Abstract
Proper functioning of the human circadian timing system is crucial to physical and mental health. Much of what we know about this system is based on experimental protocols that induce the desynchronization of behavioral and physiological rhythms within individual subjects, but the neural (or extraneural) substrates for such desynchronization are unknown. We have developed an animal model of human internal desynchrony in which rats are exposed to artificially short (22-h) light–dark cycles. Under these conditions, locomotor activity, sleep–wake, and slow-wave sleep (SWS) exhibit two rhythms within individual animals, one entrained to the 22-h light–dark cycle and the other free-running with a period >24 h (τ>24 h). Whereas core body temperature showed two rhythms as well, further analysis indicates this variable oscillates more according to the τ>24 h rhythm than to the 22-h rhythm, and that this oscillation is due to an activity-independent circadian regulation. Paradoxical sleep (PS), on the other hand, shows only one free-running rhythm. Our results show that, similarly to humans, (i) circadian rhythms can be internally dissociated in a controlled and predictable manner in the rat and (ii) the circadian rhythms of sleep–wake and SWS can be desynchronized from the rhythms of PS and core body temperature within individual animals. This model now allows for a deeper understanding of the human timekeeping mechanism, for testing potential therapies for circadian dysrhythmias, and for studying the biology of PS and SWS states in a neurologically intact model.
Keywords: suprachiasmatic
In mammals, a master circadian pacemaker localized within the hypothalamic suprachiasmatic nucleus (SCN) governs overt circadian rhythms of physiology and behavior. The SCN is constituted by a network of single-cell neuronal oscillators that regulates circadian rhythms through direct and indirect output pathways to brain regions controlling specific physiological and behavioral processes (1, 2). The SCN master regulation of circadian rhythms can potentially take place through control of circadian oscillators elsewhere in the brain and in virtually all peripheral tissues, which presumably act as local pacemakers for specific rhythmic modalities (3, 4).
Although the evidence clearly indicates that the circadian rhythms of locomotor activity, core body temperature (CBT), and sleep–wake share a common circadian pacemaker within the SCN (1, 2, 5), some features of these rhythmic modalities suggest that they might be differentially regulated. The first indication that the rhythms of CBT, rest–activity, and sleep structure could be independently regulated came from studies in humans that show “spontaneous internal desynchronization” (6, 7). Human subjects under temporal isolation sometimes exhibit a circadian rhythm of CBT with a near-24-h period, whereas their self-imposed rest–activity cycle (and associated sleep–wake cycle) oscillates with a considerably longer period (generally >30 h). Desynchronization between the rest–activity cycle and the CBT rhythm can be also experimentally induced through so-called “forced desynchrony protocols,” in which the experimenter imposes a rest–activity cycle that is different from 24 h. Typically, in such studies the rhythms of CBT and other physiological variables including plasma melatonin and cortisol, sleep propensity, and rapid eye-movement sleep, also referred to as paradoxical sleep (PS), oscillate, out of synchrony with the imposed rest–activity cycle, with a period near 24 h (6, 7).
It is still a matter of controversy whether internal desynchronization of physiological and behavioral rhythms represents the activity of two independent oscillators and, if it does, whether these oscillators are anatomically identifiable. In fact, the anatomical basis of internal desynchronization, whether spontaneous or induced by forced desynchrony protocols, remains unknown and the lack of animal models of forced desynchronization has slowed progress toward determining the neural and molecular basis of circadian desynchrony. Here, we report an animal model of circadian desynchronization, in which the rhythms of CBT and PS can be dissociated from those of rest–activity, sleep–wake, and slow-wave sleep (SWS).
