared with their wild-type littermate controls (P < 0.05, Fig. 2B), suggesting that E14.5 neural stem cells with mutated PS1 alleles show less self-renewal capacity.
To assess neural stem-cell multipotentiality, primary neurospheres were cultured on a basement matrix in the presence of 1% serum for 7 d. The cells, which spread out from the spheres, were immunostained for MAP2 (neurons), GFAP (astrocytes), and O4 (oligodendrocytes) (Fig. 2C). The single neurosphere cells from PS1+/+ and PS1−/− mice differentiated into neurons, astrocytes, or oligodendrocytes in vitro, showing that the neural stem cells were multipotent. However, the neurospheres derived from E14.5 PS1−/− neural stem cells gave rise to more neuronal progeny, as well as more astroglial progeny, than those from wild-type neural stem cells (Fig. 2D).
These observations suggest that neural stem cells are depleted in the E14.5 PS1−/− and PS1−/−;PS2+/− brains and that neural stem cells in the E14.5 PS1−/− brains have an impaired capacity to self-renew, one of the cardinal properties of neural stem cells. The remaining E14.5 PS1−/− neural stem cells maintain their multilineage potential, and actually give rise to more neuronal and astroglial progeny when differentiated in vitro.
We next determined the size of neural stem-cell population in PS1 mutant brains across development and ask whether the PS1 gene has a gene-dosage effect (Fig. 2E). The PS1−/− neural stem-cell population, which was generated and found to have a normal size at E9.5, was reduced 85% over the next 24 h (P < 0.05) and by 93% at E14.5 as compared with wild-type controls. The PS1+/− neural stem-cell population, which showed a statistically nonsignificant decrease during embryogenesis, was reduced by 27% at 12 wk and by 40% at 24 wk (both P < 0.05), relative to their appropriate wild-type controls. Thus, a mutation of the PS1 gene has a clear gene dosage effect on the maintenance of the neural stem-cell population.
Introduction of active Notch1 rescues the self-renewal capacity of E14.5 PS1−/− neural stem cells
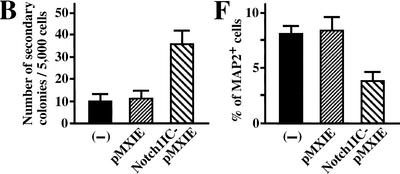
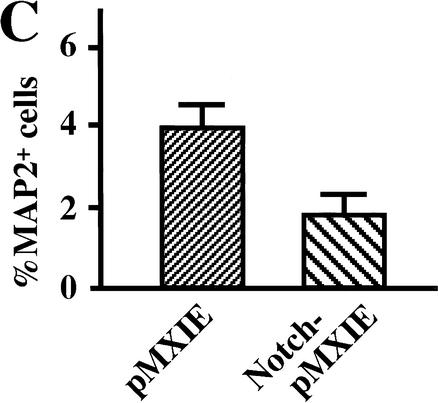
Presenilins cleave a number of membrane-bound proteins and may participate in signaling pathways other than Notch signaling (Niwa et al. 1999; Soriano et al. 2001). To test whether diminished Notch signaling is responsible for the depleted neural stem cells in E14.5 PS1−/− brains, we introduced an active form of Notch1 (Myc-Notch1IC, Fig. 3A) into PS1−/− precursor cells via a retroviral construct carrying a green fluorescent protein (GFP) marker gene in vitro, and cultured the cells in the neurosphere assay. The resultant primary neurospheres showed various patterns of GFP expression, because infection and retroviral gene integration may occur at several different times during sphere formation. We picked up individual primary neurospheres containing GFP-positive cells under a fluorescent microscope, dissociated them, and then plated them to generate secondary neurospheres. More secondary clonal neurospheres were grown from Notch1IC-pMXIE-infected PS1−/− progeny neurospheres than from pMXIE-infected or nonretrovirus-infected PS1−/− neurospheres (Fig. 3B). Although the secondary neurospheres (either with Notch1IC-pMXIE or with control retrovirus) consisted of GFP+ and GFP− spheres, most of the secondary neurosphere colonies of Notch1IC-pMXIE-infected primary neurospheres were GFP+ (Fig. 3C), and were verified to express Notch1IC protein by anti-Myc immunostaining (Fig. 3D). The expression levels of GFP and Myc in cells of clonal GFP+ secondary neurospheres still varied, possibly related to partial suppression of the transgene expression. Using GFP+ secondary neurospheres, we next asked whether introduction of Notch1IC could rescue the effect of the PS1 mutation on neuronal differentiation (Fig. 3E,F). The percentages of differentiated MAP2+ cells were quantified, and Notch1IC expression was found to decrease the percentage of MAP2+ neurons in PS1−/− secondary neurospheres (Fig. 3F). These results suggest that diminished Notch signaling is responsible, at least in part, for the decrease of E14.5 PS1−/− neural stem cells and for the increase in their neuronal differentiation.
Figure 3.
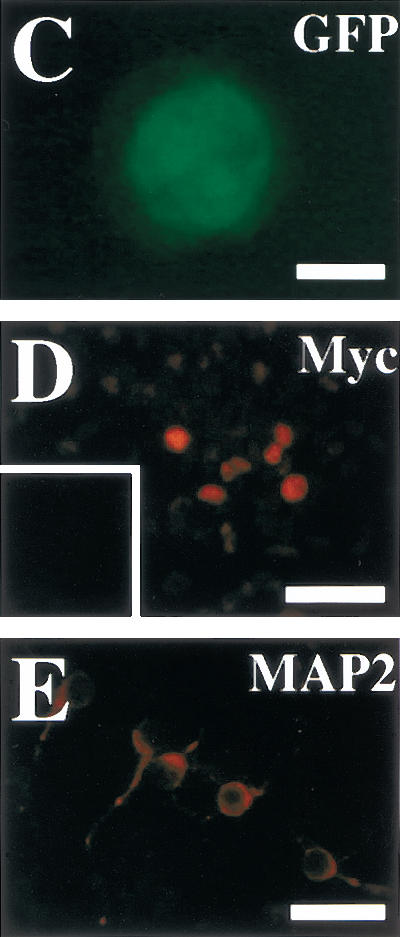
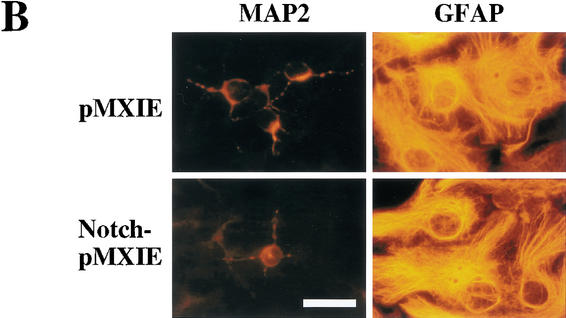
Introduction of the constitutively active form of the Notch1 gene partially rescues the phenotype of PS1−/− neural stem cells. (A) The structures of the retroviral vectors are shown. An amino-terminal Myc epitope-tagged Notch1IC encoding residues 1753–2531, which contains a putative nuclear localization signal sequence, was inserted to create Notch1IC-pMXIE. (B) The numbers of secondary neurosphere colonies derived from PS1−/− primary sphere colonies with or without retroviral infection were counted (n = 4 or more for each group). An increase in the numbers of secondary neurosphere colonies was observed in Notch1IC-pMXIE-infected PS1−/− sphere colony group (P < 0.05 vs.pMXIE-infected group); (−) the noninfected group. (C) Most of the secondary sphere colonies infected with the Notch1IC-pMXIE were GFP positive. (D) The GFP+ spheres were plated and immunostained with anti-Myc antibody (Santa Cruz). Nuclear Myc staining was detected, verifying expression of the Notch1IC protein. An inset shows the lack of anti-Myc immunostaining of a sphere infected with pMXIE. (E,F) Single secondary spheres derived from PS1−/− primary spheres and single GFP+ secondary spheres derived from PS1−/− primary spheres infected with retrovirus were plated separately under differentiating conditions and immunolabeled for MAP2. (F) The introduction of Notch1IC decreased the percentage of MAP2+ neurons in PS1−/− sphere colonies (n = 3 colonies per group, P < 0.05 Notch1IC-pMXIE vs. pMXIE-infected groups). Bar in C, 100 μm;, in D, 40 μm;, in E, 25 μm. The graphed results are shown as means ± S.E.M.
