Abstract
Toll-like receptors (TLRs) and NK receptors are the two most important receptor families in innate immunity. Although it has been observed that TLR signaling can induce or up-regulate the expression of the ligands for stimulatory NK receptors on monocytes or muscle cells, there is not yet a report indicating whether TLR signaling can break down self-tolerance through NK receptors. The present work reports that TLR3 signaling by polyinosinic–polycytidylic acid stimulation induces intestinal epithelial cells (IECs) to express retinoic acid early inducible-1 (a ligand for NKG2D) and to induce NKG2D expression on CD8αα intestinal intraepithelial lymphocytes by IL-15 derived from TLR3-activated IECs. The blockade of interaction between NKG2D and Rae1 inhibits the cytotoxicity of intraepithelial lymphocytes against IECs in a cell–cell contact-dependent manner and therefore alleviates polyinosinic–polycytidylic acid-induced epithelial destruction and acute mucosal injury of small intestine. These results demonstrate that TLR signaling induces tissue injury through the NKG2D pathway, suggesting that TLR signaling may break down self-tolerance through induction of abnormal expression of ligands for stimulatory NK receptors.
Keywords: intestinal injury, NK receptor, Rae1
Toll-like receptors (TLRs) are one of the most important types of receptors in innate immunity. TLRs generally recognize foreign molecular patterns from bacterial cell-wall structures or viral RNA intermediates and play a critical role in pathogen recognition and host defense (1). However, inappropriate TLR signaling can contribute to loss of tolerance, resulting in tissue injury and even autoimmune diseases (2–8). It is thought that TLR signaling induces autoimmune tissue injury possibly by promoting the production of proinflammatory cytokines or modulating the function of dendritic cells (8–11). Indeed, our recent study has shown that abnormal TLR3 signaling breaks down the mucosal homeostasis through an IL-15-dependent manner (12). However, the detailed mechanisms of how TLR signaling breaks down self-tolerance remain to be further investigated.
NK receptors, including inhibitory receptors and stimulatory receptors, are another type of important receptor in innate immune system. Normally, the activity of NK cells is controlled by inhibitory receptors that recognize ligands for the inhibitory receptors (mostly MHC class I molecules) expressed on normal cells. If the expression of ligands for the inhibitory receptors is diminished or expression of the ligands for the stimulatory receptors is increased, normal cells will become the targets for NK cell-mediated killing (13). NKG2D is the best characterized stimulatory NK receptor until now and recognizes autologous ligands that are up-regulated by transformation, infection, or cell stress (14). Because NKG2D is expressed on NK cells and T cells (14), the inappropriate expression of ligands on normal cells may lead to the breakdown of tolerance of NK and/or T cells to self-parenchyma cells. Indeed, the nonspecific induction or inappropriate expression of NKG2D ligands has been reported to be involved in the initiation or exacerbation of autoimmune diseases such as rheumatoid arthritis, celiac disease, or autoimmune diabetes (15–18), although the underlying mechanisms of the inappropriate expression of NKG2D ligands remain unclear. In mice, the expression of Rae1 (retinoic acid early inducible-1), a family of proteins that have been identified as high-affinity ligands for NKG2D, is strictly regulated in normal cells and minimally detected on healthy adult tissues (19–21). Here, we report that TLR3 signaling induces intestinal epithelial cells (IECs) to express Rae1, which mediate epithelial destruction and mucosal injury by interacting with NKG2D expressed on intestinal intraepithelial lymphocytes (IELs). These results suggest that TLR signaling may break down self-tolerance through induction of abnormal expression of ligands for stimulatory NK receptors on parenchyma cells.
Results
Cell–Cell Contact Is Necessary in the Killing of IECs by Polyinosinic–Polycytidylic Acid [poly(I:C)]-Treated IELs.