Results and Discussion
We recently developed an animal model of forced desynchrony: Rats exposed to 22-h light–dark (LD) cycles exhibit two stable locomotor activity rhythms with different period lengths in individual animals (8). We determined that one of these rhythms, with a period of 22 h (T22 h) and entrained to the LD cycle, is associated with the expression of clock genes in the ventrolateral (VL) SCN. The other rhythm, with a period longer than 24 h (τ>24 h) and not entrained to the LD cycle, is associated with clock gene expression in the dorsomedial (DM) SCN (9). This finding suggests that the uncoupling of anatomically identifiable subpopulations of neuronal oscillators within the SCN itself could lead to the desynchronization of different circadian physiological and behavioral processes, similar to that observed in human subjects. To test this hypothesis, we monitored rhythms of locomotor activity, CBT, and electrocorticographic (ECoG) sleep–wake activity in forced desynchronized rats.
Adult male Wistar rats housed individually under a 22-h LD cycle (11 h of light:11 h of dark) were implanted under deep anesthesia during the light phase with an i.p. temperature sensor or with both an i.p. temperature sensor and ECoG electrodes. Animals were returned to their home cages where locomotor activity by infrared beam interruptions, and ECoG activity were recorded. At the end of the experiment, animals were killed and the temperature sensors were removed to acquire the temperature data. Fig. 1A depicts the rhythms of locomotor activity and temperature of a typical animal under a 22-h LD cycle. χ2 periodogram analysis indicated two statistically significant rhythmic components with periods of 22 h and >24 h (τ>24 h = 25 h ± 5 min for all rats) for both locomotor activity and CBT. Sixteen animals (of 25 animals studied) showed this pattern of rhythmicity, with stable rhythms of locomotor activity and CBT for both T22 h and τ>24 h. Notably, six of the remaining nine animals showed statistically significant CBT rhythm only for the τ>24 h component despite the fact that they showed a statistically significant T22 h locomotor activity rhythm (Table 1). In the 16 animals with dual locomotor activity rhythms and dual CBT rhythms, the percentage of variance of locomotor activity explained by T22 h component in the periodogram (16 ± 1.46) was significantly higher than the percentage of variance explained by the τ>24 h component (10.2 ± 0.84; t test, P < 0.005). In contrast, the percentage of variance of CBT data explained by the T22 h component (11.5 ± 1.38) was significantly smaller than that explained by the τ>24 h component (18.8 ± 2.2; t test, P < 0.01). This analysis indicates that, whereas the circadian oscillation of activity shows higher cycle-to-cycle phase stability under the T22 h period, the circadian oscillation of temperature is more stable under the τ>24 h period. This, together with the fact that six animals showed solely a τ>24 h CBT rhythm despite having a significant T22 h locomotor activity oscillation demonstrates that the circadian rhythm of CBT can be dissociated from rhythmic locomotor activity. The more robust oscillation of locomotor activity under a 22-h period may reflect the fact that this behavioral process is under stronger masking (10) by the LD cycle than CBT is, as it is clearly suggested by the reactive peak of activity after lights off (Fig. 2B).
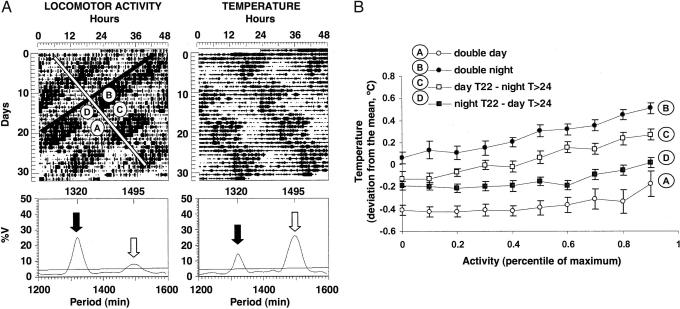
Fig. 1.
Desynchronization of locomotor activity and CBT in the forced desynchronized rat. (A) (Upper) Double plotted actograms for motor activity and temperature of a representative forced desynchronized rat under a 22-h LD cycle. The white and black diagonal bars indicate the onset of the τ>24 h and the T22 h locomotor activity rhythms, respectively. The circled letters represent the four phases (defined by the two activity rhythms) on which the analysis of CBT was performed. (Lower) χ2 periodograms of the time series represented on the actograms. The analysis yielded statistically significant peaks for the τ>24 h (white arrow) and the T22 h (black arrow) rhythms. The numbers on top indicate the period of the significant peaks in minutes. (B) Mean temperature levels (as deviation from the individual mean temperature) as a function of different levels of locomotor activity (as percentile of maximum values) for each of the four phases indicated in A. Each value represents the mean ± SE drawn from 16 animals with dual activity and dual CBT rhythms. General linear models with repeated measures yielded significant differences between phases A, B, C, and D in all possible compared pairs (P < 0.001) and linear regression analysis significant slopes within each phase (P < 0.001).