Active Notch1 maintains neural stem cells in vivo
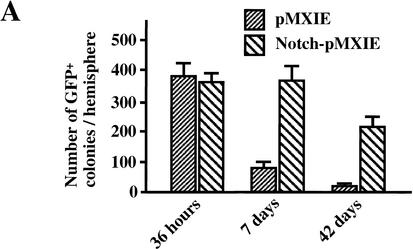
The results of the in vitro retroviral rescue experiment suggest that the active form of Notch1 enhances the symmetric division in vitro of neural stem cells from the E14.5 PS1−/− brain, which have self-renewal defects. We asked whether similar gain-of-function effects of Notch1IC would be seen in wild-type neural stem cells in vivo. Retroviral particles (1 × 104 cfu in 0.5 μL) were injected into the right lateral ventricle of P1 mice, and the mice sacrificed 36 h, 7 d, or 42 d later. Cells surrounding the forebrain lateral ventricle were cultured in the neurosphere assay for 7 d. Dissections performed 36 h after injection yielded comparable numbers of in vitro GFP+ neurosphere colonies from Notch1IC-pMXIE-injected and control pMXIE-injected hemispheres (Fig. 4A), which suggests that the two types of retrovirus were of similar titer and had a similar efficiency in infecting proliferating neural precursor cells in the brain.
Figure 4.
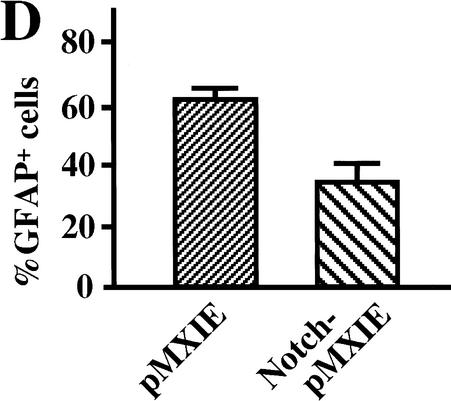
The active form of Notch1 maintains neural stem cells in vivo. Retroviral particles (1 × 104 cfu in 0.5 μL) generated from pMXIE or Notch1IC-pMXIE plasmids were injected into the right lateral ventricle of P1 mice. (A) The numbers of GFP+ neurosphere colonies from the hemispheres of injected sides were determined 36 h, 7 d, or 42 d after the injection. Significantly more GFP+ sphere colonies were found in the hemispheres injected with the Notch1IC-pMXIE retrovirus than with the pMXIE retrovirus after 7-d survival (pMXIE; n = 8 vs. Notch1IC-pMXIE; n = 9, P < 0.05) as well as after 42-d survival (pMXIE; n = 8 vs. Notch1IC-pMXIE; n = 8, P < 0.05). (B) Single GFP+ sphere colonies derived from brains 7 d after infection with retrovirus at P1, were induced to differentiate and immunolabeled for MAP2 and GFAP. Bar, 25 μm. (C,D) The percentages of the cells expressing MAP2 (C) and GFAP (D) were quantified to reveal that smaller percentages of neurons expressing MAP2 and astrocytes expressing GFAP were observed differentiating from the brain injected with Notch1IC-pMXIE (n = 8) compared with those with pMXIE (n = 8) (P < 0.05). The graphed results are shown as means ± S.E.M.
At P8 (7 d after injection), six times more GFP+ neurosphere colonies were produced from dissections of Notch1IC-pMXIE-injected mice than from dissections of the control pMXIE-injected mice (Fig. 4A). Given that most of the proliferating cells at P1 in vivo are the progeny of neural stem cells and not the stem cells themselves, these results suggest that the expression of Notch1IC may prevent progenitor cells from differentiating further or may revert them from progenitor cells to neural stem cells. The GFP+ neurosphere colonies from both groups were passaged to produce GFP+ secondary neurospheres (showing self-renewal, data not shown) or were plated and differentiated into neurons and astroglia (showing multipotentiality, Fig. 4B). The expression of Notch1IC suppressed the differentiation of sphere colony progenitor cells both into neurons (Fig. 4C) and into astroglia (Fig. 4D).
Over the 5-wk survival between P8 and P43, GFP+ neurosphere colonies from the control pMXIE-injected mouse brains were reduced from 82 spheres/hemisphere to 15 (82% reduction). This reduction was proportional to the loss in total number of neurospheres (GFP+ and non-GFP+) in the control pMXIE-injected mouse brains (82%; 6533 spheres/hemisphere at P8 to 1149 at P43). This loss could be due to death of the stem cells or to the fact that many of the early postnatal neurospheres were actually progenitor cell colonies that showed some self-renewal ability in vitro, but had no long-term self-renewal ability in vivo. On the contrary, the relative reduction in the number of GFP+ neurospheres from the Notch1IC-pMXIE-injected mouse brains was much smaller (371 spheres/hemisphere to 215, 42% reduction), and this reduction was lower than that of total neurospheres (82%; 7416 spheres/hemisphere at P8 to 1327 at P43). These results are consistent with the notion that the expression of Notch1IC either converts some neural progenitor cells into neural stem cells or enhances the survival of neural progenitor cells in the early postnatal brains (ideas that are difficult to discriminate between). The expression of Notch1IC in authentic, definitive neural stem cells also may modify cell kinetics, mechanisms of which include increasing the cell cycle times and/or promoting the symmetric division.
