Abstract
A consideration of the morphological aspects of the earliest modern humans in Europe (more than ≈33,000 B.P.) and the subsequent Gravettian human remains indicates that they possess an anatomical pattern congruent with the autapomorphic (derived) morphology of the earliest (Middle Paleolithic) African modern humans. However, they exhibit a variable suite of features that are either distinctive Neandertal traits and/or plesiomorphic (ancestral) aspects that had been lost among the African Middle Paleolithic modern humans. These features include aspects of neurocranial shape, basicranial external morphology, mandibular ramal and symphyseal form, dental morphology and size, and anteroposterior dental proportions, as well as aspects of the clavicles, scapulae, metacarpals, and appendicular proportions. The ubiquitous and variable presence of these morphological features in the European earlier modern human samples can only be parsimoniously explained as a product of modest levels of assimilation of Neandertals into early modern human populations as the latter dispersed across Europe. This interpretation is in agreement with current analyses of recent and past human molecular data.
Keywords: crania, dentition, Late Pleistocene, postcrania
The evolutionary fate of the Neandertals has preoccupied paleoanthropologists with varying degrees of intensity for approximately a century, emerging with the human paleontological discoveries of the early 20th century and the resultant syntheses. Although present throughout the 20th century, considerations of the fate of the Neandertals have intensified in the past quarter-century starting with the paleontological emergence of Out-of-Africa models of modern human origins (1, 2) that combined the early appearance of modern human anatomy in Africa (2, 3) with the presence of equatorial anatomical patterns among the earliest modern Europeans (1). These interpretations were joined half a decade later by inferences from extant human molecular analyses, which rapidly polarized the field into two opposing models, best termed the Replacement (Out-of-Africa with total replacement of all other indigenous human populations) and Regional Continuity (variable regional transitions to modern human morphology among genetically interconnected human populations).†
After two decades of debate, it is now recognized that these polarized models, Replacement (sensu stricto) vs. Regional Continuity (sensu stricto) are intellectually dead. Repeated attempts to refute one or the other model have shown that it is easy, on a global basis, to refute both scenarios with paleontological and/or extant human biological (both anatomical and molecular) data. It is time to move on from these models, and, particularly in the past half-decade, the emergent consensus model of modern human emergence is one of Out-of-Africa with temporally and geographically varying degrees of absorption of regional late archaic populations [the Assimilation Model (10)]. The issue is no longer whether human populations outside of the African homeland of modern humans contributed to subsequent modern human populations. Indeed, given the interfertility of sibling species separated for <1–2 million years (11, 12), the Assimilation Model should be the null hypothesis for modern human phylogenetic emergence. Nonetheless, the issue as to how much assimilation occurred remains, with interpretations ranging from trivial amounts to complete population blending.
In this context, uncertainties continue regarding the phylogenetic position of the western Eurasian Neandertals, promoting more and more refined analyses of their remains. However, resolution of this issue lies more with their potential issue, the early modern Europeans. And despite steadily growing numbers of samples of late archaic and especially early modern human fossils elsewhere in the Old World (7, 13, 14), it is principally within Europe that the best fossil record exists to evaluate what happened, in at least one region, when early modern humans dispersed across Eurasia.
The Relevant Fossils
To evaluate human reproductive patterns when indispersing modern humans met indigenous Neandertal populations in Europe, it is necessary to establish the currently known potential ancestral populations. It is only from these lineages that the European early modern humans (EEMHs) are likely to have acquired their phylogenetically informative characteristics.
The first sample comprises the oxygen isotope stage 4 and 3 Neandertals, established as a regional lineage in western Eurasia since the Middle Pleistocene. They occupied all of Mediterranean Europe and much of Europe north of the Alps and Balkans until at least 42–43 thousand calendar years before the present (ka B.P.), possibly persisting later in pockets of central and northwestern Europe but remaining throughout most of Iberia until ≈35 ka B.P. (all dates in calendar years B.P.). They are also known from southwestern Asia and eastward into central Asia.
Second are the east and northeast African earliest modern humans, currently known principally from the sites of Aduma, Bouri, Haua Fteah, Herto, and Omo-Kibish, and dating between ≈75 to perhaps in excess of 160 ka B.P. They are joined by the Qafzeh and Skhul samples, largely if not exclusively dating to between 80 and 100 ka B.P. in extreme southwestern Asia. Multiple lines of evidence (15, 16) indicate that the Qafzeh–Skhul sample represents a temporary northward expansion of these earliest modern humans into that region, after which they were replaced by Neandertal populations dispersing southward. This combined sample is referred to as the Middle Paleolithic modern humans (MPMHs).
Two other samples of Late Pleistocene remains are sometimes considered relevant; they are not. The northwest African Aterian remains (principally from Dar-es-Soltane and Témara) are regional late archaic humans, who present a complex mosaic of archaic and possibly derived modern human characteristics and may be too recent to be pertinent (7). The southern African Middle Stone Age remains from Blombos, Die Kelders, Klasies River Mouth, Mumbwa, Pinnacle Point, and Sea Harvest present few distinctly modern human features (small teeth do not so qualify), have a series of archaic aspects of the cranium, mandible, dentition, and postcranium, and may represent the product of admixture between regional late archaic humans and southward dispersing modern humans after ≈100 ka B.P. (17–19). Hofmeyr 1 (13) is younger than the earliest EEMHs and therefore cannot be ancestral to them.
