Abstract
Mammalian cells adapt to hypoxic conditions through a transcriptional response pathway mediated by the hypoxia-inducible factor, HIF. HIF transcriptional activity is suppressed under normoxic conditions by hydroxylation of an asparagine residue within its C-terminal transactivation domain, blocking association with coactivators. Here we show that the protein FIH-1, previously shown to interact with HIF, is an asparaginyl hydroxylase. Like known hydroxylase enzymes, FIH-1 is an Fe(II)-dependent enzyme that uses molecular O2 to modify its substrate. Together with the recently discovered prolyl hydroxylases that regulate HIF stability, this class of oxygen-dependent enzymes comprises critical regulatory components of the hypoxic response pathway.
Keywords: Asparaginyl hydroxylase, hypoxia, oxygen sensing, HIF
Almost all mammalian cells possess the ability to recognize changes in the local availability of oxygen. When oxygen levels are low (hypoxia), a conserved hypoxic response pathway is activated. At the center of this pathway lies the ubiquitously expressed transcription factor hypoxia-inducible factor (HIF) (Semenza 1999). HIF is a heterodimer composed of an alpha subunit, HIF-1α or the paralogs HIF-2α or HIF-3α (Tian et al. 1997; Gu et al. 1998; O'Rourke et al. 1999; Srinivas et al. 1999), and the HIF-1β subunit, also known as the aryl hydrocarbon receptor nuclear translocator (ARNT) (Wang et al. 1995). Whereas HIF-1β expression and activity levels remain largely unaffected by changes in oxygen levels, the HIF-α subunit is strongly induced following exposure to hypoxic conditions.
Two primary mechanisms by which HIF-α activity is regulated by oxygen have been identified. Under normoxic conditions, the oxygen-dependent degradation domain (ODD) within the HIF-α subunit is recognized by the product of the von-Hippel Lindau tumor suppressor gene (pVHL) (Maxwell et al. 1999). pVHL is a component of a protein–ubiquitin ligase complex that targets the alpha subunit for degradation by the proteasome (Maxwell et al. 1999; Cockman et al. 2000; Ohh et al. 2000; Tanimoto et al. 2000). pVHL recognition of HIF-α is dependent on hydroxylation of proline residues within the ODD (Ivan et al. 2001; Jaakkola et al. 2001; Yu et al. 2001). Under hypoxic conditions, prolyl hydroxylation is blocked, resulting in increased HIF-α stability and accumulation (Ivan et al. 2001; Jaakkola et al. 2001; Yu et al. 2001). This posttranslational modification is carried out by a family of prolyl hydroxylase enzymes that bear structural and functional similarities to previously characterized hydroxylases (Bruick and McKnight 2001; Epstein et al. 2001). Like these enzymes, the HIF prolyl hydroxylase enzymes use Fe(II) to bind O2 to hydroxylate both 2-oxoglutarate and the target proline residue (Bruick and McKnight 2001; Epstein et al. 2001). Because these enzymes bind oxygen directly, it has been speculated that they may be critical oxygen sensors within the hypoxic response pathway.
In addition to inducing HIF stability, hypoxic conditions promote the ability of the HIF-α C-terminal transactivation domain (CAD) to interact with coactivators such as p300 (Ema et al. 1999; Carrero et al. 2000; Kung et al. 2000; Gu et al. 2001). It was recently shown that HIF-α association with p300 is blocked under normoxic conditions by hydroxylation of a conserved asparagine residue within the CAD (Lando et al. 2002). Asparagine hydroxylation is abrogated by hypoxia, allowing HIF to recruit the larger transcriptional apparatus to hypoxia-responsive target genes (Lando et al. 2002). As with HIF prolyl hydroxylases, endogenous HIF asparaginyl hydroxylase activity can be blocked with competitive inhibitors of 2-oxoglutarate and with iron chelators (Lando et al. 2002), suggesting that this enzyme might also resemble known hydroxylase enzymes. Such asparaginyl hydroxylase enzymes would be expected to bind O2, potentially playing the role of a second oxygen sensor within the hypoxic response pathway. In this study we identify an asparaginyl hydroxylase enzyme capable of modifying the key asparagine residue within the HIF-1α and HIF-2α CADs, thereby suppressing HIF activity.