Table 1.
Most rats exposed to a 22-h LD cycle express dual locomotor activity rhythms and dual temperature rhythms
| Locomotor activity | CBT |
||
|---|---|---|---|
| T22 h component | τ>24 h component | Both components | |
| T22 h component | 0 | 1 | 1 |
| τ>24 h component | 0 | 0 | 1 |
| Both components | 1 | 5 | 16 |
Number of animals that showed either both or only one of the T22 h and τ>24 h components for locomotor activity and CBT.
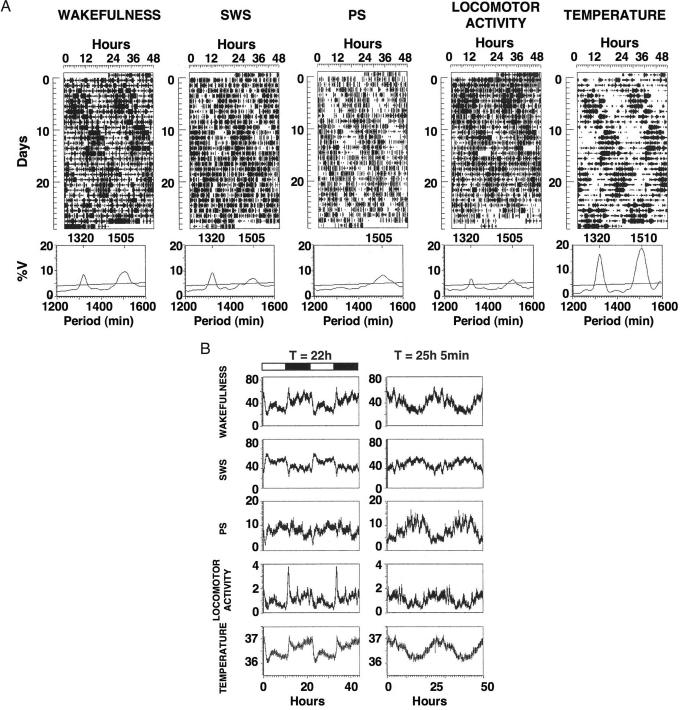
Fig. 2.
Desynchronization of sleep stages in the forced desynchronized rat. (A) Double plotted actograms of wakefulness, SWS, PS, locomotor activity, and CBT of a representative rat, and their corresponding periodograms. (B) Circadian variation of all variables in the same animal shown in A, plotted in modulo of the two significant periods (T22 h = 22 h; τ>24 h = 25 h, 5 min) obtained in the periodogram. Values represent the mean ± SE of the 10-min interval values for each successive cycle, smoothed by running averages of three data points. The dark and white horizontal bars on the left indicate the dark and light phases of the 22-h LD cycle, respectively.
Because in the animals with dual CBT rhythms, locomotor activity also oscillates both with 22- and >24-h periods, the oscillations of CBT with these respective periods could represent a true circadian modulation of heat-producing mechanisms or a by-product of activity-induced heat production. Accordingly, human subjects under a rest–activity (and respective dark and light phases) forced desynchrony protocol show both an endogenous free-running modulation of CBT but also behaviorally induced changes in CBT that are associated to the experimenter-imposed rest–activity cycle (11, 12). In animals, an imposed rest–activity cycle is not feasible and genuine circadian modulation of CBT must be statistically dissected from locomotor activity-induced rhythmic CBT (13).