The only other directly relevant specimen is Nazlet Khater 2, from ≈42 ka B.P. in Egypt (20). Approximately contemporaneous with the earliest EEMHs (21), it may represent the morphology of modern humans dispersing out of Africa after ≈50 ka B.P. However, in some features it is more archaic than the MPMHs, which raises questions as to the degree to which its ancestry was purely from the MPMHs and therefore whether it represents the ancestral modern human morphology.
The primary sample of analysis consists of the EEMHs, those before ≈33 ka B.P. and therefore predating the Gravettian (or Middle Upper Paleolithic) populations of Europe. As a result of an ongoing cleansing of the fossil record through direct radiometric dating, a series of obviously modern, and in fact Late Upper Paleolithic or Holocene, human remains have been removed from consideration (7). This cleansing has helped to dilute the impression that the earliest modern humans in Europe were just like recent European populations. The resultant sample, temporally secured through direct dating and/or careful excavation, consists of specimens from the sites of Brassempouy (22), Cioclovina (23), Mladeč (24–27), Muierii (28), Oase (21, 29), Les Rois (30), and La Quina Aval (31).
Given the modest size of the EEMH sample and the potential for evidence of diverse ancestry to persist in subsequent European populations, the more abundant and complete human remains from the European Gravettian are also considered. Given their often elaborate burials, these remains are known from sites spanning Europe (32).
The Relevant Framework
It is assumed that the EEMHs were derived principally from the MPMHs, expanding and dispersing through southwestern Asia and then westward across Europe subsequent to at least ≈41 ka B.P. (the date of the oldest EEMH, Oase 1). This hypothesis is supported by the first appearance of a long list of autapomorphic modern human character states in the MPMH sample (33) and their persistence in the EEMH sample. Among others, these EEMH autapomorphic traits include absence of a supraorbital torus, distinct canine fossae, narrow nasal apertures, chisel-shaped maxillary incisors, expanded parietal arcs, prominent parietal bosses, laterally bulbous mastoid processes, projecting mentum osseum, narrow mandibular corpus, marked gluteal buttress, pilastric femoral diaphysis, and angular tibial and fibular diaphyses. In addition, their nasal aperture inferior margins and the body proportions inferred biomechanically from femoral diaphyseal proportions (15, 27–29) indicate evolutionarily recent tropical ancestry, similar to that seen in the Qafzeh–Skhul sample (15, 16).
The abundant autapomorphic modern human characteristics in the EEMH sample are therefore inferred to have come from that MPMH lineage. The question is the extent to which they had productive reproductive interactions with the Neandertal populations. Evidence for such encounters should consist principally of autapomorphic Neandertal characteristics in the EEMH or subsequent Gravettian samples. In addition, if any of the EEMHs exhibit plesiomorphic traits that had been lost in the MPMHs, then those EEMHs should have acquired the traits from the lineage retaining those otherwise plesiomorphic features, namely, the Neandertals in western Asia and/or Europe.
In this analysis, the issue is not whether these EEMHs exhibit a suite of derived characteristics of modern humanity; by definition and appellation they do. Focusing on such traits obscures the real question. Nor is it relevant whether “archaic” and “Neandertal” features present in the EEMHs appear in more recent human populations, either Late Pleistocene or Holocene; one cannot inherit from one's descendants.
In this analysis, the traits chosen have been evaluated, to the extent possible given their paleontological nature, to be epigenetic (sensu ref. 34) and independent. Some are likely to be indirect reflections of underlying epigenetic traits or developmental processes, and most are likely to be multifactorial in their underlying biological bases. However, they should serve, much like haplotypes, as genetic markers. Moreover, unlike extant human DNA, they have real time depth, and, unlike ancient DNA, diagenesis does not affect the perceptibility of alternative character states (cf. ref. 35).
The Morphological Mosaic of EEMHs
There are two aspects of the EEMH neurocranial vault that are unusual relative to the MPMH sample. Both of these character states, frontal flattening and occipital bunning, are the secondary effects of differential cerebral-squamous bone formation growth rates in infancy in the context of varying cranial proportions and are homologous across these Late Pleistocene samples (36, 37).
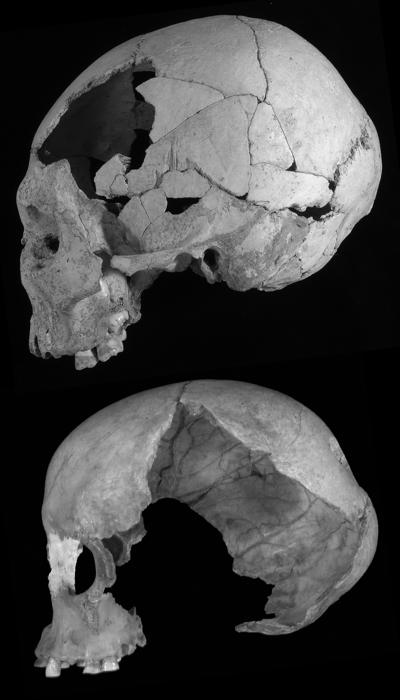
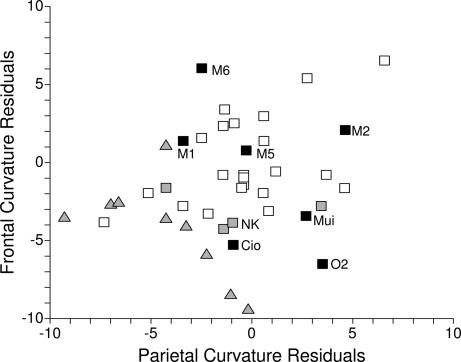
The undeformed Oase 2 cranium (Fig. 1) has parietal bones that exhibit the marked curvature of modern humans but a frontal bone that is exceptionally long and flat, despite the absence of a supraorbital torus or a projecting glabella. A comparison of these curvatures (Fig. 2) places Oase outside of the MPMH and Gravettian ranges of variation and closest in frontal curvature to three Neandertals, Shanidar 1, Spy 1, and La Chapelle-aux-Saints 1. Oase 2 is approached in this aspect by Cioclovina 1 and Muierii 1, who are close to two of the three MPMH specimens, plus Nazlet Khater 2. Moreover, the EEMH sample is highly variable, spanning the complete range of the Late Pleistocene samples, whereas those comparative samples are significantly differently from each other in both frontal and parietal residuals (both ANOVA, P < 0.001).