Results and Discussion
FIH-1 is predicted to fold like 2-oxoglutarate- and Fe(II)-dependent oxygenases
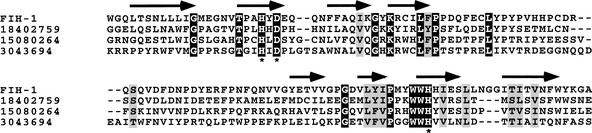
Fe(II)-dependent enzymes that use O2 to oxidize both 2-oxoglutarate and either polypeptide or metabolite substrates are found throughout nature. Structures have been determined for several members of this family, revealing a conserved double-stranded β-helix that composes the enzyme core (Roach 1995; Valegård et al. 1998; Zhang et al. 2000; Clifton et al. 2001). Within this fold lies a critical His-X-Asp/Glu dyad and C-terminal His residue responsible for binding the iron atom (Hegg and Que 1997). Sequence profile searches have been used to identify previously undetected members of this family of enzymes (Aravind and Koonin 2001). Using these guidelines, additional sequence analysis was undertaken to identify candidate hydroxylase enzymes. Of particular interest was the finding that the protein factor inhibiting HIF-1 (FIH-1) is predicted to contain a β-helix core featuring the 2-His-1-carboxylate triad diagnostic of 2-oxoglutarate- and Fe(II)-dependent oxygenases (Fig. 1). FIH-1 was recently shown to inhibit the activity of the CAD through direct interactions with both the CAD of HIF-1α and pVHL (Mahon et al. 2001). These properties strongly suggested that FIH-1 may suppress CAD activity via hydroxylation of the asparagine residue that mediates coactivator recruitment (Lando et al. 2002).
Figure 1.
Predicted secondary structure of FIH-1. A multiple sequence alignment was generated using Jpred (Cuff et al. 1998) for amino acid residues 179–300 of human FIH-1. Related proteins are indicated by their gi accession numbers. Segments predicted to be in an extended conformation (β-strand) are denoted by arrows. Identical residues are shaded in black, and similar residues are shaded in gray. Asterisks indicate critical amino acids for Fe(II)-binding in characterized hydroxylase enzymes.
FIH-1 inhibits HIF-α CAD activity
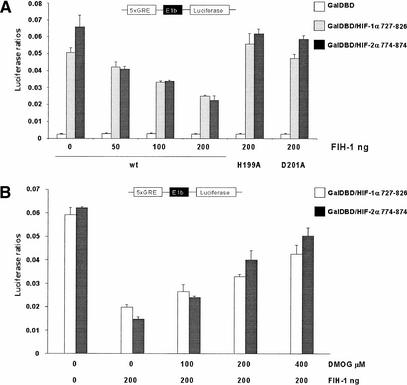
O2-dependent regulation of CAD activity can be uncoupled from O2-dependent regulation of HIF-α stability (Jiang et al. 1997; Ema et al. 1999; O'Rourke et al. 1999; Carrero et al. 2000; Gu et al. 2001) by fusing the CAD to the O2-insensitive Gal4 DNA-binding domain (GalDBD). Transfection of the GalDBD fusion with the terminal 100 amino acids from either HIF-1α or HIF-2α drives expression of a luciferase reporter gene under the control of a Gal4 reporter (Fig. 2A). As observed by Mahon and colleagues (Mahon et al. 2001), cotransfection of wild-type (wt) FIH-1 suppressed CAD-dependent expression of the luciferase reporter in a dose-dependent fashion (Fig. 2A). Because the asparaginyl hydroxylase activity that inhibits CAD activity is dependent upon Fe(II) (Lando et al. 2002), mutation of the predicted HXD dyad necessary for iron chelation would be expected to eliminate effect of FIH-1 on the Gal4–CAD chimera. Consistent with this hypothesis, mutation of either His 199 (H199A) or Asp 201 (D201A) to Ala prevents FIH-1 suppression of HIF CAD activity (Fig. 2A). 2-Oxoglutarate-dependent hydroxylase enzymes can also be inhibited in vivo by the competitive inhibitor dimethyl-oxalylglycine (DMOG), a cell-permeable analog of 2-oxoglutarate (Jaakkola et al. 2001). As shown in Figure 2B, FIH-1-dependent suppression of CAD activity is inhibited by DMOG. Together, these data indicate that FIH-1 may be a 2-oxoglutarate- and Fe(II)-dependent oxygenase.