Multipotent neural stem cells can be generated without RBP-Jκ, but cannot be maintained in vivo or in vitro
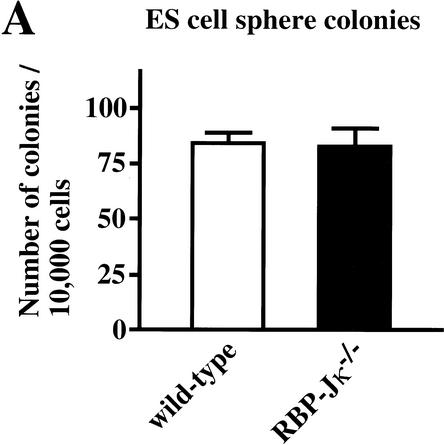
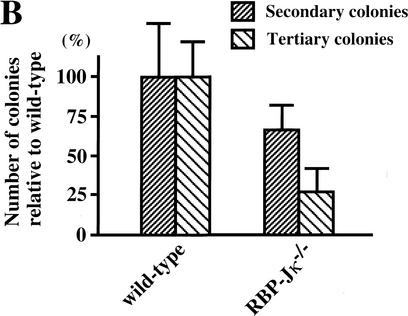
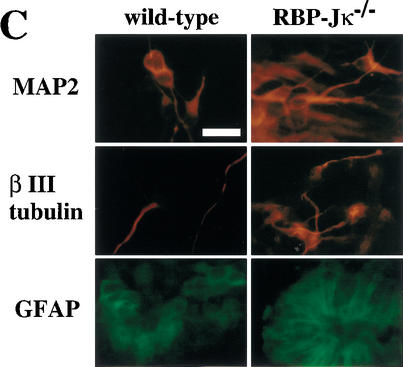
To investigate whether neural stem cells can be generated initially in the absence of all Notch signaling, a PS1−/−;PS2−/− genotype, or alternatively a RBP-Jκ−/− genotype, is required. However, the fact that we currently are unable to detect multipotent neural stem cells in embryonic brains earlier than E8.5 (Tropepe et al. 1999), when a neural plate already exists, makes it difficult to analyze the generation of neural stem cells. Recently, we reported a clonal colony-forming assay, in which ES cells can be induced in serum-free medium to make sphere colonies (referred to as primitive neural stem cell-derived spheres), which contain multipotent neural lineage cells with self-renewal ability (Tropepe et al. 2001). Therefore, the initial generation of neural stem cells in vitro was assayed by use of RBP-Jκ−/− ES cells. There were no differences between wild-type and RBP-Jκ−/− ES cells (Fig. 5A) in the generation of primitive neural stem cell-derived sphere colonies. To assess self-renewal capability, single ES cell-derived sphere colonies were serially dissociated to generate secondary and then tertiary sphere colonies. The RBP-Jκ−/− ES cell-derived sphere colonies formed fewer secondary and tertiary sphere colonies than wild type (Fig. 5B), suggesting that RBP-Jκ−/− primitive neural stem cells have a decreased self-renewal capacity. To verify multipotentiality, primary ES-derived spheres with wild-type or RBP-Jκ−/− genotypes were plated on the basement matrix and induced to differentiate in the presence of 1% serum for 7 d. The cells were immunostained to assess the differentiation into immature neurons (βIII tubulin), neurons (MAP2), astrocytes (GFAP), and undifferentiated neural lineage cells (Nestin). Whereas most of the cells derived from ES cell-derived spheres remained Nestin+-undifferentiated neural lineage cells, some of the remaining cells differentiated into neurons and astroglia (Fig. 5C). Robust differentiation into βIII tubulin+ or MAP2+ neuronal cells was observed from RBP-Jκ−/− sphere colonies (Fig. 5C). Although clustering and neurite bundle formation by the MAP2+ cells made it difficult to quantify the percentage of neuronal differentiation, more neuronal cells appeared to differentiate from RBP-Jκ−/− than wild-type primitive neural stem-cell sphere colonies.
Figure 5.
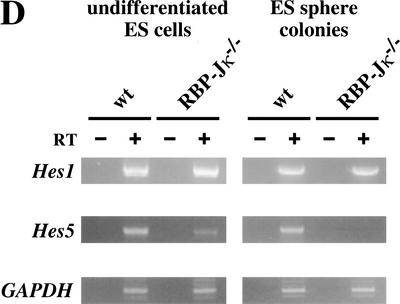
Generation of sphere colonies from RBP-Jκ−/− ES cells. (A) Multipotent neural lineage cell colonies are clonally derived from single ES cells. Comparable numbers of ES cell-derived sphere colonies were formed from wild-type and RBP-Jκ−/− ES cells. Data represent six cultures per group from four separate experiments. (B) RBP-Jκ−/− ES cell-derived sphere colonies show less self-renewal. The numbers of secondary and tertiary sphere colonies revealed a reduced self-renewal capacity of RBP-Jκ−/− ES cell-derived primitive neural stem cells compared with wild-type controls (P < 0.05 tertiary RBP-Jκ−/− vs. wild-type sphere colonies, n = 6 for both groups). (C) ES cell-derived sphere colonies show multipotentiality. Single ES cell-derived sphere colonies were induced to differentiate and immunolabeled for neurons (MAP2+), immature neurons (βIII tubulin+), and astroglia (GFAP+). More neuronal differentiation was observed in RBP-Jκ−/− ES cell-derived sphere colonies compared with wild type. Bar, 25 μm. (D) RT–PCR analysis of Hes1 and Hes5 expression. Comparable levels of Hes1 expression were observed in wild-type and RBP-Jκ−/− ES cells as well as between wild-type and RBP-Jκ−/− ES cell-derived sphere colonies. Weak expression of Hes5 was detected in undifferentiated RBP-Jκ−/− ES cells. However, none of the wild-type Hes5 expression seen in wild-type ES cell-derived sphere colonies was present in RBP-Jκ−/− sphere colonies. GAPDH was used for standardization of the samples. RT (+ and −) indicate that the RNA samples were treated with or without reverse transcriptase. The graphed results are shown as means ± S.E.M.
Notch activation is known to enhance Hes1 and Hes5 expression, and the expression of these genes was assessed by RT–PCR. Comparable expression of Hes1 was seen in wild-type and RBP-Jκ−/−-undifferentiated ES cells, as well as between wild-type and RBP-Jκ−/− ES cell-derived sphere colonies (Fig. 5D). Similar levels of Hes1 expression were observed in E14.5 PS1−/− and wild-type ganglionic eminence neurospheres (data not shown). These findings suggest that signaling pathways other than the Notch pathway may activate Hes1 expression. On the other hand, no Hes5 expression was present in RBP-Jκ−/− ES cell-derived sphere colonies (Fig. 5D). The weaker expression of Hes5 detected in undifferentiated RBP-Jκ−/− ES cells than in wild-type ES cells (in which Notch signaling drives Hes5 expression in addition to the baseline nonspecific expression) might be an example of the low level nonspecific expression of a variety of genes in undifferentiated ES cells (Elefanty et al. 1997).
These observations suggest that multipotent neural lineage cells (primitive neural stem cells) can be generated in vitro in the absence of RBP-Jκ. The RBP-Jκ−/− ES cell-derived primitive neural stem cells showed less self-renewal and more neuronal differentiation; data that are consistent with the results from PS1−/− definitive neural stem cells.
Decrease in the division times of neural stem cells in adult PS1+/− mice
Neural stem cells expand their population during mid-to-late embryogenesis by dividing symmetrically; they also increase the length of their cell cycle times as development proceeds in vivo (Martens et al. 2000). For example, the average cell cycle time of the FGF2-responsive stem cells of the E11.5 embryo was estimated to be 17.6 h and that of E17.5 embryo to be 48.4 h. After birth, forebrain neural stem cells further slow their cell cycle times to become the relatively quiescent adult neural stem cells, which divide asymmetrically once every 15 d to self-renew and give rise to mostly short-lived, constitutively proliferating progenitor cells (Morshead et al. 1998). The effects of a null mutation of one of the PS1 alleles on the in vivo cell cycle times of the adult neural stem cells and their progeny were investigated. We used two versions of an in vivo BrdU-labeling paradigm to test whether first, the cell cycle time of adult PS1+/− neural progenitor cells is altered, and second, to estimate the relative cell cycle time of adult PS1+/− neural stem cells compared with those in wild-type mice.
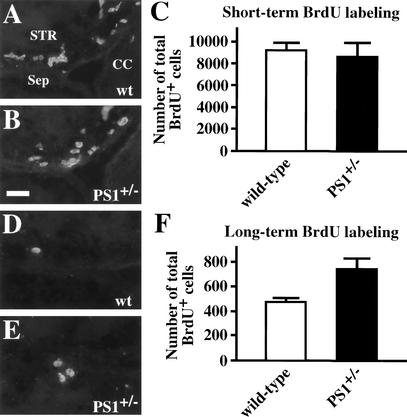
First, a short-term BrdU-labeling procedure (five injections of BrdU every 3 h with sacrifice 1 h after the last injection) was used to label the entire constitutively proliferating progenitor population in the adult forebrain subependyma over its estimated cell cycle (12.7 h) (Morshead et al. 1998). There were comparable numbers of BrdU-labeled progenitor cells in adult PS1+/− subependyma and in their wild-type controls (Fig. 6A–C). Given that the number of neural stem cells in the adult PS1+/− brains was decreased by 40% compared with wild type (Fig. 2E), the results showing no differences in the numbers of the short-term BrdU-labeled cells (the progeny of the stem cells) in adult PS1+/− and wild-type brains can be interpreted in two ways as follows: PS1+/− neural stem cells may divide with a shorter cell cycle time than wild type in an attempt to replenish the constitutively proliferating progenitor population, or alternatively, the PS1+/− constitutively proliferating population may reside longer within the forebrain subependyma. To distinguish between these alternatives, we used a second, long-term BrdU-labeling paradigm (five injections every 3 h as above, but with sacrifice 30 d after the last injection). In this paradigm, the constitutively proliferating cells divide during the 30 d after the BrdU injections, thereby diluting the BrdU label below the threshold of immunofluorescent detection, or leave the subependyma to become differentiated post-mitotic neurons and glia, or die (Morshead et al. 1998). Thus, the BrdU-labeled cells remaining within the subependyma 30 d after the BrdU injections should be neural stem cells (Morshead et al. 1998). It is important to note that these 30-day-survival BrdU+ cells represent only a small subpopulation (usually <5%) of all of the neural stem cells, and that the number of BrdU+ cells depends on the cell cycle time of neural stem cells. Surprisingly, there were 57% more 30-day-survival BrdU+ cells in the adult PS1+/− brains than in their wild-type controls (Fig. 6D–F).