Fig. 1.
The Oase 2 (Upper) and Muierii 1 (Lower) crania in norma lateralis left.
Fig. 2.
Bivariate plot of linear residuals from the Gravettian least-squares lines of frontal (Fr) and parietal (Pa) arc versus chord (Ch) (FrArc = 1.170 × FrCh – 1.76, r2 = 0.853, n = 22; PaArc = 1.014 × PaCh + 10.27, r2 = 0.871, n = 25, respectively). Samples sizes exceed plotted values because isolated frontal and parietal arcs were used to compute the regression lines. Black squares, EEMHs (Cio: Cioclovina 1; M#, Mladeč; Mui: Muierii 1; O2: Oase 2); gray squares, MPMHs and Nazlet Khater 2 (NK); open squares, Gravettians; gray triangles, Neandertals.
The Muierii 1 (Fig. 1) and Mladeč 3, 5, and 6 crania exhibit prominent occipital buns, with Cioclovina 1, Mladeč 1, and Oase 2 having smaller hemi-buns (38); occipital buns are the product of posterior displacement of the occipital squamous portion above the tentorium cerebelli as a result of differential lambdoid suture bone deposition during infancy (36). Similar occipital buns are ubiquitous among European Neandertals (93.3%, n = 15) but absent from MPMHs (n = 13). Fifty-seven percent of the EEMH crania, therefore, exhibit such reflections of differential neurocranial growth, and the variation is both within (Mladeč) and across site samples.
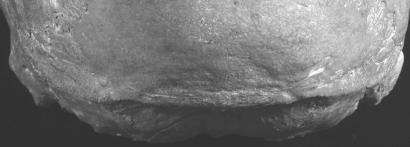
All of the Neandertal occipital bones (n = 23) exhibit the absence of an external occipital protuberance, a nuchal torus limited to the median half of the superior nuchal line, and especially a horizontal oval suprainiac fossa (39). Among the MPMHs (n = 16), 87.5% of the specimens lack any evidence of a suprainiac fossa. The immature Qafzeh 10 has a slight supra-iniac porosity but no fossa (40). Aduma 3 has a suprainiac fossa (41), but it lacks a nuchal torus and has a prominent external occipital protuberance. Therefore, none (0.0%) of the MPMHs have the full Neandertal iniac morphology. The Neandertal complex of features is, however, present in its entirety on Cioclovina 1 (Fig. 3), even though its clear superior nuchal line is on (rather than below) the nuchal torus and the superomedial confluence of the semispinalis capitis insertions produces a small spine below (rather than part of) the superior nuchal lines (23). Mladeč 6 also had a small horizontal oval suprainiac fossa, but its nuchal torus extends across the occipital bone (24). Both of these present a Neandertal morphology within the context of otherwise modern human nuchal regions, and they are distinct from the supranuchal fossa morphology (42) present on Mladeč 5 and common among Gravettian and later crania. The Neandertal occipital morphology appears to be absent from the subsequent Gravettian remains (n = 19), although Lagar Velho 1 has a pattern similar to that of Qafzeh 10 (43).
Fig. 3.
Posterior view of the Cioclovina 1 occipital squamous, illustrating the suprainiac fossa, nuchal torus, and absence of an external occipital protuberance, despite the clear superior nuchal line on, rather than below, the nuchal torus.
Middle Pleistocene archaic Homo crania characteristically exhibit a prominent juxtamastoid eminence along the occipitomastoid suture, one approaching or exceeding the tip of the mastoid process. Among the Neandertals, all sufficiently preserved mature crania exhibit a distinct juxtamastoid eminence, with 78.5% (n = 14) having large ones that approximate the tips of their mastoid processes, mastoid processes that are indistinguishable in porion height from those of modern humans (44). The remainder of the Neandertal crania have eminences that project to about half of their mastoid process heights. Among the MPMHs, 85.7% (n = 7) have no eminence, only a slightly raised line along the suture; the remaining specimen, Qafzeh 3, has a crest that approaches half of the mastoid height, but it is not separated medially from its nuchal plane. Among the EEMHs, Cioclovina 1 and Muierii 2 have no crest, Mladeč 2 and Oase 2 have medium crests, and Mladeč 1 and 5 evince large ones. The Mladeč 2 and Oase eminences fall within the overlap range of the Neandertal and MPMH variation, and Mladeč 1 and 5 are outside the MPMH range of variation.
Neandertal mandibular rami variably have three features that are unusual in Late Pleistocene modern humans: lingular bridging of the mandibular foramen (horizontal-oval form), a high coronoid process with an asymmetrical mandibular notch, and the notch crest in the middle third of the mandibular condyle (45).