Although a proportional increase of CBT with increased activity is present under all phases of the circadian cycle, a given level of activity may yield higher values of CBT at specific circadian phases. This activity-independent increase in temperature is interpreted as direct modulation of temperature control systems by a circadian pacemaker. In our forced desynchronized animals, the two overlapping rhythms of locomotor activity define a T22 h night and day, and a τ>24 h subjective night and subjective day, respectively. The relationship between activity levels and CBT at the phase representing “day” for both the T22 h and τ>24 h rhythms (double day; Fig. 1A, phase A), which corresponds to the light phase for the T22 h rhythm and the rest phase for the τ>24 h rhythm, yielded the lowest temperature values for any specific level of activity (Fig. 1B). The same relationship estimated at a phase representing “night” for both the T22 h and τ>24 h rhythms (double night; Fig. 1A, phase B), which corresponds to the dark phase for the T22 h rhythm and the active phase for the τ>24 h rhythm, yielded the highest temperature values for any specific level of activity. The relationship between temperature and activity in both conflicting phases, in which the lights are on (T22 h day) but the τ>24 h rhythm is in subjective night (Fig. 1A, phase C), and vice versa, in which the lights are off (T22 h night) but the τ>24 h rhythm is in subjective day (Fig. 1A, phase D) yielded higher values of temperature than the double-day phase but lower than the double-night phase (Fig. 1B). Interestingly, phase C showed higher values than phase D (general linear models with repeated measures, P < 0.001 in all comparisons). Of note, the double-day and double-night curves were not statistically different from those for the day and night, respectively, of control animals housed under a 12:12 LD cycle (data not shown), suggesting that circadian regulation of CBT during the nonconflicting phases of the forced desynchrony protocol is similar to that of 24-h LD-synchronized animals. Our analysis also indicated that, in all four phases (Fig. 1, phases A–D), an increase in locomotor activity levels induced a proportional increase in temperature (linear regression, P < 0.001, in all four phases). Thus, CBT is both under circadian control and influenced by activity-induced heat.
The differences in activity-independent CBT indicate that CBT is truly oscillating according to two rhythms within individual animals, generating four basic levels of temperature for any given level of locomotor activity. This activity-independent, daily or circadian regulation of temperature has been previously described in several rodent species (13, 14), including the rat in which it relies on an intact SCN (15). Furthermore, our analysis clearly demonstrates that activity-independent CBT in the forced-desynchronized rat oscillates more robustly in accordance to a τ>24 h rhythm than to the entrained T22 h rhythm. The activity-independent oscillation of CBT in the 22-h domain could represent the output of an oscillator and/or a masking effect of light on CBT. Light is known to produce a reduction in CBT that depends on the phase of the circadian cycle (15). On the other hand, the analysis of both locomotor activity rhythms (8, 16) and clock gene expression patterns (9) in the forced desynchronized rat has indicated that the T22 h locomotor activity rhythm, associated with the T22 h CBT rhythm here described, likely represents the output of a true, entrainable oscillator within the VL-SCN.
The robust τ>24 h circadian oscillation in CBT, independent of the imposed 22-h LD cycle, in our forced desynchronized rats is reminiscent of human CBT rhythms under similar forced desynchrony conditions. In the rat, this τ>24 h oscillation, as judged by the locomotor activity rhythm, is associated with the clock gene activity within the DM-SCN (9), and our results suggest that the expression of forced desynchronized rhythms of locomotor activity and CBT in humans could also be associated with uncoupling of dual oscillators within the SCN master circadian oscillator. Although humans do not exhibit two rhythms of rest–activity as our rats do, this may be a consequence of the experimenter-imposed forced rest–activity cycle. Notably, human subjects under spontaneous internal desynchronization do show evidence of two rest–activity periodicities, one that free-runs with a much longer than 24-h period and another that is in phase with the circa 24-h rhythm in CBT (17, 18).