Figure 6.
Short-term and long-term BrdU labeling of the adult PS1+/− brain. Adult mice were labeled with five injections of BrdU (65 mg/kg for each injection) every 3 h and sacrificed 1 h (A–C) or 30 d (D–F) after the last injection. Cryosections cut at 14 μm thick were immunostained for BrdU, and the total numbers of BrdU-positive cells from the rostral tip of the crossing of the corpus callosum to the rostral tip of the crossing of the anterior commissure were counted. (C) In the short-term BrdU-labeling paradigm, most of the BrdU-positive cells were considered to be constitutively proliferating progenitor cells. Comparable numbers of short-term labeled BrdU-positive cells were found in adult PS1+/− (n = 6) and wild-type (n = 5) brains. (F) The BrdU-positive cells, which had been labeled and stayed at the subependymal zone in adult brains for 30 d post-BrdU, were considered to be neural stem cells. There was a 57% increase of BrdU-positive cells in adult PS1+/− (n = 5) brains as compared with wild-type (n = 4) controls (P < 0.05). (STR) Striatum; (Sep) septum; (CC) corpus callosum. Bar, 20 μm. The graphed results are shown as means ± S.E.M.
The only way to interpret the decrease of neurosphere-forming cells in vitro (an assay for total population of neural stem cells) and the increase of 30-day-survival BrdU+ cells in the adult PS1+/− brains in vivo (an assay for a dividing subpopulation of neural stem cells) is to conclude that the smaller numbers of PS1+/− stem cells divide more quickly. The total number of long-term survival BrdU+ cells in vivo will be proportional to the labeling period (constant between two groups) and the number of neural stem cells (60% in adult PS1+/− brains relative to wild type), and inversely proportional to the cell cycle time of the neural stem cells. Accordingly, the estimated relative cell cycle time of adult PS1+/− neural stem cells in vivo is shorter (∼38% of that of wild-type mice). One may still argue that given the tendency of PS1+/− neural stem cells to differentiate prematurely, some of the 30-day-survival BrdU-labeled cells in the adult PS1+/− brains could be postmitotic neurons or glia rather than neural stem cells. This possibility seems unlikely, however, because no accumulation of the cells was observed in the subependyma of the adult PS1+/− brains (data not shown).
Thus, a heterozygous null mutation of PS1 depletes the numbers of adult neural stem cells by producing a deficit in their self-renewal ability, but the remaining adult forebrain neural stem cells proliferate more quickly in an attempt to compensate for the deficit.
Discussion
Although multipotent neural lineage cells (primitive neural stem cells) can be generated in vitro in the complete absence of RBP-Jκ, a mediator of Notch receptor signaling, activation of the Notch-signaling system is indispensable for the in vivo maintenance of the definitive neural stem-cell population in the tissue surrounding the forebrain lateral ventricle over the entire life span. Attenuated Notch signaling by means of a homozygous mutation in the Notch1, RBP-Jκ, or PS1 genes disrupts embryonic neural stem-cell self-renewal, and because there are less symmetrical and self-renewing divisions by the Notch-signaling deficient neural stem cell, there is more neuronal and more astroglial differentiation of the neural progenitor cells that are the asymmetric progeny of the neural stem cells. A heterozygous mutation in the PS1 gene reduces the number of adult neural stem cells, and drives those remaining to proliferate more rapidly in vivo.
Generation of multipotent neural lineage cells in the absence of RBP-Jκ
An important conclusion of the present study is that primitive neural stem cells can be generated from single RBP-Jκ−/− ES cells, and form clonally derived floating sphere colonies in vitro. Although accumulating evidence suggests the existence of RBP-Jκ-independent Notch-signaling pathway (Shawber et al. 1996; Matsuno et al. 1997), RBP-Jκ is a major mediator of Notch signaling. Thus, initial neural induction appears to be independent of Notch signaling in mammals. We have suggested that neuralization from pluripotent ES cells happens through a default mechanism in mammals (Tropepe et al. 2001). The RBP-Jκ−/− ES-derived sphere colonies contain stem cells that are capable of proliferating and making new neurospheres at least twice (self-renewal) and contain progenitor cells that are capable of differentiating into neurons and glia (multipotentiality). Thus, the RBP-Jκ−/− ES-derived sphere colony cells show the cardinal features of neural stem cells that are displayed by neural stem cells isolated from the brain in vivo. However, it is worth noting that there are important differences between ES cell-derived sphere colonies and neurospheres derived from the embryonic and adult forebrain (Tropepe et al. 2001). ES cell-derived sphere colony cells retain a greater degree of pluripotency as assayed by their ability to colonize various neural and non-neural embryonic tissues in chimeric embryos in vivo (Clarke et al. 2000; Tropepe et al. 2001). We suggest that the primitive neural stem cell derived from ES cells may be a transient cell in vivo (as ES cells themselves are), or perhaps even more of a potential cell in vivo, in that the primitive neural stem cell is a default state that is inhibited in vivo by factors that may keep neural stem-cell generation in check until definitive neural stem cells are created as the neural plate forms later in embryogenesis.
Notch signaling is essential for the maintenance of definitive neural stem cells in vivo and in vitro
We suggest that the Notch receptor-signaling pathway participates in the maintenance of the neural stem-cell population for several reasons. First, neural stem cells are missing almost completely in the E10.5 Notch−/− and E8.5 RBP-Jκ−/− brains, whereas they exist in the appropriate control brains. Second, the relative number of neural stem cells in the PS1−/− brains relative to their wild-type littermate controls decreases across embryogenesis. The size of neural stem cell pools in PS1−/− brains, which is comparable to wild-type at E9.5 (92% of wild type), decreases to 15% of wild type only 24 h later, and then to 7% of wild type at E14.5. It should be noted that the neural stem-cell population of wild-type brains rapidly expands by symmetric divisions during this period (Martens et al. 2000). Thus, the neural stem-cell population in the PS1−/− brain still may increase in absolute size through embryogenesis, but at a much slower pace compared with the wild-type brain. This suggests that PS1−/− neural stem cells may divide more asymmetrically, in contrast to the predominant symmetric division of wild-type neural stem cells during embryogenesis. Third, the dissociation of single primary PS1−/− neurospheres from E14.5 brains produced fewer secondary neurospheres as compared with wild-type spheres, further suggesting that fewer numbers of neural stem cells were generated by symmetric division in vitro in primary PS1−/− spheres. This decline of the self-renewal ability of PS1−/− neural stem cells was partially rescued by transducing a constitutively active form of the Notch1 gene, suggesting that the diminished Notch signaling is responsible for the attenuated self-renewal of PS1−/− neural stem cells.