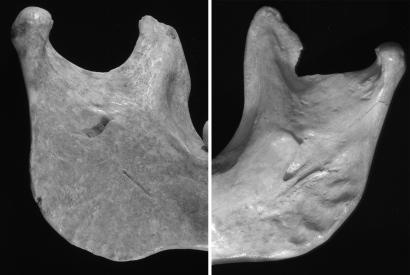
Lingular bridging is absent from the MPMH sample (n = 6), Nazlet Khater 2, and all Early Pleistocene and pre-200 ka B.P. European Homo mandibles (n = 15). It appears in 46.2% (n = 13) of late Middle Pleistocene Neandertal lineage fossils and then in 40.9% (n = 22) of the Neandertals. It is absent (n = 16) from Gravettian mandibles. Muierii 1 lacks this Neandertal trait, but Oase 1 exhibits it unilaterally (Fig. 4).
Fig. 4.
Medial views of the Oase 1 and Muierii 1 mandibular rami.
Although relative coronoid height and mandibular notch symmetry vary among recent humans, all MPMH (n = 5) and all Gravettian (n = 17) mandibles exhibit symmetrical notches with the coronoid at the level of the mandibular condyle. Among Neandertals, 66.7% (n = 12) have asymmetrical notches, perpetuating a pattern that emerged in the European Middle Pleistocene (59.4% asymmetrical, n = 16). Oase 1 has the MPMH pattern, but Muierii 1 has the Neandertal form (Fig. 4).
A mandibular notch crest that is lateral relative to the mandibular condyle appears to be plesiomorphic for the genus Homo (n = 7) and is found in all of the MPMHs (n = 8) and Nazlet Khater 2. Among Middle Pleistocene Europeans and the Neandertals, 61.5% (n = 13) and 47.1% (n = 17), respectively, have a medially positioned crest. Oase 1 has a lateral crest position, but Muierii 1 has a medially displaced one. All Gravettian mandibles (n = 17) have the lateral crest pattern.
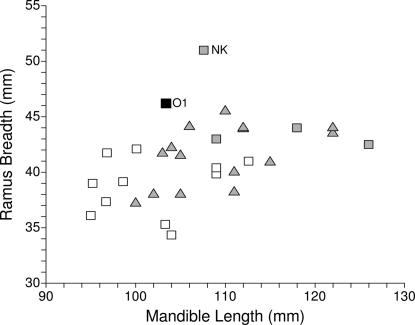
The Muierii 1 mandible has an absolutely narrow ramus (35 mm), but the Oase 1 rami are exceptionally wide (45.9 and 46.2 mm) (Fig. 4). Wide rami are common in the Middle Pleistocene (Arago, Baringo-Kapthurin, Mauer, and Tighenif) but rare in the Late Pleistocene. Only Nazlet Khater 2 exceeds Oase 1 in absolute ramal breadth and relative to mandible length, but Oase 1 is approached in absolute dimensions by four Neandertals and by Shanidar 4 and Zafarraya 2 in relative size (Fig. 5). MPMH and Gravettian mandibles all have smaller rami. Moreover, the anteriorly positioned zygomatic bones and long temporal fossae of Oase 2 (Fig. 1) match the wide rami of Oase 1. Even though this feature is present in Nazlet Khater 2, it is plesiomorphic and absent from all MPMHs.
Fig. 5.
Bivariate plot of mandible ramus breadth versus superior length. Symbols are as in the legend of Fig. 2. O1, Oase 1; NK, Nazlet Khater 2.
A mandibular transverse torus with a planum alveolare on the lingual symphysis is plesiomorphic for Late Pleistocene humans. The former feature is present in 81.5% of the Neandertals (n = 27), although only 51.9% have the latter trait. Moreover, when present, they are evident in juvenile as well as in mature mandibles (46). Among the MPMHs, only Qafzeh 9 has a planum alveolare (n = 10), and none of them has a transverse torus; Nazlet Khater 2 retains both features (20). Oase 1 and La Quina Aval 4 lack both features, but both are present on Les Rois 1 and probably Les Rois 2.
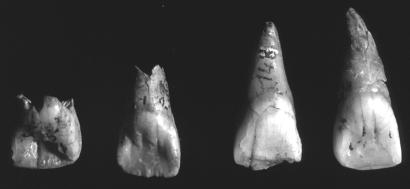
The maxillary central incisors (I1s) of the MPMH (n = 14) are chisel-shaped, with only a hint of marginal ridges on two of them. All Neandertals, as with all known archaic Homo, have shovel-shaped I1s, with most Neandertal I1s being notable for large lingual tubercles and marked labial convexity (they are poorly developed on at least Le Moustier 1 and Subalyuk 2). The Brassempouy I1s have the autapomorphic chisel-shape, but the Les Rois I1s show the full range from chisel-shaped to pronounced lingual tubercles and marginal ridges (but not labial convexity) (Fig. 6).
Fig. 6.
Lingual views of the Les Rois I1s.
Neandertal maxillary canines (C1s) variably present strong marginal ridges, a central lingual ridge, and a prominent lingual tubercle, features that are absent from the MPMH sample. The Mladeč 9 canine has all three of these features (24). Neandertal secondary mandibular premolars (P4s) have a high frequency of lingual metaconids, lingual-distal accessory cusps, asymmetrical occlusal contours, and transverse crests (47). Similar features are absent from the MPMH sample. The EEMH P4s vary, with Brassempouy 3040 probably lacking these features, La Quina Aval 3 possessing the first two features with moderate development of the third, and Les Rois 2 showing an intermediate form. Only the last feature is lacking from the EEMH sample.