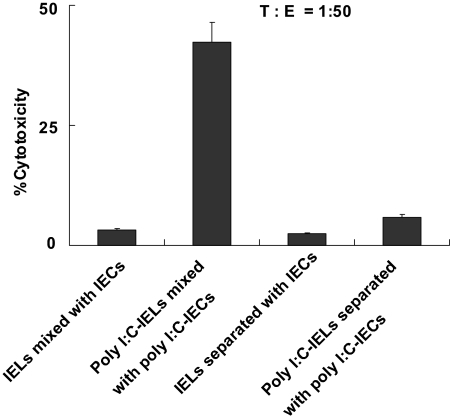
Our recent study has shown that TLR3 signaling can break down the epithelial homeostasis of the small intestine (12); however, the mechanisms remain unclear. To investigate how TLR3 signaling promotes the loss of tolerance of epithelial cells, we determined whether the cytotoxicity of IELs against IECs is mediated by cell–cell contact. For the 51Cr release assay, we used poly(I:C)-treated IECs as target cells and poly(I:C)-treated IELs as effectors. When effectors and target cells were placed in the same well, a high level of cytotoxicity was observed. However, when effectors and target cells were separated by a membrane, no lysis of the labeled target cells occurred (Fig. 1). These results demonstrate that the killing of IECs by IELs depends on cell–cell contact.
Fig. 1.
The killing of IECs by IELs depends on cell–cell contact. IECs (4 × 104) were incubated for 24 h in the lower compartment of a transwell chamber coated with rat tail collagen (Sigma–Aldrich, St. Louis, MO) and then were stimulated with 100 μg/ml poly(I:C) or PBS for 6 h. IELs were isolated from C57BL/6J mice previously treated with 30 μg/g poly(I:C) or PBS for 6 h and then were placed in the upper compartment or in the same well with IECs. IELs and IECs were used as effectors (E) and target cells (T), respectively, in the 51Cr release assay. Values are shown as means ± SEM from three independent experiments.
NKG2D Expression on CD8αα+ IELs Is Up-Regulated by IEC-Derived IL-15 After poly(I:C) Treatment.
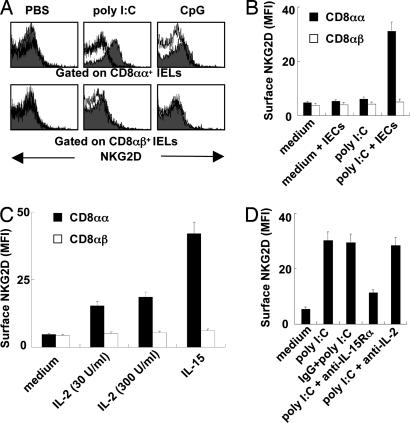
Because cytotoxicity of IELs against IECs depends on cell–cell contact, to more thoroughly investigate the mechanisms of epithelial destruction, we examined the expression of NKG2D on IELs. As shown in Fig. 2A, CD8αα+ IELs or CD8αβ+ IELs did not express NKG2D; however, treatment with poly(I:C) in vivo induced the expression of NKG2D on CD8αα+ IELs but not on CD8αβ+ IELs. Because our recent results have shown that the enhanced cytotoxicity of IELs induced by poly(I:C) depends on IEC-derived IL-15 (12), we investigated whether NKG2D expression on IELs induced by poly(I:C) needs the presence of IECs. As shown in Fig. 2B, elevated NKG2D expression on IELs stimulated by poly(I:C) in vitro depended on the presence of IECs. We also found that IL-15 could stimulate CD8αα+ IELs to express NKG2D in vitro but had little effect on CD8αβ+ IELs (Fig. 2C). Moreover, anti-IL-15Rα neutralizing Ab abrogated the increase of NKG2D expression on CD8αα+ IELs induced by poly(I:C) in the presence of IECs (Fig. 2D), whereas anti-IL-2 neutralizing Ab did not. These results demonstrate that IL-15, which is derived from poly(I:C)-treated IECs, induces the expression of NKG2D on CD8αα+ IELs.
Fig. 2.