Finally, the size of the neural stem-cell population in adult PS1+/− brains decreased with age relative to their littermate wild-type controls. Because the number of sphere colony-forming cells in adult forebrains is stably maintained over the life span (Tropepe et al. 1997), a decrease in the absolute number of adult neural stem cells in adult PS1+/− brains could be explained as follows: in wild-type brains, individual adult neural stem cells divide asymmetrically to produce one daughter neural stem cell with a long cell cycle time (>15 d) and one neural progenitor that divides once every 12 h for 15 d, producing progeny that die or differentiate into neurons that migrate to the olfactory bulb (Lois and Alvarez-Buylla 1994; Morshead et al. 1998). Some PS1+/− adult neural stem cells may be lost through postnatal life, through death, or by division into two progenitor cells, and this could account for the decreased self-renewal ability of PS1+/− adult neural stem cells. In addition, if wild-type adult neural stem cells are also occasionally lost by death or by division into two progenitor cells, then another wild-type neural stem cell may replenish the lost stem cell by dividing symmetrically to maintain the size of the adult neural stem-cell population. Although there is no evidence for such wild-type adult neural stem-cell replenishment in vivo under baseline conditions, PS1+/− neural stem cells would have less capacity to replenish the lost neural stem cells by symmetric division than wild-type neural stem cells.
Our findings are consistent with a previous study showing that the numbers of neural stem cells in E10.5 Hes1−/− brains (and their self-renewal ability) were decreased in comparison with controls (Nakamura et al. 2000). Given that Hes1 is one of the target genes of Notch signaling and its expression is enhanced by the activation of the Notch pathway, the decrease of neural stem cells in embryonic PS1−/− brains might be ascribed to the suppression of Hes1 expression. This is unlikely, however, because comparable levels of Hes1 expression were observed in PS1−/− and control brains by in situ hybridization and Northern blotting (Handler et al. 2000). Comparable Hes1 levels were seen as well in PS1−/− and wild-type neurospheres (data not shown), and even in RBP-Jκ−/− and wild-type ES cell sphere colonies by RT–PCR (this study). It may be the activation of Hes1 that is important in Notch signaling, rather than its baseline expression levels. On the other hand, a decreased expression of Hes5 was observed in PS1−/− brains (Handler et al. 2000) and in RBP-Jκ−/− ES cell sphere colonies (this study). Given that a deficit of either Hes1 (Nakamura et al. 2000) or Hes5 (this study) expression could result in a reduced self-renewal of neural stem cells, cooperative expression of both of the Hes1 and Hes5 genes might be essential for Notch effects on neural stem cells.
PS1 mutation may affect the cell cycle time of adult neural stem cells in vivo
The present data provide the first suggestion that the cell cycle times of neural stem cells may be altered in mice with PS1 mutations. By using long-term BrdU-labeling as an assay for adult forebrain neural stem cells in vivo, a 57% increase of a BrdU-labeled, dividing subpopulation of neural stem cells in PS1+/− brains as compared with wild-type controls was seen. Given the 40% decrease in the number of entire PS1+/− neural stem cells compared with wild type as assayed in vitro at the same age in the same mutant mice, the only way to interpret all of these findings is that PS1+/− neural stem cells may divide more quickly in the adult in vivo, with an estimated cell cycle time that is 38% of the cell cycle time of wild-type neural stem cells. Neural stem cells extend their cell cycle times during embryogenesis (Martens et al. 2000) and lengthen them even further in adulthood, when the cell cycle time of neural stem cells is estimated to be >15 d (Morshead et al. 1998). Our findings can be interpreted in at least two ways. First, attenuated Notch (or other cascade) signaling in PS1+/− neural stem cells may directly release the adult neural stem cells from relative quiescence. A deficiency of PS1 results in the accumulation of cytosolic β-catenin, which, in turn, increases cyclin D1 transcription and accelerates entry into the S phase of the cell cycle (Soriano et al. 2001). Consistent with this idea, a gain-of-function study showed that Notch activation induces quiescence in precursor cells from the E14.5 mouse telencephalon (Chambers et al. 2001), although Notch activation may, on the contrary, induce cyclin D1 and promote proliferation in at least one cell line (Ronchini and Capobianco 2001). A second possible explanation suggests that the decrease in neural stem cells and, subsequently, in their progeny (the constitutively proliferating subependymal progenitors) indirectly may induce the proliferation of neural stem cells to replenish the progenitor population through unknown feedback mechanisms. Killing the adult subependymal constitutively proliferating cells (the immediate progeny of neural stem cells) with high doses of tritiated thymidine in vivo, induces most of the neural stem cells to enter S phase within a few days (Morshead et al. 1998). In either case, shortened cell cycle times, and, hence, more cell divisions, may enhance the chance that a PS1−/− neural stem cell will give rise to two progenitor cells instead of one progenitor and one neural stem cell, and subsequently being lost as a neural stem cell.
Notch signaling may affect the maintenance of the neural stem state rather than providing an instructive differentiation signal
Historically, Notch signaling in Drosophila was thought to maintain cells in an undifferentiated state (Artavanis-Tsakonas et al. 1995; Kimble and Simpson 1997). More recently, gain-of-function evidence in mammals has suggested that Notch signaling directly and instructively induces glial differentiation (Furukawa et al. 2000; Gaiano et al. 2000; Morrison et al. 2000). Some Notch-signaling loss-of-function studies in mammals seem consistent with this neuronal/glial fate switch idea, in that there is a premature appearance and increased number of postmitotic neurons expressing MAP2 or βIII tubulin between E10.5 and E13.5 in the PS1−/− brain (Shen et al. 1997; Handler et al. 2000). Similarly, mice with mutations in other Notch-signaling molecules such as Notch1, RBP-Jκ, or Hes1/5 have revealed premature neuronal differentiation (Ishibashi et al. 1995; de la Pompa et al. 1997; Ohtsuka et al. 1999). However, it is worth noting that such mice with null mutations in Notch-signaling genes die in mid-to-late embryogenesis, when neurogenesis predominates over gliogenesis in vivo. A clonal analysis of E10 cortical cells in vitro showed that neuronal differentiation from single neural stem cells preceded gliogenesis in clonal cell colonies (Qian et al. 2000). Thus, the in vivo analyses of Notch mutants may not allow sufficient time to assess whether gliogenesis is increased or decreased.
The present study of the loss-of-function and gain-of-function in Notch pathway molecules in vitro revealed that the PS1 homozygous mutation drives E14.5 neural stem cells to differentiate both into more neurons and more astroglia, and that the expression of the active form of Notch1 suppressed the differentiation of postnatal neural stem-cell progeny both into neurons and into astroglia. Our findings are therefore inconsistent with the idea that Notch signaling controls a neuronal/glial fate switch of neural stem cells in the central nervous system (Gaiano et al. 2000), although it remains possible that the different times of the introduction of active Notch in neural stem cells (and thus the different in vivo progenitor cell environments) result in the apparently contradictory findings. Our data are more consistent with the idea that Notch signaling keeps cells in an undifferentiated state. PS1−/− neural stem cells have a greater probability of dividing asymmetrically to produce neuronal progenitors early in vivo (and neuronal and glial progenitors in vitro), rather than of dividing symmetrically to produce two daughter neural stem cells as wild-type neural stem cells often do during early embryogenic development. Hence, neuronal progenitor cells in the PS1−/− brain may differentiate prematurely from early asymmetric neural stem-cell divisions. Note that this hypothesis of premature neuronal division as a by product of the failure of symmetric divisions of forebrain neural stem cells with deficits in Notch signaling can be seen as an alternative to the idea that Notch signaling is directly and instructively involved in the fate choice between neuronal and glial differentiation in the mammalian central nervous system (Gaiano et al. 2000). Our findings, therefore, are consistent with the idea of a primary defect in symmetric stem-cell self-renewal within the central nervous system.