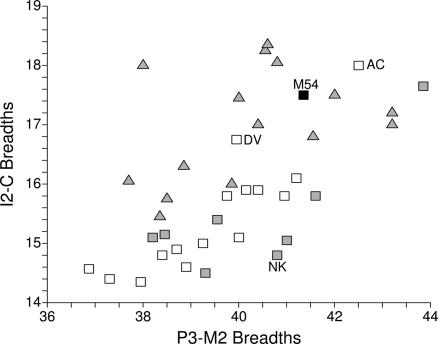
Oase 1 and 2 have exceptionally large distal molars. Their crown “areas” (length × breadth) are large for the M2s but exceptional for the M3s (21, 29). They are approached only by a few Early and Middle Pleistocene Homo fossils. The Mladeč 8 maxilla lacks its M3s, but its M2 is similar to that of Oase 2 at the top of the Late Pleistocene range of variation. In addition, Mladeč 54 has mesiodistal mandibular dental proportions [(I2–C)/(P3–M2) × 100: 42.3] on the Neandertal mean (42.3 ± 2.3, n = 15) and separate from that of MPMH (38.5 ± 1.4; n = 7) and Nazlet Khater 2 (36.3) (Fig. 7).
Fig. 7.
Bivariate plot of summed mesial (I2–C) versus summed postcanine (P3–M2) dental crown breadths. Symbols as in the legend to Fig. 2. M54, Mladeč 54; NK, Nazlet Khater 2; AC, Arene Candide 1; DV, Dolní V̆estonice 13.
The Morphological Mosaic of Gravettian Modern Humans
It is appropriate to query the extent to which the better preserved and larger sample of Gravettian human remains might show derived Neandertal features and/or plesiomorphic traits lost in the MPMH sample. Only in Iberia were these populations close in time to the latest Neandertals, but any persistence of this morphological mosaic would only reinforce the pattern seen among the EEMHs.
Occipital buns are less common than among the EEMHs. However, prominent ones are present in 18.9% (n = 37) of the individuals, including Brno 2, Cro-Magnon 3, Dolní Vĕstonice 11, Pavlov 1, and Předmostí 1, 2, and 7. In addition, hemi-buns are present in 29.7% of the sample.
There is a marked difference across mature Late Pleistocene mandibles in anterior symphyseal angles (infradentale-pogonion versus the alveolar plane) (P < 0.0001), with the Neandertal sample having vertical (90°) to retreating symphyses (81 ± 7°, n = 18), the Gravettian sample having projecting symphyses (97 ± 6°, n = 12), and the MPMH sample (including Nazlet Khater 2) being essentially vertical (89 ± 1°, n = 5). However, four immature Gravettian individuals (Kostenki 3, Lagar Velho 1, Předmostí 2, and Sunghir 2) have markedly retreating symphyseal angles; because this angle changes little through development in recent humans (slope: 0.17; r2 = 0.019; n = 245; ages: 1 year–adult), this reflects a symphyseal orientation variation overlapping that of the Neandertals and distinct from that of the MPMH. Most (95.7%, n = 23) of the Gravettian mandibles have essentially vertical lingual mandibular symphyses. However, Kostenki 4 has both a planum alveolare and a pronounced transverse torus, contrasting with all MPMHs.
Maxillary incisor shoveling remains in the Gravettian, but it appears in varying degrees of development of the marginal ridges and lingual tubercles. It is present, for example, in Lagar Velho 1, Fanciulli 6, Dolní Věstonice 15, and Sunghir 2. In mandibular mesiodistal dental proportions, most of the specimens (n = 14) are similar to the MPMHs, but Arene Candide 1 and Dolní Vĕstonice 13 have proportions (42.4 and 41.9) close to the Neandertal mean (see above; Fig. 7).
Neandertals exhibit relatively and absolutely long clavicles (48). Both MPMH (n = 2) and 93.8% of the Gravettian remains (n = 16) exhibit the abbreviated clavicles of recent humans, distinct from the Neandertals. Sunghir 1, however, despite its linear body proportions, high crural indices, and biomechanically inferred narrow trunk (49, 50), has a clavicle length indistinguishable from that of the Neandertals.
Neandertals and their western Eurasian predecessors exhibit a derived morphology of the scapular axillary border, the dorsal sulcus pattern, present in 77.3% (n = 11) of Neandertal scapulae. This configuration is absent from the MPMHs, but it occurs in 24.0% of the Gravettian sample (n = 25), including Barma Grande 2, Dolní Věstonice 14, Předmostí 14, and Sunghir 1, 2, and 3.
The opponens pollicis muscle, in both early Homo and recent humans, forms a variably marked insertion crest on the distoradial metacarpal 1 diaphysis, but among Neandertals from infancy to adulthood (n = 15) it forms a pronounced radial flange. MPMH (n = 4) and Nazlet Khater 2 have the plesiomorphic pattern, as do most Gravettian individuals. However, Sunghir 1 exhibits a pronounced Neandertal-like flange.
Finally, most Gravettian remains evince the high crural indices of equatorial humans, also seen in the MPMHs (16, 51). In addition, biomechanical analysis of the Mladeč 27 femur indicates that it had linear body proportions (27). Yet, Lagar Velho 1 has a low crural index close to that of similarly aged Neandertals and contrasting with the high values of immature Gravettian children such as Balla 1 and Sunghir 2 (49, 52). In addition, the Cro-Magnon postcrania, despite uncertainties in associating the mixed femora and tibiae, can only have had Neandertal-like or intermediate crural indices.