Figure 2.
Effect of FIH-1 on HIF-1α and HIF-2α transactivation domain activity. (A) 293T cells were cotransfected with expression vectors for either the Gal4 DNA binding domain (GalDBD) or the indicated GalDBD/HIF-1α 727–826 or GalDBD/HIF-2α 774–874 chimeras, a Gal4 response element-driven luciferase reporter, and wild-type FIH-1 (wt) or H199A or D201A substitution mutants as indicated. (B) 293T cells were cotransfected with either GalDBD/HIF-1α 727–826 or Gal/HIF-2α 774–874 chimeras, a Gal4 response element-driven luciferase reporter, and 200 ng of wild-type FIH-1 plasmid. Transfected cells were subjected to increasing (0–200 μM) dimethyl-oxalylglycine (DMOG) treatment. Data are the average of three transfections +/− S.D.
FIH-1 suppresses HIF-α transcriptional activity by preventing CAD association with p300
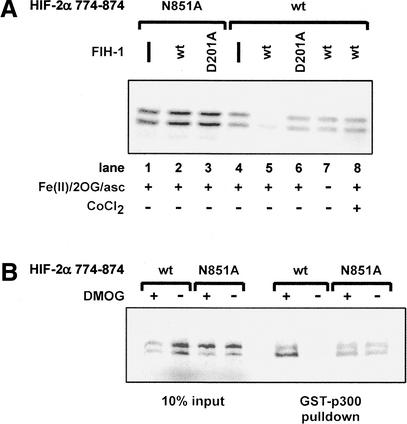
Under normoxic conditions, HIF-α CAD association with the CH1 domain of the coactivator p300 is blocked by hydroxylation of a conserved asparagine residue (Lando et al. 2002). To test the hypothesis that FIH-1 is the asparaginyl hydroxylase responsible for mediating p300 interaction with the CAD, recombinant CH1 domain of p300 was expressed as a glutathione S-transferase (GST) fusion and used to pull down 35S-labeled HIF-2α CAD. As shown in Figure 3A (lane 4), GST-p300 does associate with the CAD (HIF-2α residues 774–874). However, preincubation of the CAD with recombinant wild-type FIH-1, expressed as a maltose-binding protein (MBP) fusion, severely inhibits association with GST-p300 (Fig. 3A, lane 5). FIH-1-dependent inhibition requires addition of 2-oxoglutarate, Fe(II), and ascorbate, consistent with putative hydroxylase activity (Fig. 3A, lanes 5,7). Disruption of FIH-1's putative Fe(II)-binding site (D201A) blocks recombinant FIH-1 activity (Fig. 3A, lane 6). DMOG and Co(II), competitive inhibitors of hydroxylase activity, also prevent FIH-1 from disrupting the CAD–p300 interaction (Fig. 3A,B). Mutation of the asparagine residue to alanine (N851A for HIF-2α) prevents CAD hydroxylation in vivo and leads to constitutive CAD association with p300, even under normoxic conditions (Lando et al. 2002). The N851A mutation likewise renders the CAD–p300 interaction insensitive to FIH-1 in vitro (Fig. 3A,B). FIH-1 was equally capable of blocking p300 association with the HIF-1α CAD (data not shown). In addition to preventing p300 association with the CAD via hydroxylation, FIH-1 could also directly compete with p300 for CAD binding. However, FIH-1 fails to block p300 binding to the CAD in the absence of excess Fe(II) and 2-oxoglutarate (Fig. 3A, lane 7), although it remains capable of binding the CAD under these conditions (Mahon et al. 2001).
Figure 3.
In vitro hydroxylation of HIF-2α 774–874 by FIH-1 inhibits interaction with p300. 35S-labeled HIF-2α 774–874 wild-type (wt) or the N851A mutant were treated with MBP-FIH-1 (wt or D201A mutant) in the presence of Fe(II), ascorbate (asc), and 2-oxoglutarate (2OG) or the hydroxylase inhibitor DMOG, then incubated with immobilized GST–p300 CH1. 35S-labeled HIF-2α 774–874 bound to the GST–p300 CH1 domain was visualized following SDS-polyacrylamide gel electrophoresis. FIH-1 activity is inhibited by 1 mM CoCl2 (A) or 2 mM DMOG (B).