Our gain-of-function study in vivo showed that enhanced Notch signaling (by transducing an active form of Notch1 via retroviral infection) increased the number of postnatal neural stem cells in the subependyma of the forebrain lateral ventricle. The cells expressing the active form of Notch1 showed self-renewal and multipotentiality, and, thus, they were neural stem cells. Our data suggest that Notch signaling encourages neural stem cells to divide symmetrically to increase the size of the neural stem-cell population, rather than to divide asymmetrically to produce progenitor cells in the embryonic brain, consistent with other gain-of-function studies showing that constitutively active Notch signaling inhibits the differentiation of neural progenitor cells in mammals (Ishibashi et al. 1994; Lardelli et al. 1996; Bao and Cepko 1997). This hypothesis that Notch signaling enhances the symmetric and self-renewing division of neural stem cells predicts less glial differentiation from neural stem cells in the postnatal nervous system. This prediction appears inconsistent with recent studies suggesting an instructive role for Notch signaling to produce glia from neural progenitor cells in the mammalian central nervous system (Gaiano et al. 2000). However, this inconsistency disappears if adult forebrain neural stem cells take on glial features but do not differentiate into unipotential glial cells. Notch signaling enhances GFAP transcription in adult hippocampus-derived progenitor cells (Tanigaki et al. 2001), and at least some of the GFAP-expressing astrocytes in the adult forebrain subependyma appear to be neural stem cells (Doetsch et al. 1999).
Materials and methods
Animals and genotyping
The generation of Notch1, RBP-Jκ, and PS1/2 mutant mice have been described previously (Conlon et al. 1995; Oka et al. 1995; Wong et al. 1997; Donoviel et al. 1999). Notch1−/− and their heterozygous and wild-type littermates or RBP-Jκ−/− and their heterozygous and wild-type littermates on a CD1 background, or PS1−/−, PS1+/−, and their wild-type littermates on a C57B16/129 F1 hybrid background, were used. Midday of the day the vaginal plug was found was termed embryonic day 0.5 (E0.5). They were genotyped as described (Conlon et al. 1995; Oka et al. 1995; Wong et al. 1997; Donoviel et al. 1999).
Isolation of forebrain neural stem cells
The protocol used to generate neurospheres from embryonic brain in vitro has been described (Tropepe et al. 1999). Briefly, timed-pregnant mice at the specified gestational ages were killed and head primordia of embryos were excised into fresh PBS. From E8.5 to E10.5 embryo, the head primordia were dissected and anterior neural tube tissue was teased gently away from surrounding head mesenchyme and overlying epidermal ectoderm. E14.5 brains were dissected by the removal of the epidermis and calvarium and tissues from the ganglionic eminence were excised. Postnatal day (P) 0–3 mice were anesthetized on ice and decapitated. The same protocol used for E14.5 brains was used to dissect striatal tissue from these mice. The collected tissue was dissociated mechanically in serum-free medium (see below) into a cell suspension.
P8 or adult brains were dissected as described previously (Tropepe et al. 1997). Briefly, mice were killed via cervical dislocation, and their brains were aseptically excised. Medial and lateral portions of lateral ventricle subependyma were dissected from both hemispheres and then cut into 1-mm3 pieces in oxygenized artificial cerebrospinal fluid. The tissue was digested with 1.33 mg/mL trypsin (Sigma), 0.67 mg/mL hyaluronidase (Sigma), and 0.2 mg/mL kynurenic acid (Sigma) to dissociate tissue at 37°C for 1 h. Tissue was transferred to serum-free medium containing 0.7 mg/mL trypsin inhibitor (Roche) and triturated with a fire-polished Pasteur pipette.
Cell culture
Cells were cultured in a neural stem cell colony-forming (neurosphere) assay (Reynolds et al. 1992). Cells from embryonic forebrain were plated at 10 cells/μL in 24-well (0.5 mL/well) uncoated plates (Nunclon) in serum-free medium (Tropepe et al. 1999) containing 10 ng/mL FGF2 (human recombinant; Sigma) and 2 μg/mL heparin (Upstate Biotech). Cells from postnatal brains were plated as above in serum-free medium containing FGF2, heparin, and 20 ng/mL EGF (mouse submaxillary; Upstate Biotech).
To assess self-renewal, primary floating neurosphere colonies were subcloned by mechanically dissociating a single neurosphere colony in 0.2 mL of serum-free medium containing FGF2, heparin, and EGF, and plated in uncoated 96-well (0.2 mL/well) plates (Nunclon). Stem-cell self-renewal was assessed by identifying new neurospheres after a further 7 d in vitro.
Immunocytochemistry
Seven days after primary culture, single neurosphere colonies were transferred to individual wells of a 24-well culture plate (Nunclon) coated previously with Matrigel basement membrane matrix (0.6 mg/mL in serum-free medium; Becton-Dickinson) in 0.5 mL/well of the medium containing 1% FBS (GIBCO). Wells were processed 7 d later by use of immunocytochemistry as described previously (Tropepe et al. 1999). We used anti-MAP-2 mouse monoclonal (IgG) (1:1000; Roche), anti-GFAP rabbit polyclonal (IgG) (1:400; Chemicon), anti-O4 mouse monoclonal (IgM) (1:40; Roche), anti-nestin rabbit polyclonal (IgG) (1:400; a kind gift of Dr. R. McKay, NIH, Bethesda, MD), or anti-βIII tubulin mouse monoclonal (IgG) (1:400; Sigma) antibodies as primary, then followed by appropriate FITC- or TRITC-conjugated secondary antibodies. Cultures were counterlabeled with the nuclear stain Hoechst 33258 (1 μg/mL; Sigma).
Retrovirus preparation and infection
A replication-incompetent retroviral vector containing an internal ribosome entry site (IRES) sequence followed by enhanced GFP, pMXIE, was constructed from pMX (DNAX, Ohnishi et al. 1996) and pIRES2-EGFP (Clontech). An amino-terminal Myc epitope-tagged Notch1IC (Nye et al. 1994) encoding residues 1753–2531 was inserted to create Notch1IC-pMXIE. A packaging cell line, ΦNX-Eco (ATCC 3443), was transfected transiently with the retroviral plasmids using FuGene 6 (Roche) and cultured for 3 d at 32°C. The supernatant was filtered and centrifuged to yield a titer >4 × 107 cfu/mL for both pMXIE and Notch1IC-pMXIE viruses. The virus solution was stored at −80°C until used. Ganglionic eminence cells were spin infected at 1000 rpm with 2 × 105 of retrovirus in the presence of 5 μg/mL Polybrene (Sigma) at room temperature for 1.5 h. The retrovirus solution was then removed and the cells were plated in the usual sphere colony-forming condition. For in vivo infection experiments, CD1 P1 pups were anesthetized by ice-water immersion. Retrovirus (1 × 104 cfu in 0.5 μL) together with 0.1 mg/mL of Polybrene and Trypan Blue (Sigma) was manually injected into the right lateral ventricle of the brains using a 32-gauge needle (Hamilton). The needle was kept in the position for more than 1 min after injection to minimize leakage of the virus solution.
Bromodeoxyuridine labeling and detection
Mice were injected with bromodeoxyuridine (BrdU, Sigma) (65 mg/kg, i.p., dissolved in PBS) every 3 h for five injections and killed 1 h or 30 d after the last injection. Animals were overdosed with anesthetic and perfused transcardially with 4% paraformaldehyde/0.4% picric acid. Brains were removed and postfixed with the same fixative overnight, cryoprotected with 20% sucrose in PBS at 4°C, and then cryosectioned at 14-μm thickness. Sections were incubated in 1N HCl at 60°C for 30 min, then rinsed in PBS, and subsequently incubated in rat anti-BrdU antibody (1:100, Seralab) at 4°C overnight, follwed by FITC donkey anti-rat antibody (1:200, Jackson). The total number of BrdU-labeled cells was estimated using the optical dissector method (Coggeshall and Lekan 1996) in every eighth section from the rostral tip of the crossing of the corpus callosum rostrally and extending caudally to the rostral tip of the crossing of the anterior commissure.