Discussion
The assessment of Neandertal features in EEMH and Gravettian samples is not new (e.g., refs. 4, 10, and 53). However, previous attempts have focused on single samples, have been limited by the available fossil sample, and/or were ambiguous in the polarities of traits. Critiques of them have been misguided in using recent human trait presence as a guide, confusing the polarities of traits, applying different criteria of trait definition depending upon the overall morphology of the specimen, using inappropriate combinations of distinct characters (especially through multivariate morphometrics), and/or focusing on the overall “modern” morphology of these fossils rather than specific deviations from that pattern.
This reassessment of the polarities of EEMH and subsequent Gravettian human populations in Europe, based on an analysis of trait polarity in the potential ancestral samples (33), should make it clear that the overall morphological gestalt of the EEMH and Gravettians is the apomorphic modern human pattern, but there are numerous craniofacial, dental, and postcranial traits that are unlikely to have been derived from MPMH. Some of these latter traits are distinctly Neandertal in form and others are plesiomorphic traits lost in the MPMH sample. Given preservation, these non-MPMH traits are limited to the skull and dentition in the EEMH sample, but they are found in the postcrania as well in the Gravettian sample. Despite small sample sizes, these non-MPMH characteristics are common among the EEMHs, if less pervasive in the larger Gravettian sample. Moreover, the morphological complexes exhibiting the non-MPMH patterns are polymorphic within the EEMH and Gravettian samples, the pattern that would be expected should two morphologically distinct populations blend to some degree.
This pattern can only be parsimoniously explained as a result of variable assimilation of Neandertals into EEMH populations as the latter dispersed across Europe. If the associated admixture were rare, then one would expect only a few of these non-MPMH traits in a few of the EEMHs; this is not the case. One would also expect any substantial evidence of non-MPMH features to be absent by the time of the Gravettian, 10 millennia or more after the initial spread of EEMHs (except in Iberia); the fossils indicate otherwise. Yet, the overwhelming phenetic and cladistic modernity of these EEMH (and Gravettian) human remains indicates that the majority of their ancestry was that of the MPMHs. Indeed, this is the pattern that is also emerging from current interpretations of the human molecular record, both extant (8, 9, 54) and ancient (55, 56), despite the small sample sizes used in most of the latter analyses.
One could debate whether the traits are morphologically primary or secondary, but all appear to be epigenetic and developmentally stable. Indeed, similar traits are routinely used for recent human interpopulational relationships (34) and for justifying new hominid paleospecies (e.g., refs. 57–60). One could question whether some of the EEMH/Gravettian traits are homologous to the Neandertal ones, but repeated assessments of the critical elements (and not their phenetic contexts) support homology across the samples for these features. One could note the presence of some of these features in earlier, pre-MPMH African fossils, yet all of these traits were lost in the MPMHs. One could query whether the MPMH sample is sufficient to evaluate its range of variation, even though it greatly exceeds the sample sizes on which primate species (or even genera) are routinely named. And one could question whether other African pre-50 ka B.P. purportedly modern specimens should have been included in the MPMH sample; inclusion of these specimens would modestly reduce the relevance of I1 shoveling and mandibular symphyseal morphology, expand the sample for the MPMH mandibular ramal pattern, have no effect on the other traits, and have little effect on the overall pattern. The role of the Nazlet Khater 2 skeleton is also debatable; it has two plesiomorphic mandible features absent in the MPMHs, suggesting that its post-MPMH ancestors may have experienced admixture with regional late archaic humans.
Through such reevaluations of traits and relevant samples, a few of the archaic and/or Neandertal features in the EEMH/Gravettian samples might be discounted. However, there is a sufficient number of features among the EEMHs/Gravettians that either links them directly to the Neandertals or does so indirectly through their plesiomorphic form, such that rejecting several of the features would not significantly alter the pattern. Given a low probability of any one of these Neandertal/plesiomorphic features appearing in the EEMH/Gravettian sample being derived from the MPMHs, the cumulative probability across these traits rapidly becomes so small as to make a strict MPMH ancestry for these early modern Europeans extremely unlikely.
Conclusions
The human paleontological record of EEMHs is the ultimate test of the phylogenetic fate of the Neandertals. Its indications are clear. Early modern Europeans reflect both their predominant African early modern human ancestry and a substantial degree of admixture between those early modern humans and the indigenous Neandertals. Given the tens of millennia since then and the limitations inherent in ancient DNA, this process is largely invisible in the molecular record. It is readily apparent in the paleontological record.
Materials and Methods
All morphometric dimensions follow Martin (61), and discrete traits are referenced. Data are from personal examination of original fossils and primary paleontological descriptions. For traits that remain stable through development, immature and mature specimens are used; for traits that change during development, the samples are restricted to adults.
Acknowledgments
J. C. M. Ahern, S. Athreya, S. E. Churchill, L. W. Cowgill, T. W. Holliday, F. H. Smith, and J. Zilhão provided helpful comments. This work was supported by National Science Foundation Grants BCS-0409194 and BCS-0509072, Wenner–Gren Grants 7111 and 7290, and the Leakey Foundation.
Abbreviations
- ka B.P.
thousands of years before present
- EEMHs
European early modern humans
- MPMHs
Middle Paleolithic modern humans.
Footnotes
The authors declare no conflict of interest.