Desynchronization of PS from the rest–activity cycle and its associated sleep–wake cycle is yet another signature of humans under forced desynchrony protocols. Under these circumstances, PS propensity increases shortly after the circadian minimum of CBT (12), a feature that is present also in spontaneously desynchronized human subjects (17, 19). The robust τ>24 h oscillation of CBT in the present study hinted to the possibility that this tight correlation between CBT circadian rhythmicity and PS may also be present in the forced desynchronized rat. We explored this possibility in four animals in which we performed long-term ECoG recordings and simultaneously monitored locomotor activity and CBT. Fig. 2 shows a representative animal. Whereas locomotor activity, CBT, wakefulness, and SWS presented dual T22 h and τ>24 h rhythms, PS only showed a significant τ>24 h oscillation (Fig. 2A). The temporal profiles for slow-wave activity and the theta power were similar to the SWS and PS, respectively [supporting information (SI) Fig. 3]. The oscillation of SWS, slow-wave activity, and temperature in synchrony with the 22-h LD cycle could represent a masking phenomenon. Behavioral analysis (8, 16) indicates that the 22-h locomotor activity rhythm in the forced desynchronized rat represents an entrained rhythm that can predict the phase of the free-running rhythm when animals are released into constant darkness after desynchrony. Furthermore, the 22-h oscillation of clock gene expression within the VL-SCN in desynchronized animals persists under constant darkness conditions (9), although masking by the LD cycle could contribute to the expression rhythm of otherwise weak VL-SCN oscillators. These results suggest that the regulation of sleep stages and temperature in the 22-h domain here reported may emerge from the dual contribution of autonomous VL-SCN oscillators and masking processes.
The peak of PS propensity occurred during the nadir of the τ>24 h CBT rhythm (Fig. 2B). Although with lower amplitude, there was a progressive increase of PS propensity across the light phase of the 22-h LD cycle, a feature also observed in the scheduled sleep phase in human forced desynchrony protocols (12). The overall temporal distribution of sleep structure and activity was observed in all four animals studied by ECoG recordings (Table 2). Our results clearly demonstrate that the circadian timing of PS in the forced desynchronized rat is, as it is in humans, tightly associated to the free-running (τ>24 h) CBT rhythm but not with the activity-induced CBT rhythm that results from the 22-h forced desynchrony protocol.
Table 2.
Desynchronization of sleep stages, CBT, and locomotor activity in the forced desynchronized rat
| Variables | Period, h |
|||
|---|---|---|---|---|
| Rat 14 (19 days) | Rat 16 (34 days) | Rat 21 (14 days) | Rat 23 (31 days) | |
| Wakefulness | ||||
| T22 h | 22 | 22.1 | 22.4 | 22 |
| τ>24 h | 24.8 | 25.1 | 25.4 | 25.2 |
| SWS | ||||
| T22 h | 22 | 22 | 22.3 | 22 |
| τ>24 h | 24.8 | 25.1 | 25.2 | 25.1 |
| PS | ||||
| T22 h | NS | NS | NS | NS |
| τ>24 h | 24.9 | 25.1 | 25.7 | 25.2 |
| Locomotor activity | ||||
| T22 h | 21.9 | 22.1 | 22.2 | 22 |
| τ>24 h | 24.8 | 25.1 | 25.3 | 25.2 |
| CBT | ||||
| T22 h | 22 | 22.1 | 22.2 | 22 |
| τ>24 h | 25.1 | 25.1 | 25.5 | 25.2 |
For each animal, the periods indicated correspond to the statistically significant periods obtained by periodogram analysis. For each variable, periods are shown for the rhythmic component associated with the 22-h LD cycle (T22 h) or the free-running component (τ>24 h). The days in parentheses indicate the duration of the study for each animal. NS, The variable did not show a statistically significant oscillation for that specific component.