FIH-1 hydroxylates the key asparagine residue within the HIF-α CAD
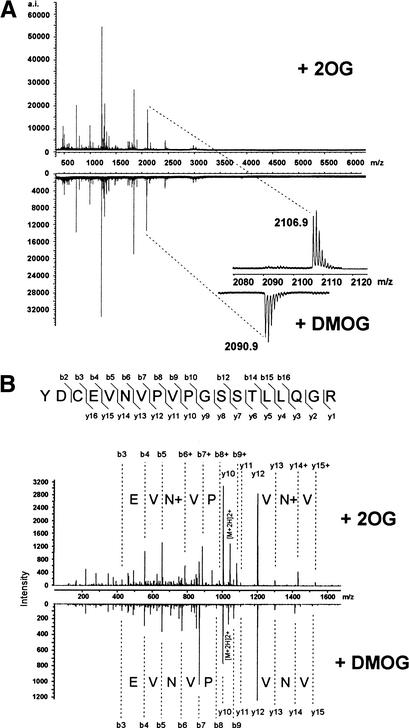
To confirm that FIH-1 possesses asparaginyl hydroxylase activity, recombinant MBP–FIH-1 was incubated (+/− DMOG) with purified HIF-2α CAD (residues 774–874) expressed as a Trx6H fusion protein in Escherichia coli. Using matrix-assisted laser desorption time-of-flight mass spectrometry (MALDI-TOF-MS), a tryptic peptide fragment containing Asn 851 was shown to contain an additional mass of 16 daltons, consistent with hydroxylation, in the sample prepared with active FIH-1 (Fig. 4A, +2OG). The fragment from the DMOG-containing reaction lacked the mass increase. To identify the modified residue, the relevant tryptic peptides from each sample were further analyzed by tandem mass spectrometry. Comparison of the fragment patterns revealed that Asn 851 contained the additional oxygen atom (Fig. 4B). Together these data show that FIH-1 is an asparaginyl hydroxylase that inhibits HIF transcriptional activity by preventing CAD association with the coactivator p300.
Figure 4.
HIF-2α 774–874 is efficiently hydroxylated at residue Asn 851 by FIH-1 in vitro. (A) MALDI-TOF-MS spectra of trypsin-digested Trx6H HIF-2α 774–874 after treatment with FIH-1 in the presence of 2-oxoglutarate (2OG, upper spectrum) or DMOG (lower spectrum). The tryptic peptide YDCEVNVPVPGSSTLLQGR (846–864) is hydroxylated (+16 daltons) after treatment in the presence of 2OG, but not DMOG (magnified below). (B) Tandem MS sequencing of the hydroxylated and nonhydroxylated HIF-2α 846–864 tryptic peptides after FIH-1 treatment with 2-oxoglutarate (2OG) or DMOG, respectively, shows the hydroxylated residue is Asn 851. For instance, b- and y-type fragment ions were observed with the 2090.9 unmodified peptide that covered nearly the entire peptide sequence (lower spectrum). The modified 2106.9 peptide produced some fragment ions (upper spectrum) at the same m/z values as the unmodified peptide. These coincidental fragments correspond to portions of the sequence that did not contain Asn 851. Fragments were also observed for the modified sequence at m/z values of +16 daltons more than the Asn 851-containing fragments of the unmodified sequence (indicated by b+ and y+ in the upper panel). The boundary of the coincident and +16-dalton fragment ions is indicated by the symbol N+ on the upper panel.
The role of FIH-1 in the hypoxic response pathway
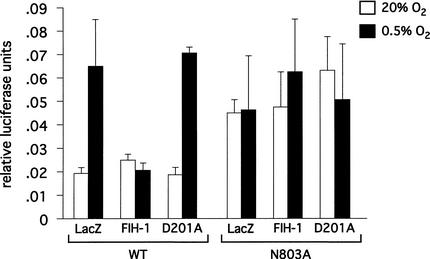
Because both the HIF prolyl and asparaginyl hydroxylases use molecular O2 as a substrate, it is reasonable to predict that these enzymes might be direct oxygen sensors within the hypoxic response pathway. Additional studies will be necessary to determine if the affinity of these enzymes for oxygen could account for the sensitivity of the hypoxic response to changes in O2 in vivo. When overexpressed at high levels in 293T cells, FIH-1 does suppress CAD activity under normoxic conditions (Fig. 2). If FIH-1 activity is an oxygen sensor, this inhibitory activity should be suppressed under hypoxic conditions. Indeed, suppression of the inhibitory effects of the HIF prolyl hydroxylase on HIF activity has been observed under hypoxic conditions (Bruick and McKnight 2001). However, when transfected at high levels, FIH-1 was still able to suppress HIF-1α CAD activity at low oxygen concentrations (Mahon et al. 2001). Similar effects are observed when FIH-1 is transfected at much lower levels (Fig. 5). Although even low levels of overexpressed FIH-1 may still retain enough activity to suppress the CAD under hypoxic conditions, this result raises the possibility that the regulation of FIH-1 activity may be not be regulated solely by its affinity for O2.