Generation of ES cell sphere colonies
The establishment of embryonic stem (ES) cell lines carrying a heterozygously or homozygously disrupted allele of RBP-Jκ gene in D3 cells has been described previously (Oka et al. 1995). The ES cells were grown on mitotically inactive fibroblast feeder layers maintained in 15% fetal calf serum/Dulbecco's modified Eagle's medium containing LIF (1000 U/mL). Harvested ES cells were washed twice, centrifuged, and resuspended in chemically defined serum-free medium, and were subsequently plated at 20 cells/μL in 24-well plates (0.5 mL) containing LIF (1000 U/mL), FGF2, heparin, and B27 supplement (Life Technologies), as described previously for assaying primitive neural stem cell-derived sphere colonies (Tropepe et al. 2001).
RT–PCR
Total RNA was isolated (RNeasy extraction kit, QIAGEN) and 1 μg of total RNA was used to synthesize cDNA with oligo-d(T)12–18 primers and MoMLV reverse transcriptase (Superscript II; Roche) in a 25-μL reaction mixture at 42°C for 1 h. The PCR reaction mixture (20 μL) consisted of 1 μL of cDNA, 16 pmole each of 5′ and 3′ primers, 0.2 mM dNTP, 1.5 mM Mg2+, 2 μL of PCR reaction buffer, and 0.8 units of Taq polymerase (Promega). cDNA was amplified in a thermal cycler (Perkin-Elmer) with denaturation at 95°C for 30 sec, annealing at 56°C for 40 sec and extension at 72°C for 40 sec for 40 cycles. The sense and antisense primers used were as follows: Jag1, sense 5′-GTCCACG GCACCTGCAATG-3′; antisense 5′-CAAGGTTTGGCCTCG CACT-3′; Dll1, sense 5′-ACTCCTTCAGCCTGCCTGA-3′, antisense 5′-TATCGGATGCACTCATCGC-3′; Dll3, sense 5′-CTGGTGTCTTCGAGCTACA-3′, antisense 5′-ACACGTGC TAGCAGGTTCC-3′; PS1, sense 5′-AGCCAAGAACGGCAG CAGCAGCATGACAGGCAGAG-3′, antisense 5′-TACTGA AATCACAGCCAAGATGAGCCATGC-3′; PS2, sense 5′-AGC GTCATCGTAGTCATGACCA-3′, antisense 5′-CACCATG GCAGATGAGTATATC-3′; Notch1, sense 5′-CCAGCATGG CCAGCTCTGG-3′, antisense 5′-CATCCAGATCTGTGGCC CTGTT-3′; RBP-Jκ, sense 5′-TGGCACTGTTCAATCGCCTT-3′, antisense 5′-AATCTTGGGAGTGCCATGCCA-3′; Hes1, sense 5′-AAAGACGGCCTCTGAGCACA-3′, antisense 5′-TCATG GCGTTGATCTGGGTCA-3′; Hes5, sense 5′-AAGTACCGTG GCGGTGGAGATGC-3′, antisense 5′-CGCTGGAAGTGG TAAAGCAGCTT-3′; GAPDH, sense 5′-ACCACAGTCCAT GCCATCAC-3′, antisense 5′-TCCACCACCCTGTTGCTGT A-3′. These pairs of primers for all genes are designed to encompass at least one intron to avoid false positive amplification from contaminated genomic DNA.
Acknowledgments
We thank Dr. Garry P. Nolan for the ΦNX-Eco cell line, Dr. Yasumichi Hitoshi for advise on retroviral experiments, Dr. Janet Rossant for LIF, Dr. Ron McKay for anti-nestin antibody, and Brenda L.K. Coles and Andrew Suri for their technical contributions. This work was supported by the Canadian Institutes of Health Research. S.H. and V.T. are CIHR fellows.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL shitoshi-tky@umin.ac.jp; FAX 81-3-5800-6548.
E-MAIL derek.van.der.kooy@utoronto.ca; FAX (416) 978-3844.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.975202.
References
- Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995;268:225–232. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Bao Z-Z, Cepko C. The expression and function of Notch pathway genes in the developing rat eye. J Neurosci. 1997;17:1425–1434. doi: 10.1523/JNEUROSCI.17-04-01425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows RC, Wancio D, Levitt P, Lillien L. Response diversity and the timing of progenitor cell maturation are regulated by developmental changes in EGFR expression in the cortex. Neuron. 1997;19:251–267. doi: 10.1016/s0896-6273(00)80937-x. [DOI] [PubMed] [Google Scholar]
- Chambers CB, Peng Y, Nguyen H, Gaiano N, Fishell G, Nye JS. Spatiotemporal selectivity of response to Notch1 signals in mammalian forebrain precursors. Development. 2001;128:689–702. doi: 10.1242/dev.128.5.689. [DOI] [PubMed] [Google Scholar]
- Clarke DL, Johansson CB, Wilbertz J, Veress B, Nilsson E, Karlström H, Lendahl U, Frisén J. Generalized potential of adult neural stem cells. Science. 2000;288:1660–1663. doi: 10.1126/science.288.5471.1660. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: A case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Conlon RA, Reaume AG, Rossant J. Notch1 is required for the coordinate segmentation of somites. Development. 1995;121:1533–1545. doi: 10.1242/dev.121.5.1533. [DOI] [PubMed] [Google Scholar]
- de la Pompa JL, Wakeham A, Correia KM, Samper E, Brown S, Aguilera RJ, Nakano T, Honjo T, Mak TW, Rossant J, et al. Conservation of the Notch signalling pathway in mammalian neurogenesis. Development. 1997;124:1139–1148. doi: 10.1242/dev.124.6.1139. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Donoviel DB, Hadjantonakis A-K, Ikeda M, Zheng H, St George Hyslop P, Bernstein A. Mice lacking both presenilin genes exhibit early embryonic patterning defects. Genes & Dev. 1999;13:2801–2810. doi: 10.1101/gad.13.21.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elefanty AG, Robb L, Birner R, Begley CG. Hematopoietic-specific genes are not induced during in vitro differentiation of scl-null embryonic stem cells. Blood. 1997;90:1435–1447. [PubMed] [Google Scholar]
- Furukawa T, Mukherjee S, Bao Z-Z, Morrow EM, Cepko CL. rax, Hes1, and notch1 promote the formation of Müller glia by postnatal retinal progenitor cells. Neuron. 2000;26:383–394. doi: 10.1016/s0896-6273(00)81171-x. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Gaiano N, Nye JS, Fishell G. Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron. 2000;26:395–404. doi: 10.1016/s0896-6273(00)81172-1. [DOI] [PubMed] [Google Scholar]
- Handler M, Yang X, Shen J. Presenilin-1 regulates neuronal differentiation during neurogenesis. Development. 2000;127:2593–2606. doi: 10.1242/dev.127.12.2593. [DOI] [PubMed] [Google Scholar]
- Herreman A, Hartmann D, Annaert W, Saftig P, Craessaerts K, Serneels L, Umans L, Schrijvers V, Checler F, Vanderstichele H, et al. Presenilin 2 deficiency causes a mild pulmonary phenotype and no changes in amyloid precursor protein processing but enhances the embryonic lethal phenotype of presenilin 1 deficiency. Proc Natl Acad Sci. 1999;96:11872–11877. doi: 10.1073/pnas.96.21.11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi M, Moriyoshi K, Sasai Y, Shiota K, Nakanishi S, Kageyama R. Persistent expression of helix-loop-helix factor HES-1 prevents mammalian neural differentiation in the central nervous system. EMBO J. 1994;13:1799–1805. doi: 10.1002/j.1460-2075.1994.tb06448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama R, Nakanishi S. Helix-loop-helix factors in growth and differentiation of the vertebrate nervous system. Curr Opin Genet Dev. 1997;7:659–665. doi: 10.1016/s0959-437x(97)80014-7. [DOI] [PubMed] [Google Scholar]