Treatment with poly(I:C) increases NKG2D expression on CD8αα+ IELs in an IEC-derived IL-15-dependent manner. (A) IELs were isolated from 30 μg/g poly(I:C)- or CpG-treated C57BL/6J mice at 6 h and then subjected to flow- cytometry analysis. The expression of NKG2D on CD8αα+ IELs or CD8αβ+ IELs is shown. Cells stained with isotype-matched control Ig demonstrated the specificity of mAb binding (white histogram). (B) Equal numbers of IECs were added to IEL cultures in the presence of 100 μg/ml poly(I:C). The IELs were incubated for 24 h, and the surface expression of NKG2D on CD8αα+ IELs or CD8αβ+ IELs was analyzed by flow cytometry. MFI, mean fluorescence intensity. (C) IELs cultured in the presence of IL-2 or IL-15. The surface expression NKG2D on CD8αα+ IELs or CD8αβ+ IELs was analyzed by flow cytometry at 48 h. (D) poly(I:C) (100 μg/ml) was added to the cocultures of IELs and equal numbers of IECs in the presence of 20 μg/ml anti-IL-2 or 20 μg/ml anti-IL-15Rα Ab, which had been added into the cultures 1 h before poly(I:C). IELs were incubated for 24 h, and the surface expression of NKG2D on CD8αα+ IELs was analyzed by flow cytometry. (B–D) Values are shown as means ± SEM from three independent experiments.
Expression of Rae1 on IECs Is Up-Regulated by poly(I:C) Treatment.
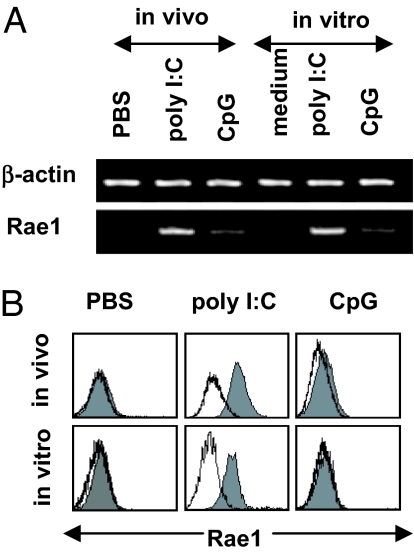
We next investigated whether poly(I:C) induces NKG2D ligands' expression on IECs. RT-PCR was used to detect IEC gene expression of all known NKG2D ligands: H60, Mult1, and Rae1. H60 was not expressed on IECs either before or after poly(I:C) treatment, and Mult1 was constitutively expressed on IECs without changes (data not shown). Rae1 transcripts were not detected in control IECs but were detected in IECs treated with poly(I:C) (Fig. 3A). Flow-cytometry analysis confirmed that poly(I:C) treatment induced Rae1 expression on IECs (Fig. 3B).
Fig. 3.
Treatment with poly(I:C) induces Rae1 expression on IECs. (A) IECs were treated with poly(I:C) in vivo at 5 μg/g for 1.5 h and in vitro at 100 μg/ml for 6 h. The mRNA levels of Rae1 or β-actin on IECs were detected by RT-PCR. (B) IECs were treated with poly(I:C) in vivo at 5 μg/g for 6 h or in vitro at 100 μg/ml for 12 h. The surface expression of Rae1 was analyzed by flow cytometry. Cells stained with isotype-matched control Ig demonstrated the specificity of mAb binding (white histogram).
Blockade of NKG2D–Rae1 Interaction Inhibits the Cytotoxicity of IELs Against IECs and Prevents Mice from dsRNA-Induced Acute Small Intestinal Injury.