The emergence of modern humans and the fate of the Neandertals has generated an enormous literature. To avoid omissions and misrepresentation through generalization of subtle differences in interpretation, the background framework is presented here largely devoid of references. For recent reviews, see refs. 4–9.
References
- 1.Trinkaus E. In: Aspects of Human Evolution. Stringer CB, editor. London: Taylor & Francis; 1981. pp. 187–224. [Google Scholar]
- 2.Bräuer G. Humanbiol Budapest. 1982;9:69–78. [Google Scholar]
- 3.Day MH, Stringer CB. In: L'Homo erectus et la Place de l'Homme de Tautavel parmi les Hominidés Fossiles. de Lumley H, editor. Paris: Centre National de la Recherche Scientifique; 1982. pp. 814–846. [Google Scholar]
- 4.Trinkaus E, Zilhão J. Trabalhos Arqueol. 2002;22:497–518. [Google Scholar]
- 5.Pearson OM. Evol Anthropol. 2004;13:145–159. [Google Scholar]
- 6.Smith FH, Jankovié I, Karavanié I. Quartern Intl. 2005;137:7–19. [Google Scholar]
- 7.Trinkaus E. Annu Rev Anthropol. 2005;34:207–230. [Google Scholar]
- 8.Templeton AR. Yrbk Phys Anthropol. 2005;48:33–59. doi: 10.1002/ajpa.20351. [DOI] [PubMed] [Google Scholar]
- 9.Garrigan D, Hammer MF. Nat Gen Rev. 2006;7:669–680. doi: 10.1038/nrg1941. [DOI] [PubMed] [Google Scholar]
- 10.Smith FH, Falsetti AB, Donnelly SM. Yrbk Phys Anthropol. 1989;32:35–68. [Google Scholar]
- 11.Jolly CJ. Yrbk Phys Anthropol. 2001;44:177–204. doi: 10.1002/ajpa.10021. [DOI] [PubMed] [Google Scholar]
- 12.Holliday TW. In: Neanderthals Revisited. Harvati K, Harrison T, editors. New York: Springer; 2006. pp. 289–306. [Google Scholar]
- 13.Grine FE, Bailey RM, Harvati K, Nathan RP, Morris AG, Henderson GM, Ribot I, Pike AWG. Science. 2007;315:226–229. doi: 10.1126/science.1136294. [DOI] [PubMed] [Google Scholar]
- 14.Shang H, Tong H, Zhang S, Shen F, Trinkaus E. Proc Natl Acad Sci USA. 2007;104:6573–6578. doi: 10.1073/pnas.0702169104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franciscus RG. J Hum Evol. 2003;44:701–729. doi: 10.1016/s0047-2484(03)00062-9. [DOI] [PubMed] [Google Scholar]
- 16.Holliday TW. Am Anthropol. 2000;102:54–68. [Google Scholar]
- 17.Smith FH. In: The Origin of Modern Humans and the Impact of Chronometric Dating. Aitken MJ, Stringer CB, Mellars PA, editors. Princeton: Princeton Univ Press; 1993. pp. 234–248. [Google Scholar]
- 18.Churchill SE, Pearson OM, Grine FE, Trinkaus E, Holliday TW. J Hum Evol. 1996;31:213–237. doi: 10.1006/jhev.1998.0227. [DOI] [PubMed] [Google Scholar]
- 19.Lam YM, Pearson OM, Smith CM. Am J Phys Anthropol. 1996;100:545–557. doi: 10.1002/(SICI)1096-8644(199608)100:4<545::AID-AJPA8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 20.Crevecoeur I. Bordeaux, France: Université Bordeaux 1; 2006. PhD thesis. [Google Scholar]
- 21.Trinkaus E, Moldovan O, Milota Ş, Bîlgăr A, Sarciňa L, Athreya S, Bailey SE, Rodrigo R, Gherase M, Higham T, et al. Proc Natl Acad Sci USA. 2003;100:11231–11236. doi: 10.1073/pnas.2035108100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henry-Gambier D, Maureille B, White R. Bull Mém Soc Anthropol Paris. 2004;16:49–87. [Google Scholar]
- 23.Soficaru A, Petrea C, Doboş A, Trinkaus E. Curr Anthropol. 2007 in press. [Google Scholar]
- 24.Frayer DW, Jelínek J, Oliva M, Wolpoff MH. In: Early Modern Humans at the Moravian Gate. Teschler-Nicola M, editor. Vienna: Springer; 2006. pp. 185–272. [Google Scholar]
- 25.Wolpoff MH, Frayer DW, Jelínek J. In: Early Modern Humans at the Moravian Gate. Teschler-Nicola M, editor. Vienna: Springer; 2006. pp. 273–340. [Google Scholar]
- 26.Minugh-Purvis N, Viola TB, Teschler-Nicola M. In: Early Modern Humans at the Moravian Gate. Teschler-Nicola M, editor. Vienna: Springer; 2006. pp. 357–383. [Google Scholar]
- 27.Trinkaus E, Smith FH, Stockton TC, Shackelford LL. In: Early Modern Humans at the Moravian Gate. Teschler-Nicola M, editor. Vienna: Springer; 2006. pp. 385–445. [Google Scholar]