In summary, circadian rhythms can be uncoupled in a predictable and stable manner in our rat forced desynchrony model. Under these circumstances, the circadian regulation of CBT and PS shows the same properties as that in forced desynchronized humans. In the rat, the entrained and free-running locomotor activity rhythms are associated with the independent activities of the VL- and DM-SCN (9), respectively, and our present results strongly suggest that the free-running oscillation of CBT and PS may be also associated with the DM-SCN activity. We propose that, in humans, desynchronization of these rhythms may also be associated with the uncoupling of dual oscillators within the hypothalamic master circadian clock. The association between the circadian rhythm of PS and the DM-SCN activity is particularly interesting. The SCN has been recognized for decades as the pacemaker for the sleep–wake cycle (5), and the output pathways that sustain this function are beginning to be mapped (20). However, so far there is no evidence that the SCN governs the timing of specific sleep stages independently. Our results point to a more protagonistic role of the SCN in the regulation of sleep stages, one in which SWS, which is more responsive to acute effects of light, would be governed by LD-associated clock gene expression of VL-SCN oscillators, and PS, typically under robust circadian control, would be governed by DM-SCN oscillators.
The VL-SCN and DM-SCN are recognized as areas that present different cytoarchitecture, chemoarchitecture, and topography of afferent and efferent connections (21), as well as different clock gene expression patterns (22, 23) and responses to light or abrupt phase shifts (refs. 24–26 and reviewed in ref. 27). Our findings add a new layer of complexity to this subregional organization, because they suggest that the VL- and DM-SCN independently control circadian rhythmicity of specific physiological and behavioral variables. Specifically, the circadian oscillation of CBT and PS in association with DM-SCN activity strongly suggests that this region controls, in a rather LD cycle-independent manner, these two rhythms, whereas the rhythms of locomotor activity and SWS are associated with the activity of either the VL- or DM-SCN.
Internal desynchronization of circadian rhythms is a common feature in most circadian pathologies, including those associated with aging, seasonal affective disorder, jet lag, nocturnal shift work, and work under non-24-h LD cycles (6, 28). Our findings in the forced desynchronized rat indicate that desynchronization of circadian rhythms within the same individual could emerge from uncoupling of neuronal oscillators within the SCN itself and may represent an entrée to explore potential treatments for these ailments.
Materials and Methods
Animals and Surgery.
All experiments were approved by the Animal Care and Use Committee of the University of Washington and the University of Barcelona. Male Wistar rats, 2 months old on arrival, were purchased from Charles River [Raleigh, NC (for rats used at University of Washington); Les Oncines, France (for rats used at University of Barcelona)] and housed individually in transparent polycarbonate cages (20 × 25 × 22 cm) fitted with infrared beam detectors. Approximately one-half of the animals for temperature and activity recordings were studied at University of Barcelona and one-half at University of Washington. Given that no significant differences were seen between the two groups, the data were pooled. All sleep studies were performed at University of Washington.
Forced desynchronized animals were maintained under a symmetrical LD cycle of 11 h of light and 11 h of dark. Control animals were maintained under a 24-h symmetrical LD cycle. Light consisted of cool white light (100–300 lux) and darkness of dim red light (<1 lux). Locomotor activity was continuously monitored by means of a system with two crossed infrared beams and, after 10–15 days, once the rhythms were clearly visible, rats were anesthetized during the light phase of the LD cycle and implanted with i.p. temperature sensors (Thermochrone iButtons; Dallas Semiconductor, Dallas, TX) (29). Some of the rats were implanted with ECoG electrodes for sleep recording (see below). Temperature and motor activity were simultaneously detected and recorded in 15-min data bins.
ECoG electrodes were placed over the frontal and parietal cortices as previously described (30). The leads from the ECoG electrodes were routed to a Teflon pedestal, which was attached to the skull with dental cement. Animals were returned to their home cages where locomotor activity was monitored through infrared beam interruptions. After 5 days of recovery, the ECoG electrodes were connected to an amplifier through a wire attached to a swivel. ECoG signals (128-Hz sampling rate) were amplified, passed through filters, and digitized. Recordings lasted a minimum of 14 days and a maximum of 30 days.
Analysis of CBT and Locomotor Activity.
CBT and activity were plotted as actograms to visualize rhythmic components. The χ2 periodogram (31) was used to estimate the period of statistically significant oscillations in the circadian range.