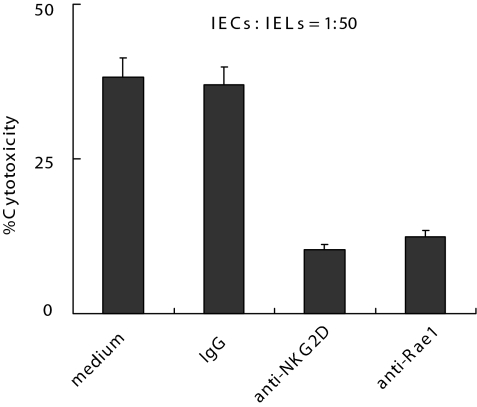
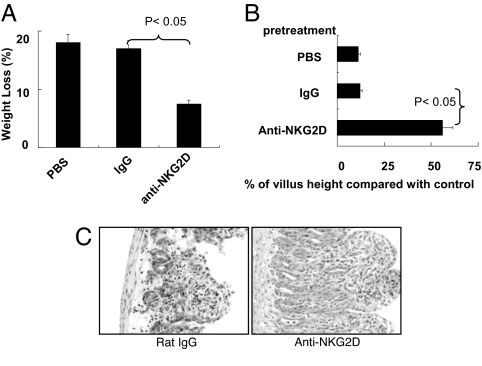
To further investigate whether the interaction between NKG2D and Rae1 is involved in poly(I:C)-induced small intestinal injury, we used anti-Rae1 or anti-NKG2D Ab to block NKG2D–Rae1 interaction. First, we evaluated whether anti-Rae1 or anti-NKG2D Ab could inhibit the cytotoxicity of IELs against IECs. As shown in Fig. 4, blockade of NKG2D-Rae1 interaction in vitro inhibited the cytotoxicity of IELs against primary IECs. Indeed, the blockade also inhibited the cytotoxicity of IELs against YAC-1 cells (data not shown). Second, we examined the effect of anti-NKG2D Ab on poly(I:C)-induced small intestinal injury. As shown in Fig. 5, the blockade of NKG2D–Rae1 interaction in vivo by anti-NKG2D Ab protected mice from the poly(I:C)-induced weight loss (Fig. 5A), villous atrophy (Fig. 5B), and mucosal erosion (Fig. 5C). Taken together, these results indicate that NKG2D–Rae1 interaction is involved in the epithelial destruction in poly(I:C)-induced small intestinal injury.
Fig. 4.
Blockade of NKG2D–Rae1 interaction inhibits the cytotoxicity of IELs against IECs. IECs were stimulated with 100 μg/ml poly(I:C) for 12 h in vitro, and IELs were isolated from C57BL/6J mice treated with 30 μg/g poly(I:C) for 6 h. IELs and IECs were used as target cells and effectors, respectively, in the 51Cr release assay. Anti-NKG2D (20 μg/ml) or anti-Rae1 (20 μg/ml) Ab also was added, respectively. Values are shown as means ± SEM from three independent experiments.
Fig. 5.
Blockade of NKG2D–Rae1 interaction prevents mice from poly(I:C)-induced small intestinal injury. C57BL/6J mice (n = 3) were pretreated with control IgG Ab at 400 μg per mouse or anti-NKG2D Ab at 400 μg per mouse 24 h before poly(I:C) injection. (A) Thirty-six hours after poly(I:C) injection at 30 μg/g, the weight of mice was measured, and the percentage of weight loss (referring to the weight at 0 h) is shown. (B and C) Morphometric analysis of villous height (B) and representative photographs for H&E-stained, paraffin-embedded sections (C) are shown at 12 h after 30 μg/g poly(I:C) injection. (Magnification: ×200.) Values in A and B are shown as means ± SEM from three independent experiments.
Discussion
The results presented here demonstrate that NKG2D–Rae1 interaction is involved in the IEL-mediated epithelial destruction by inappropriate TLR3 signaling and provide evidence that TLR signaling may promote the loss of tolerance through a NKG2D-dependent manner. On the one hand, poly(I:C) induces CD8αα IELs to express NKG2D through IEC-derived IL-15. On the other hand, TLR3 signaling stimulates IECs to express Rae1, the ligands of NKG2D. Similarly, it has also been reported that TLR signaling can induce the expression of ligands for NKG2D receptor on macrophage or muscle cells (22, 23), although the in vivo relevance of this observation has not been investigated. Overall, these results suggest that TLR signaling can lead to the aberrant expression of NKG2D ligands on target cells. In fact, TLR signaling also up-regulates the expression of ligands for other stimulatory NK receptors. A recent paper demonstrates that TLR activation up-regulates the expression of AICL, a ligand of NKp80, on monocytes (24). These results suggest that TLR signaling can break down the self-tolerance by inducing the abnormal expression of ligands for stimulatory NK receptors.