Figure 5.
Effect of hypoxia on FIH-1 inhibition of transactivation domain activity. Human embryonic kidney 293 cells were transfected with a Gal4-tk-luc reporter DNA, the HIF-1α 727–826/Gal4 chimera (wild-type or the N803A substitution mutant) and FIH-1 (wild-type or the D201A substitution mutant) or a LacZ control. Cells were incubated for 19 h under normoxic conditions or for 5 h under normoxic conditions followed by 14 h under hypoxic conditions (0.5% O2). FIH-1, but not the D201A mutant, suppresses CAD activation under hypoxic (0.5% O2) conditions. FIH-1 suppression of CAD activity under normoxic conditions is not observed under these conditions as baseline luciferase activity levels are at the level of detection. Mutation of the asparagine to alanine renders the CAD insensitive to either FIH-1 or oxygen concentration.
Full induction of the hypoxic response pathway upon exposure to low oxygen levels requires both stabilization of the HIF-α transcription factor and activation of the CAD (Lando et al. 2002). Each event depends on abrogation of a hydroxylase enzyme that negatively regulates HIF under normoxic conditions. Although the two modes of regulation can be apparently uncoupled from each other through the construction of chimeras containing either the ODD or the CAD, the machinery regulating the two appears to be linked (Mahon et al. 2001). In vitro binding studies suggest that FIH-1 and pVHL interact and may be part of a larger complex that represses HIF by multiple mechanisms under normoxic conditions (Mahon et al. 2001), although regulation of the CAD by hydroxylation does not appear to require pVHL (Sang et al. 2002). Such cooperative interactions may serve to tightly control HIF activity and prevent misexpression of HIF-target genes, many of which promote solid tumor formation (Maxwell et al. 2001).
Materials and methods
Plasmid construction
The HIF CADs (mouse HIF-2α 774–874 and human HIF-1α 727–826) were generated by PCR and cloned into pET-32a (Novagen) for bacterial expression with an N-terminal thioredoxin-6 histidine tag (Trx6H). The CH1 domain of human p300 (amino acids 300–528) was cloned into pGEX 4T3 (Amersham) for bacterial expression with an N-terminal GST fusion. The human FIH-1 coding region was generated by RT–PCR and cloned into both the pcDNA3.1/V5-HIS vector (Invitrogen) and the pMBP-parallel1 vector (Sheffield et al. 1999).
Transfections
HEK 293T cells grown in DMEM/10% fetal bovine serum in 24-well trays were transfected with Gal4 or HIF CADs/Gal4 chimeras (Lando et al. 2002; 200 ng/well), G5E1b-Luc reporter (100 ng/well), control renilla luciferese reporter (pRL-TK; 10 ng/well), FIH-1 pcDNA3.1/V5-HIS (0–200 ng/well), and Efbos (510 ng DNA/well total) using LipofectAMINE 2000 (Life Technologies). After 6 h, transfected cells were subjected to increasing (0–200 μM) dimethyl-oxalylglycine (DMOG) treatment for 16 h. Luciferase activity was measured using the dual luciferese assay (Promega) and reported as the ratio of luciferase activity relative to control renilla reporter activity. Western blot analysis confirmed that transfected wild-type, H199A, and D201A FIH-1 constructs expressed an equivalent amount of protein (data not shown).