To study the relationship between CBT and locomotor activity, within each animal temperature data points were expressed as deviation from the mean and activity data were transformed to a percentile of the maximum (10 levels of activity). For rats under a 22-h LD cycle, separate data sets were generated for each of the four phases outlined in Fig. 1: double day, double night, T22 h day–τ>24 h subjective night, and T22 h night–τ>24 h subjective day. For control (24-h LD cycle) rats, separate data sets were generated for the light and the dark phases. CBT (as a deviation from the individual's mean) was analyzed as a function of the different levels of activity (as a percentile of the individual's maximum activity) for each of these phases separately. Statistical analysis was carried out by means of general linear models with repeated measures to study the effect of the stages on body temperature, considering the levels of activity as an intersubject factor.
Analysis of Sleep Stages.
The vigilance states of wakefulness, SWS, and PS were determined off-line in 10-s epochs by an operator blind to the circadian phase at which the recording was taken. Wakefulness was characterized by fast low-amplitude ECoG waves in coincidence with locomotor activity recorded through infrared beams. SWS was associated with slow high-amplitude ECoG waves and lack of locomotor activity. In contrast, PS is characterized by fast low-amplitude ECoG waves, appearance of theta ECoG (visualized through a fast Fourier transform), and lack of locomotor activity. Slow-wave activity (ECoG frequencies of 0.5–4.0 Hz) and theta (ECoG frequencies of 4.0–8.0 Hz) powers were calculated through fast Fourier transform, normalizing to the power calculated for SWS and PS episodes, respectively. The percentage of time spent in each state was calculated for every 10 min. The percentage data were plotted as actograms to visualize rhythmic components. The χ2 periodogram was used to estimate the period of statistically significant oscillations in the circadian range.
Supplementary Material
Acknowledgments
We thank R. Refinetti for advice with temperature data analysis and D.-J. Dijk, M. González, B. Schwartz, and C. Wotus for comments on the manuscript. This study was supported by National Institutes of Health Grant R01 MH075016 and University of Washington Department of Biology start-up funds (to H.O.d.l.I.), the Ministerio de Ciencia y Tecnología, Spain (BFI 2003-03489), and a travel grant from Generalitat de Catalunya (AGAUR 2005-BE 00042) (to T.C.).
Abbreviations
- SCN
suprachiasmatic nucleus
- CBT
core body temperature
- PS
paradoxical sleep
- SWS
slow-wave sleep
- LD
light–dark
- VL
ventrolateral
- DM
dorsomedial
- ECoG
electrocorticographic.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702424104/DC1.
References
- 1.Moore RY, Leak RK. In: Handbook of Behavioral Neurobiology: Circadian Clocks. Takahashi JS, Turek F, Moore RY, editors. New York: Kluwer Academic/Plenum Publishers; 2001. pp. 141–179. [Google Scholar]
- 2.Klein DC, Moore RY, Reppert SM. Suprachiasmatic Nucleus. The Mind's Clock. New York: Oxford Univ Press; 1991. [Google Scholar]
- 3.Schibler U, Ripperger J, Brown SA. J Biol Rhythms. 2003;18:250–260. doi: 10.1177/0748730403018003007. [DOI] [PubMed] [Google Scholar]
- 4.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, et al. Proc Natl Acad Sci USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mistlberger RE. Brain Res Rev. 2005;49:429–454. doi: 10.1016/j.brainresrev.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Czeisler CA, Dijk DJ. In: Handbook of Behavioral Neurobiology: Circadian Clocks. Takahashi JS, Turek FW, Moore RY, editors. New York: Kluwer Academic/Plenum Publishers; 2001. pp. 531–569. [Google Scholar]