- 28.Soficaru A, Doboş A, Trinkaus E. Proc Natl Acad Sci USA. 2006;103:17196–17201. doi: 10.1073/pnas.0608443103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rougier H, Milota Ş, Rodrigo R, Gherase M, Sarcină L, Moldovan O, Zilhão J, Constantin S, Franciscus RG, Zollikofer CPE, et al. Proc Natl Acad Sci USA. 2007;104:1164–1170. doi: 10.1073/pnas.0610538104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vallois HV. Gallia Suppl. 1958;9:118–137. [Google Scholar]
- 31.Martin H. Bull Soc Préhist Franç. 1936;31:177–202. [Google Scholar]
- 32.Zilhão J, Trinkaus E. Trabalhos Arqueol. 2002;22:519–541. [Google Scholar]
- 33.Trinkaus E. Curr Anthropol. 2006;47:597–620. [Google Scholar]
- 34.Hauser G, DeStefano GF. Epigenetic Variants of the Human Skull. Stuttgart: Schweizerbart; 1989. [Google Scholar]
- 35.Zilhão J. Evol Anthropol. 2006;15:183–195. [Google Scholar]
- 36.Trinkaus E, LeMay M. Am J Phys Anthropol. 1982;57:27–35. doi: 10.1002/ajpa.1330570106. [DOI] [PubMed] [Google Scholar]
- 37.Gunz P, Harvati K. J Hum Evol. 2007;52:262–274. doi: 10.1016/j.jhevol.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Smith FH. In: The Origins of Modern Humans. Smith FH, Spencer F, editors. New York: Liss; 1984. pp. 137–209. [Google Scholar]
- 39.Trinkaus E. Am J Phys Anthropol. 2004;124:28–32. doi: 10.1002/ajpa.10336. [DOI] [PubMed] [Google Scholar]
- 40.Tillier AM. Les Enfants Moustériens de Qafzeh. Paris: Centre National de la Recherche Scientifique; 1999. [Google Scholar]
- 41.Haile-Selassie Y, Asfaw B, White TD. Am J Phys Anthropol. 2004;123:1–10. doi: 10.1002/ajpa.10330. [DOI] [PubMed] [Google Scholar]
- 42.Sládek V. Bordeaux, France: Université Bordeaux 1; 2000. PhD thesis. [Google Scholar]
- 43.Trinkaus E. Trabalhos Arqueol. 2002;22:256–286. [Google Scholar]
- 44.Trinkaus E. The Shanidar Neandertals. New York: Academic; 1983. [Google Scholar]
- 45.Stefan VH, Trinkaus E. J Hum Evol. 1998;34:443–468. doi: 10.1006/jhev.1997.0210. [DOI] [PubMed] [Google Scholar]
- 46.Madre-Dupouy M. L'Enfant du Roc de Marsal. Paris: Centre National de la Recherche Scientifique; 1992. [Google Scholar]
- 47.Bailey SE. Anat Rec. 2002;269:148–156. doi: 10.1002/ar.10116. [DOI] [PubMed] [Google Scholar]
- 48.Vandermeersch B, Trinkaus E. J Hum Evol. 1995;28:439–476. [Google Scholar]
- 49.Kozlovskaya MV, Mednikova MB. In: Homo Sungirensis. Alexeeva TI, Bader NO, editors. Moscow: Scientific World; 2000. pp. 85–144. [Google Scholar]
- 50.Mednikova M, Trinkaus E. Anthropology. 2001;39:135–141. [Google Scholar]
- 51.Holliday TW. In: Early Modern Human Evolution in Central Europe. Trinkaus E, Svoboda JA, editors. New York: Oxford; 2005. pp. 224–232. [Google Scholar]
- 52.Ruff CB, Trinkaus E, Holliday TW. Trabalhos Arqueol. 2002;22:365–391. [Google Scholar]
- 53.Frayer DW. In: Continuity or Replacement. Bräuer G, Smith FH, editors. Rotterdam: Balkema; 1992. pp. 179–188. [Google Scholar]
- 54.Evans PD, Mekel-Bobrov N, Vallender EJ, Hudson RR, Lahn BT. Proc Natl Acad Sci USA. 2006;103:18178–18183. doi: 10.1073/pnas.0606966103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Serre D, Langaney A, Chech M, Teschler-Nicola M, Paunovié M, Mennecier P, Hofreiter M, Possnert G, Pääbo S. PLoSci Biol. 2004;2:313–317. doi: 10.1371/journal.pbio.0020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noonan JP, Coop G, Kudaravalli S, Smith D, Krause J, Alessi J, Chen F, Platt D, Pääbo D, Pritchard JK, et al. Science. 2006;314:1113–1118. doi: 10.1126/science.1131412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johanson DC, White TD, Coppens Y. Kirtlandia. 1978;28:1–14. [Google Scholar]
- 58.Leakey MG, Feibel CS, McDougall I, Walker A. Nature. 1995;376:565–571. doi: 10.1038/376565a0. [DOI] [PubMed] [Google Scholar]
- 59.de Heinzelin J, Clark JD, White T, Hart W, Renne P, WoldeGabriel G, Beyene Y, Vrba E. Science. 1999;284:625–634. doi: 10.1126/science.284.5414.625. [DOI] [PubMed] [Google Scholar]
- 60.Brunet M, Guy F, Pilbeam D, Mackaye HT, Likius A, Ahounta D, Beauvilain A, Blondel C, Bocherens H, Boisserie JR, et al. Nature. Vol. 418. 2002. pp. 145–155. [DOI] [PubMed] [Google Scholar]
- 61.Bräuer G. In: Handbuch der vergleichenden Biologie des Menschen. Knussmann R, editor. Vol 1. Stuttgart: Fischer; 1988. pp. 160–232. [Google Scholar]