Human embryonic kidney 293 cells (5 × 104 cells/well) in 24-well plates (5 × 104) containing HyQ DME (High Glucose) media supplemented with 10% fetal bovine serum were transfected with DNA prepared by precipitation with 125 μM CaCl2 in the presence of 1× BES buffer (140 mM NaCl, 25 mM BES, 0.75 mM Na2PO4 at pH 6.95). A precipitation mixture containing 5 ng of Gal4-tk-luc reporter DNA, 25 ng of HIF-1α 727–826/Gal4 chimera (wild-type or N803A mutant), and 10 ng of lacZ, FIH-1, or FIH-1 D201A mutant was added to each well. Cells were incubated for 19 h under normoxic conditions or for 5 h under normoxic conditions followed by 14 h under hypoxic conditions (0.5% O2). Cells were resuspended in 100 μL of lysis buffer (30 mM Tricine at pH 7.8, 8 mM MgOAc, 0.2 mM EDTA, 1% Triton X-100, 100 mM β-Me, 1.5 mM ATP, 0.5 mM CoA, 0.5 mM luciferin). Luciferase activity was measured using a microtiter plate luminometer (Torcon Instruments).
Protein expression
GST-p300 CH1 expression in BL21(DE3) E. coli cells was induced with 0.1 mM IPTG at 25°C for 1.5 h. Cell pellets were lysed in buffer containing 20 mM Tris-HCl (pH 8), 100 mM NaCl, 10 μM ZnCl2, 0.5% NP-40, 0.5 mM DTT, 1 mM PMSF, and 0.3 μg/mL lysozyme, and sonicated on ice. After centrifugation, the supernatant was bound to glutathione-agarose resin (Scientifix) and washed with 200 volumes of lysis buffer. Trx6H HIF-2α 774–874 was expressed in BL21(DE3) E. coli cells following induction with 1 mM IPTG at 37°C for 1.5 h. Cell pellets were lysed by sonication in binding buffer (20 mM Tris-HCl at pH 7.5, 500 mM NaCl, 5 mM imidazole) containing 1 mM PMSF. After centrifugation, lysates were incubated with Ni-IDA agarose (Scientifix) at 4°C for 1 h and washed with 200 volumes of binding buffer; the Trx6H HIF-2α 774–874 protein was eluted with binding buffer containing 250 mM imidazole. BL21(DE3) E. coli cells expressing MBP–FIH-1 fusions were induced with 0.2 mM IPTG at 30°C for 4.5 h, and cell pellets were lysed by sonication in TH buffer (20 mM Tris-HCl at pH 7.9, 150 mM NaCl, 1 mM PMSF). After centrifugation, amylose agarose (Scientifix) was added, the samples were incubated at 4°C for 1 h and washed with 200 volumes of TH buffer, and the MBP–FIH-1 protein was eluted with TH buffer containing 10 mM maltose. Protein samples were desalted using a PD-10 column (Amersham).
GST pull-down assays
35S-labeled Trx6H HIF-2α 774–874 (wild-type or N851A mutant) proteins were generated using the TNT Coupled Reticulocyte Lysate System (Promega) and 35S-L-Met (Amersham Pharmacia Biotech) and treated at 30°C for 1 h with FIH-1 in buffer containing 4 mM ascorbic acid and 1.5 mM FeSO4 in the presence of either 2 mM 2-oxoglutarate or 2 mM DMOG. Approximately 1 μg of immobilized GST-p300 CH1 was added to 25 μL of 35S-Trx6H HIF-2α 774–874 in 500 μL of reaction buffer (20 mM Tris-HCl at pH 8, 150 mM NaCl, 20 μM ZnCl2, 1 mM DTT, 1 mM PMSF) and incubated at 4°C for 1 h. Protein-bound resin was washed 4 times with 1 mL of reaction buffer containing 0.1% NP-40. Bound protein was eluted with SDS-sample buffer and analyzed by SDS-polyacrylamide gel electrophoresis.
Mass spectrometry analysis of in vitro hydroxylation products
For mass spectrometry, 10 μg of purified Trx6H HIF-2α 774–874 and MBP–FIH-1 proteins was mixed in 100 μL of hydroxylation buffer (40 mM Tris-HCl at pH 7.5, 10 mM KCl, 1 mM DTT, 1 mM PMSF, 3 mM MgCl2, 4 mM ascorbic acid, 1.5 mM FeSO4) containing 4 mM 2-oxoglutarate or DMOG. After incubation at 30°C for 1 h, Trx6H HIF-2α 774–874 was affinity-purified with Ni-IDA agarose. Peptide fragments were prepared and analyzed by MALDI-TOF-MS (Lopaticki et al. 1998; Wallis et al. 2001) and subjected to partial sequence analysis by MS/MS (Wallis et al. 2001) as described previously (Lando et al. 2002).