Autoimmunity is obviously caused by the loss of self-tolerance. An increasing number of reports suggests that microbial infection might be involved in the triggering of autoimmune tissue injury (25); however, the mechanisms remain unclear. The correlation of TLRs and NK receptors might provide an explanation for how microbial infection contributes to the initiation or exacerbation of autoimmune disease; TLRs, which generally recognize molecular patterns from bacteria or viruses, can break down self-tolerance by inducing the abnormal expression of ligands for stimulatory NK receptors and promote target cells to become more susceptible to the attack of NK cells or CD8 T cells, which finally results in the progression of autoimmune disease.
NKG2D is a lectin-like activating receptor originally identified in NK cells. However, in our previous paper, we excluded the role of NK cells in the small intestinal injury induced by poly(I:C) (12). In fact, NKG2D expression also was observed on CD8+ T cells and functions as a costimulatory receptor (26–29). In humans, NKG2D is expressed by almost all human CD8+ T cells, γδ T cells, and IELs (27, 30, 31). In mice, NKG2D is expressed on activated but not resting CD8+ T cells and almost all γδ T cells in the skin (29). In this article, we show that NKG2D is minimally expressed on IELs; however, treatment with IL-15 can up-regulate the expression of NKG2D on CD8αα IELs but not on CD8αβ IELs. The different expression of NKG2D might explain why the effectors, which induce the epithelial destruction and small intestinal injury, are CD8αα IELs.
Materials and Methods
Mice.
Male C57BL/6 mice (6–8 weeks old) were obtained from the Shanghai Experimental Animal Center (Shanghai, China) and maintained under specific pathogen-free conditions. The handling of mice and experimental procedures were conducted in accordance with guidelines for experimental animals from the University of Science and Technology of China.
Reagents.
Monoclonal anti-mouse Rae1 blocking Ab (clone 199205, rat IgG2b isotype) and anti-mouse IL-15Rα neutralizing Ab (32) were purchased from R&D Systems (Minneapolis, MN). Anti-NKG2D (CX5) (17, 33) or anti-IL-2 (JES6–1A12) (34) was purchased from eBioscience (San Diego, CA). RhIL-2 and rhIL-15 were purchased from PeproTech (London, U.K.).
Injection Protocol.
poly(I:C) sodium (Sigma–Aldrich) was dissolved in pathogen-free saline and injected i.p. at 30 μg/g. The class B, 1018 CpG oligodeoxynucleotides (5′-TGACTGTGAACGTTCGAGATGA-3′; Shanghai Sangong Biological Engineering and Technology and Service, Shanghai, China) were injected i.p. at 30 μg/g.
IEL Preparation and IEC Preparation and Culture.
IELs were isolated by Percoll gradient centrifugation as described in our previous study (12). IEC preparation and culture were performed as described in our previous study (12).
51Cr Release Assay.
Cytotoxicity was assessed by a 51Cr release assay as described in our previous study (12). The percentage of target cell lysis was calculated by using the following equation: % cytotoxicity = [(experimental release cpm − spontaneous release cpm) /(maximal release cpm − spontaneous release cpm)] × 100.
RT-PCR.
Gene expression was determined by RT-PCR as described previously (12). The primer sequences for Rae1 were 5′-GCTGTTGCCACAGTCACATC-3′ (sense) and 5′-CCTGGGTCACCTGAAGTCAT-3′ (antisense).
H&E Staining.
For histology, tissue from the small intestine was fixed in 10% neutral-buffered formalin and embedded in paraffin. Five-micrometer sections were affixed to slides, deparaffinized, and stained with H&E. Morphological changes in the stained sections were examined under light microscopy.