- Kimble J, Simpson P. The LIN-12/Notch signaling pathway and its regulation. Annu Rev Cell Dev Biol. 1997;13:333–361. doi: 10.1146/annurev.cellbio.13.1.333. [DOI] [PubMed] [Google Scholar]
- Lardelli M, Williams R, Mitsiadis T, Lendahl U. Expression of the Notch 3 intracellular domain in mouse central nervous system progenitor cells is lethal and leads to disturbed neural tube development. Mech Dev. 1996;59:177–190. doi: 10.1016/0925-4773(96)00589-8. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Martens DJ, Tropepe V, van der Kooy D. Separate proliferation kinetics of fibroblast growth factor-responsive and epidermal growth factor-responsive neural stem cells within the embryonic forebrain germinal zone. J Neurosci. 2000;20:1085–1095. doi: 10.1523/JNEUROSCI.20-03-01085.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno K, Go MJ, Sun X, Eastman DS, Artavanis-Tsakonas S. Suppressor of Hairless-independent events in Notch signaling imply novel pathway elements. Development. 1997;124:4265–4273. doi: 10.1242/dev.124.21.4265. [DOI] [PubMed] [Google Scholar]
- Mayer-Proschel M, Kalyani AJ, Mujtaba T, Rao MS. Isolation of lineage-restricted neuronal precursors from multipotent neuroepithelial stem cells. Neuron. 1997;19:773–785. doi: 10.1016/s0896-6273(00)80960-5. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Perez SE, Qiao Z, Verdi JM, Hicks C, Weinmaster G, Anderson DJ. Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell. 2000;101:499–510. doi: 10.1016/s0092-8674(00)80860-0. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Craig CG, van der Kooy D. In vivo clonal analyses reveal the properties of endogenous neural stem cell proliferation in the adult mammalian forebrain. Development. 1998;125:2251–2261. doi: 10.1242/dev.125.12.2251. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Sakakibara S-i, Miyata T, Ogawa M, Shimazaki T, Weiss S, Kageyama R, Okano H. The bHLH gene Hes1 as a repressor of the neuronal commitment of CNS stem cells. J Neurosci. 2000;20:283–293. doi: 10.1523/JNEUROSCI.20-01-00283.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa M, Sidrauski C, Kaufman RI, Walter P. A role of presenilin-1 in nuclear accumulation of Ire1 fragments and induction of the mammalian unfolded protein response. Cell. 1999;99:691–702. doi: 10.1016/s0092-8674(00)81667-0. [DOI] [PubMed] [Google Scholar]
- Nye JS, Kopan R, Axel R. An activated Notch suppresses neurogenesis and myogenesis but not gliogenesis in mammalian cells. Development. 1994;120:2421–2430. doi: 10.1242/dev.120.9.2421. [DOI] [PubMed] [Google Scholar]
- Onishi M, Kinoshita S, Morikawa Y, Shibuya A, Phillips J, Lanier LL, Gorman DM, Nolan GP, Miyajima A, Kitamura T. Applications of retrovirus-mediated expression cloning. Exp Hematol. 1996;24:324–329. [PubMed] [Google Scholar]
- Ohtsuka T, Ishibashi M, Gradwohl G, Nakanishi S, Guillemot F, Kageyama R. Hes1 and Hes5 as Notch effectors in mammalian neuronal differentiation. EMBO J. 1999;18:2196–2207. doi: 10.1093/emboj/18.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka C, Nakano T, Wakeham A, de la Pompa JL, Mori C, Sakai T, Okazaki S, Kawaichi M, Shiota K, Mak TW, et al. Disruption of the mouse RBP-Jκ gene results in early embryonic death. Development. 1995;121:3291–3301. doi: 10.1242/dev.121.10.3291. [DOI] [PubMed] [Google Scholar]
- Qi H, Rand MD, Wu X, Sestan N, Wang W, Rakic P, Xu T, Artavanis-Tsakonas S. Processing of the Notch ligand Delta by the metalloprotease Kuzbanian. Science. 1999;283:91–94. doi: 10.1126/science.283.5398.91. [DOI] [PubMed] [Google Scholar]
- Qian X, Shen Q, Goderie SK, He W, Capela A, Davis AA, Temple S. Timing of CNS cell generation: A programmed sequence of neuron and glia cell production from isolated murine cortical stem cells. Neuron. 2000;28:69–80. doi: 10.1016/s0896-6273(00)00086-6. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Clonal population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronchini C, Capobianco AJ. Induction of cyclin D1 transcription and CDK2 activity by Notchic: Implication for cell cycle disruption in transformation by Notchic. Mol Cell Biol. 2001;21:5925–5934. doi: 10.1128/MCB.21.17.5925-5934.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Notch and presenilins in vertebrates and invertebrates: Implications for neuronal development and degeneration. Curr Opin Neurobiol. 2000;10:50–57. doi: 10.1016/s0959-4388(99)00054-9. [DOI] [PubMed] [Google Scholar]
- Shawber C, Nofziger D, Hsieh JJ-D, Lindsell C, Bögler O, Hayward D, Weinmaster G. Notch signaling inhibits muscle cell differentiation through a CBF1-independent pathway. Development. 1996;122:3765–3773. doi: 10.1242/dev.122.12.3765. [DOI] [PubMed] [Google Scholar]
- Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S. Skeletal and CNS defects in presenilin-1-deficient mice. Cell. 1997;89:629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Soriano S, Kang DE, Fu M, Pestell R, Chevallier N, Zheng H, Koo EH. Presenilin 1 negatively regulates β-catenin/T cell factor/lymphoid enhancer factor-1 signaling independently of β-amyloid precursor protein and Notch processing. J Cell Biol. 2001;152:785–794. doi: 10.1083/jcb.152.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigaki K, Nogaki F, Takahashi J, Tashiro K, Kurooka H, Honjo T. Notch1 and Notch3 instructively restrict bFGF-responsive multipotent neural progenitor cells to an astroglial fate. Neuron. 2001;29:45–55. doi: 10.1016/s0896-6273(01)00179-9. [DOI] [PubMed] [Google Scholar]
- Tropepe V, Craig CG, Morshead CM, van der Kooy D. Tansforming growth factor-α null and senescent mice show decreased neural progenitor cell proliferation in the forebrain subependyma. J Neurosci. 1997;17:7850–7859. doi: 10.1523/JNEUROSCI.17-20-07850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropepe V, Sibilia M, Ciruna BG, Rossant J, Wagner EF, van der Kooy D. Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev Biol. 1999;208:166–188. doi: 10.1006/dbio.1998.9192. [DOI] [PubMed] [Google Scholar]
- Tropepe V, Hitoshi S, Sirard C, Mak TW, Rossant J, van der Kooy D. Direct neural fate specification from embryonic stem cells: A primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron. 2001;30:65–78. doi: 10.1016/s0896-6273(01)00263-x. [DOI] [PubMed] [Google Scholar]
- Weiss S, Reynolds BA, Vescovi AL, Morshead CM, Craig CG, van der Kooy D. Is there a neural stem cell in the mammalian forebrain? Trends Neurosci. 1996;19:387–393. doi: 10.1016/s0166-2236(96)10035-7. [DOI] [PubMed] [Google Scholar]
- Wong PC, Zheng H, Chen H, Becher MW, Sirinathsinghji DJS, Trumbauer ME, Chen HY, Price DL, Van der Ploeg LHT, Sisodia SS. Presenilin 1 is required for Notch1 and Dll1 expression in the paraxial mesoderm. Nature. 1997;387:288–292. doi: 10.1038/387288a0. [DOI] [PubMed] [Google Scholar]