Acknowledgments
We thank S. Pyke for help with the synthesis of DMOG and Steve McKnight for helpful advice. R.K.B was funded by a National Research Service Award from the National Institutes of Health and by unrestricted endowment funds provided to Steve McKnight by an anonymous donor.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL murray.whitelaw@adelaide.edu.au; FAX 0011-1-214-648-3346.
E-MAIL bruick@biochem.swmed.edu; FAX (214) 648-3346.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.991402.
References
- Aravind L, Koonin EV. The DNA-repair protein AlkB, EGL-9, and leprecan define new families of 2-oxoglutarate- and iron-dependent dioxygenases. Genome Biol. 2001;2:1–8. doi: 10.1186/gb-2001-2-3-research0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- Carrero P, Okamato K, Coumailleau P, O'Brien S, Tanaka H, Poellinger L. Redox-regulated recruitment of the transcriptional coactivators CREB-binding protein and SRC-1 to hypoxia-inducible factor 1α. Mol Cell Biol. 2000;20:402–415. doi: 10.1128/mcb.20.1.402-415.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton IJ, Hsueh L-C, Baldwin JE, Harlos K, Schofield CJ. Structure of proline 3-hydroxylase. Evolution of the family of 2-oxoglutarate dependent oxygenases. Eur J Biochem. 2001;268:6625–6636. doi: 10.1046/j.0014-2956.2001.02617.x. [DOI] [PubMed] [Google Scholar]
- Cockman ME, Masson N, Mole DR, Jaakkola P, Chang GW, Clifford SC, Maher ER, Pugh CW, Ratcliffe PJ, Maxwell PH. Hypoxia inducible factor-α binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J Biol Chem. 2000;275:25733–25741. doi: 10.1074/jbc.M002740200. [DOI] [PubMed] [Google Scholar]
- Cuff JA, Clamp ME, Siddiqui AS, Finlay M, Barton GJ. Jpred: A consensus secondary structure prediction server. Bioinformatics. 1998;14:892–893. doi: 10.1093/bioinformatics/14.10.892. [DOI] [PubMed] [Google Scholar]
- Ema M, Hirota K, Mimura J, Abe H, Yodoi J, Sogawa K, Poellinger L, Fujii-Kuriyama Y. Molecular mechanisms of transcription activation by HLF and HIF1α in response to hypoxia: Their stabilization and redox signal-induced interaction with CBP/p300. EMBO J. 1999;18:1905–1914. doi: 10.1093/emboj/18.7.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein ACR, Gleadle JM, McNeil LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Gu J, Milligan J, Huang LE. Molecular mechanism of hypoxia-inducible factor 1–p300 interaction. J Biol Chem. 2001;276:3550–3554. doi: 10.1074/jbc.M009522200. [DOI] [PubMed] [Google Scholar]
- Gu YZ, Moran SM, Hogenesch JB, Wartman L, Bradfield CA. Molecular characterization and chromosomal localization of a third α-class hypoxia inducible factor subunit, HIF3α. Gene Expression. 1998;7:205–213. [PMC free article] [PubMed] [Google Scholar]
- Hegg EL, Que L. The 2-His-1-carboxylate facial triad—An emerging structural motif in mononuclear non-heme iron(II) enzymes. Eur J Biochem. 1997;250:625–629. doi: 10.1111/j.1432-1033.1997.t01-1-00625.x. [DOI] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara J M, Lane WS, Kaelin WG. HIFα targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian Y-M, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, et al. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Jiang BH, Zheng JZ, Leung SW, Roe R, Semenza GL. Transactivation and inhibitory domains of hypoxia-inducible factor 1α. Modulation of transcriptional activity by oxygen tension. J Biol Chem. 1997;272:19253–19260. doi: 10.1074/jbc.272.31.19253. [DOI] [PubMed] [Google Scholar]
- Kung AL, Wang S, Klco JM, Kaelin WG, Livingston DM. Suppression of tumor growth through disruption of hypoxia-inducible transcription. Nat Med. 2000;6:1335–1340. doi: 10.1038/82146. [DOI] [PubMed] [Google Scholar]
- Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain: A hypoxic switch. Science. 2002;295:858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- Lopaticki S, Morrow CJ, Gorman JJ. Characterization of pathotype-specific epitopes of Newcastle disease virus fusion glycoproteins by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and post-source decay sequencing. J Mass Spectrom. 1998;33:950–960. doi: 10.1002/(SICI)1096-9888(199810)33:10<950::AID-JMS704>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Mahon PC, Hirota K, Semenza GL. FIH-1: A novel protein that interacts with HIF-1α and VHL to mediate repression of HIF-1 transcriptional activity. Genes & Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- Maxwell PH, Pugh CW, Ratcliffe PJ. Activation of the HIF pathway in cancer. Curr Opin Gen Dev. 2001;11:293–299. doi: 10.1016/s0959-437x(00)00193-3. [DOI] [PubMed] [Google Scholar]
- Ohh M, Park CW, Ivan M, Hoffman MA, Kim T-Y, Huang LE, Pavletich N, Chau V, Kaelin WG. Ubiquitination of hypoxia-inducible factor requires direct binding to the β-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- O'Rourke JF, Tian YM, Ratcliffe PJ, Pugh CW. Oxygen-regulated and transactivating domains in endothelial PAS protein 1: Comparison with hypoxia-inducible factor-1α. J Biol Chem. 1999;274:2060–2071. doi: 10.1074/jbc.274.4.2060. [DOI] [PubMed] [Google Scholar]
- Roach PL, Clifton IJ, Fulop V, Harlos K, Barton GJ, Hajdu J, Andersson I, Schofield CJ, Baldwin JE. Structure of isopenicillin N synthase is the first from a new structural family of enzymes. Nature. 1995;375:700–704. doi: 10.1038/375700a0. [DOI] [PubMed] [Google Scholar]
- Sang N, Fang J, Srinivas V, Leshchinsky I, Caro J. Carboxyl-terminal transactivation activity of hypoxia-inducible factor 1α is governed by a von Hippel-Lindau protein-independent, hydroxylation-regulated association with p300/CBP. Mol Cell Biol. 2002;22:2984–2992. doi: 10.1128/MCB.22.9.2984-2992.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- Sheffield P, Garrard S, Derewenda Z. Overcoming expression and purification problems of RhoGDI using a family of “parallel” expression vectors. Prot Express Pur. 1999;15:34–39. doi: 10.1006/prep.1998.1003. [DOI] [PubMed] [Google Scholar]
- Srinivas V, Zhang LP, Zhu XH, Caro J. Characterization of an oxygen/redox-dependent degradation domain of hypoxia-inducible factor α (HIF-α) proteins. Biochem Biophys Res Comm. 1999;260:557–561. doi: 10.1006/bbrc.1999.0878. [DOI] [PubMed] [Google Scholar]
- Tanimoto K, Makino Y, Pereira T, Poellinger L. Mechanism of regulation of the hypoxia-inducible factor-1α by the von Hippel-Lindau tumor suppressor protein. EMBO J. 2000;19:4298–4309. doi: 10.1093/emboj/19.16.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes & Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- Valegård K, van Scheltinga ACT, Lloyd MD, Hara T, Ramaswamy S, Perrakis A, Thompson A, Lee HJ, Baldwin JE, Schofield CJ, et al. Structure of a cephalosporin synthase. Nature. 1998;394:805–809. doi: 10.1038/29575. [DOI] [PubMed] [Google Scholar]
- Wallis TP, Pitt JJ, Gorman JJ. Identification of disulfide-linked peptides by isotope profiles produced by peptic digestion of proteins in 50% 18O water. Protein Sci. 2001;10:2251–2271. doi: 10.1110/ps.15401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix–loop–helix–PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, White SB, Zhao Q, Lee FS. HIF-1α binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci. 2001;98:9630–9635. doi: 10.1073/pnas.181341498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZH, Ren JS, Stammers DK, Baldwin JE, Harlos K, Schofield CJ. Structural origins of the selectivity of the trifunctional oxygenase clavaminic acid synthase. Nat Struct Biol. 2000;7:127–133. doi: 10.1038/72398. [DOI] [PubMed] [Google Scholar]