- 7.Lavie P. Annu Rev Psychol. 2001;52:277–303. doi: 10.1146/annurev.psych.52.1.277. [DOI] [PubMed] [Google Scholar]
- 8.Campuzano A, Vilaplana J, Cambras T, Diez-Noguera A. Physiol Behav. 1998;63:171–176. doi: 10.1016/s0031-9384(97)00416-2. [DOI] [PubMed] [Google Scholar]
- 9.de la Iglesia HO, Cambras T, Schwartz WJ, Diez-Noguera A. Curr Biol. 2004;14:796–800. doi: 10.1016/j.cub.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 10.Mrosovsky N. Chronobiol Int. 1999;16:415–429. doi: 10.3109/07420529908998717. [DOI] [PubMed] [Google Scholar]
- 11.Hiddinga AE, Beersma DG, Van den Hoofdakker RH. J Sleep Res. 1997;6:156–163. doi: 10.1046/j.1365-2869.1997.00047.x. [DOI] [PubMed] [Google Scholar]
- 12.Dijk DJ, Czeisler CA. J Neurosci. 1995;15:3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Refinetti R. Am J Physiol. 1999;277:R1493–R1500. doi: 10.1152/ajpregu.1999.277.5.R1493. [DOI] [PubMed] [Google Scholar]
- 14.Refinetti R. Physiol Behav. 1994;56:829–831. doi: 10.1016/0031-9384(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 15.Scheer FA, Pirovano C, Van Someren EJ, Buijs RM. Neuroscience. 2005;132:465–477. doi: 10.1016/j.neuroscience.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Cambras T, Chiesa J, Araujo J, Diez-Noguera A. J Biol Rhythms. 2004;19:216–225. doi: 10.1177/0748730404264201. [DOI] [PubMed] [Google Scholar]
- 17.Czeisler CA, Weitzman E, Moore-Ede MC, Zimmerman JC, Knauer RS. Science. 1980;210:1264–1267. doi: 10.1126/science.7434029. [DOI] [PubMed] [Google Scholar]
- 18.Wever RA. Circadian System of Man: Results of Experiments Under Temporal Isolation. New York: Springer-Verlag; 1979. [Google Scholar]
- 19.Czeisler CA, Zimmerman JC, Ronda JM, Moore-Ede MC, Weitzman ED. Sleep. 1980;2:329–346. [PubMed] [Google Scholar]
- 20.Saper CB, Scammell TE, Lu J. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 21.Moore RY, Speh JC, Leak RK. Cell Tissue Res. 2002;309:89–98. doi: 10.1007/s00441-002-0575-2. [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H. Science. 2003;302:1408–1412. doi: 10.1126/science.1089287. [DOI] [PubMed] [Google Scholar]
- 23.Yan L, Okamura H. Eur J Neurosci. 2002;15:1153–1162. doi: 10.1046/j.1460-9568.2002.01955.x. [DOI] [PubMed] [Google Scholar]
- 24.Albus H, Vansteensel MJ, Michel S, Block GD, Meijer JH. Curr Biol. 2005;15:886–893. doi: 10.1016/j.cub.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 25.Nagano M, Adachi A, Nakahama K, Nakamura T, Tamada M, Meyer-Bernstein EL, Sehgal A, Shigeyoshi Y. J Neurosci. 2003;23:6141–6151. doi: 10.1523/JNEUROSCI.23-14-06141.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura W, Yamazaki S, Takasu NN, Mishima K, Block GD. J Neurosci. 2005;25:5481–5487. doi: 10.1523/JNEUROSCI.0889-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antle MC, Silver R. Trends Neurosci. 2005;28:145–151. doi: 10.1016/j.tins.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Waterhouse JM, Minors DS, Åkerstedt T, Reilly T, Atkinson G. In: Handbook of Behavioral Neurobiology: Circadian Clocks. Takahashi JS, Turek FW, Moore RY, editors. New York: Kluwer Academic/Plenum Publishers; 2001. pp. 571–601. [Google Scholar]
- 29.Davidson AJ, Aujard F, London B, Menaker M, Block GD. J Biol Rhythms. 2003;18:430–432. doi: 10.1177/0748730403256066. [DOI] [PubMed] [Google Scholar]
- 30.Kubota T, Kushikata T, Fang J, Krueger JM. Am J Physiol. 2000;279:R404–R413. doi: 10.1152/ajpregu.2000.279.2.R404. [DOI] [PubMed] [Google Scholar]
- 31.Sokolove PG, Bushell WN. J Theor Biol. 1978;72:131–160. doi: 10.1016/0022-5193(78)90022-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.