Flow-Cytometry Analysis.
Cellular phenotypes were analyzed by incubating cells with monoclonal antibodies conjugated to florescent labels. Double and triple immunofluorescence analyses were conducted. The monoclonal antibodies used included FITC-, phycoerythrin-, or Cy5-conjugated anti-CD3, anti-CD8α, anti-CD8β, anti-NK1.1 (PK136), anti-NKG2D (CX5), anti-Mac-1 (M1/70), anti-Rae1 (CX1), and anti-cytokeratin (PCK-26; Sigma–Aldrich). To prevent nonspecific binding, respective isotype antibodies were used as controls. Images of labeled cells were acquired by FACSCalibur and analyzed with WinMDI2.8 software.
Statistical Analysis.
Data are expressed as means ± SEM. To compare values obtained from three or more groups, one-way ANOVA was used followed by Tukey's post hoc test. To compare values obtained from two groups, Student's t tests were performed. Results were considered statistically significant when P ≤ 0.05.
Acknowledgments
We thank Xiaodong Zheng, Qun Jiang, and Huaxing Wei (Z.T.'s laboratory) for technical assistance. This work was supported by Natural Science Foundation of China Grants 30630059, 30528007, 30570819, 30571695, and 30500467, and by Ministry of Science and Technology of China 973 Basic Science Projects 2006CB504300 and 2006CB806504.
Abbreviations
- poly(I:C)
polyinosinic–polycytidylic acid
- IEL
intestinal intraepithelial lymphocyte
- IEC
intestinal epithelial cell
- TLR
Toll-like receptor.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Janeway CA, Jr, Medzhitov R. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Rakoff-Nahoum S, Hao L, Medzhitov R. Immunity. 2006;25:319–329. doi: 10.1016/j.immuni.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, Fort M, Hershberg RM. J Clin Invest. 2004;113:1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJ, Kirschning CJ, Akira S, van der Poll T, Weening JJ, Florquin S. J Clin Invest. 2005;115:2894–2903. doi: 10.1172/JCI22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang KS, Recher M, Junt T, Navarini AA, Harris NL, Freigang S, Odermatt B, Conrad C, Ittner LM, Bauer S, et al. Nat Med. 2005;11:138–145. doi: 10.1038/nm1176. [DOI] [PubMed] [Google Scholar]
- 6.Lang KS, Georgiev P, Recher M, Navarini AA, Bergthaler A, Heikenwalder M, Harris NL, Junt T, Odermatt B, Clavien PA, et al. J Clin Invest. 2006;116:2456–2463. doi: 10.1172/JCI28349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng GM, Liu ZQ, Tarkowski A. J Immunol. 2001;167:4616–4626. doi: 10.4049/jimmunol.167.8.4616. [DOI] [PubMed] [Google Scholar]
- 8.Anders HJ, Vielhauer V, Eis V, Linde Y, Kretzler M, Perez de Lema G, Strutz F, Bauer S, Rutz M, Wagner H, et al. FASEB J. 2004;18:534–536. doi: 10.1096/fj.03-0646fje. [DOI] [PubMed] [Google Scholar]
- 9.Anders HJ, Zecher D, Pawar RD, Patole PS. Arthritis Res Ther. 2005;7:215–224. doi: 10.1186/ar1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eriksson U, Ricci R, Hunziker L, Kurrer MO, Oudit GY, Watts TH, Sonderegger I, Bachmaier K, Kopf M, Penninger JM. Nat Med. 2003;9:1484–1490. doi: 10.1038/nm960. [DOI] [PubMed] [Google Scholar]
- 11.Uematsu S, Akira S. Expert Opin Biol Ther. 2006;6:203–214. doi: 10.1517/14712598.6.3.203. [DOI] [PubMed] [Google Scholar]
- 12.Zhou R, Wei H, Sun R, Tian Z. J Immunol. 2006;178:4548–4556. doi: 10.4049/jimmunol.178.7.4548. [DOI] [PubMed] [Google Scholar]
- 13.Lanier LL. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 14.Raulet DH. Nat Rev Immunol. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 15.Hue S, Mention JJ, Monteiro RC, Zhang S, Cellier C, Schmitz J, Verkarre V, Fodil N, Bahram S, Cerf-Bensussan N, et al. Immunity. 2004;21:367–377. doi: 10.1016/j.immuni.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Meresse B, Chen Z, Ciszewski C, Tretiakova M, Bhagat G, Krausz TN, Raulet DH, Lanier LL, Groh V, Spies T, et al. Immunity. 2004;21:357–366. doi: 10.1016/j.immuni.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 17.Ogasawara K, Hamerman JA, Ehrlich LR, Bour-Jordan H, Santamaria P, Bluestone JA, Lanier LL. Immunity. 2004;20:757–767. doi: 10.1016/j.immuni.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Groh V, Bruhl A, El-Gabalawy H, Nelson JL, Spies T. Proc Natl Acad Sci USA. 2003;100:9452–9457. doi: 10.1073/pnas.1632807100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerwenka A, Bakker AB, McClanahan T, Wagner J, Wu J, Phillips JH, Lanier LL. Immunity. 2000;12:721–727. doi: 10.1016/s1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- 20.Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Nat Immunol. 2000;1:119–126. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- 21.Nomura M, Zou Z, Joh T, Takihara Y, Matsuda Y, Shimada K. J Biochem (Tokyo) 1996;120:987–995. doi: 10.1093/oxfordjournals.jbchem.a021517. [DOI] [PubMed] [Google Scholar]
- 22.Hamerman JA, Ogasawara K, Lanier LL. J Immunol. 2004;172:2001–2005. doi: 10.4049/jimmunol.172.4.2001. [DOI] [PubMed] [Google Scholar]
- 23.Schreiner B, Voss J, Wischhusen J, Dombrowski Y, Steinle A, Lochmuller H, Dalakas M, Melms A, Wiendl H. FASEB J. 2006;20:118–120. doi: 10.1096/fj.05-4342fje. [DOI] [PubMed] [Google Scholar]
- 24.Welte S, Kuttruff S, Waldhauer I, Steinle A. Nat Immunol. 2006;7:1334–1342. doi: 10.1038/ni1402. [DOI] [PubMed] [Google Scholar]
- 25.Christen U, von Herrath MG. J Immunol. 2005;174:7481–7486. doi: 10.4049/jimmunol.174.12.7481. [DOI] [PubMed] [Google Scholar]
- 26.Wu J, Song Y, Bakker AB, Bauer S, Spies T, Lanier LL, Phillips JH. Science. 1999;285:730–732. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- 27.Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, Spies T. Nat Immunol. 2001;2:255–260. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- 28.Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. Immunity. 2002;17:19–29. doi: 10.1016/s1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- 29.Diefenbach A, Tomasello E, Lucas M, Jamieson AM, Hsia JK, Vivier E, Raulet DH. Nat Immunol. 2002;3:1142–1149. doi: 10.1038/ni858. [DOI] [PubMed] [Google Scholar]
- 30.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Science. 1999;285:727–729. [PubMed] [Google Scholar]
- 31.Roberts AI, Lee L, Schwarz E, Groh V, Spies T, Ebert EC, Jabri B. J Immunol. 2001;167:5527–5530. doi: 10.4049/jimmunol.167.10.5527. [DOI] [PubMed] [Google Scholar]
- 32.Gould MP, Greene JA, Bhoj V, DeVecchio JL, Heinzel FP. J Immunol. 2004;172:1754–1762. doi: 10.4049/jimmunol.172.3.1754. [DOI] [PubMed] [Google Scholar]
- 33.Zhang T, Lemoi BA, Sentman CL. Blood. 2005;106:1544–1551. doi: 10.1182/blood-2004-11-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Omori M, Ziegler S. J Immunol. 2007;178:1396–1404. doi: 10.4049/jimmunol.178.3.1396. [DOI] [PubMed] [Google Scholar]