Abstract
Multiple myeloma (MM) is an invariably fatal form of cancer characterized by clonal proliferation of malignant plasma cells in the bone marrow. The canonical Wnt signaling pathway is activated in MM cells through constitutively active β-catenin, a messenger molecule relevant to growth, survival, and migration of MM cells. The identification of a number of small molecular compounds, such as PKF115–584, which disrupt the interaction of the transcriptionally active β-catenin/TCF protein complex, provides valuable new therapeutic tools to target an alternative pathway in MM independent of the proteasome. Here we evaluated the transcriptional, proteomic, signaling changes, and biological sequelae associated with the inhibition of Wnt signaling in MM by PKF115–584. The compound blocks expression of Wnt target genes and induces cytotoxicity in both patient MM cells and MM cell lines without a significant effect in normal plasma cells. In xenograft models of human MM, PKF115–584 inhibits tumor growth and prolongs survival. Taken together, these data demonstrate the efficacy of disrupting the β-catenin/TCF transcriptional complex to exploit tumor dependence on Wnt signaling as a therapeutic approach in the treatment of MM.
Keywords: drug therapy, Wnt signaling
Multiple myeloma (MM) is characterized by clonal proliferation of plasma cells (PCs) in the bone marrow (BM), usually associated with elevated serum and urine monoclonal paraprotein. It is the second most frequent hematological cancer in the United States and remains largely incurable despite high-dose chemotherapy with stem cell support. Novel agents such as thalidomide, the immunomodulatory drug Revlimid, and the proteasome inhibitor Bortezomib can achieve responses in patients with relapsed and refractory MM; however, the median survival remains at 6 years, with only 10% of patients surviving at 10 years, highlighting the need for novel therapeutic agents (1).
The development and maintenance of MM tumors is dependent on the BM microenvironment (2). Within the BM extracellular milieu, a number of essential cytokines are secreted, including IL-6, which induces growth and drug resistance (3, 4). In addition, BM stromal cells (BMSCs) secrete Wnt ligands Wnt3A, Wnt5A, Wnt10B, and/or Wnt16, which can activate Wnt signaling in MM cells (5–7). Stimulation by these ligands induces proliferative and self-renewal capacity in hematopoietic stem cells (HSCs), which in turn give rise to the entire myelolymphoid repertoire (8–10). Wnt signaling and expression of Wnt target genes are lost in B-lineage cells derived from HSCs. However, the Wnt pathway is activated in MM cells through an unknown mechanism and can trigger tumor cell proliferation (7, 11).
The canonical Wnt pathway, mediated through the central signaling molecule β-catenin, is commonly dysregulated in many cancers (8). Binding of Wnt ligands to frizzled and low-density lipoprotein receptors (LRP5 and LRP6) results in the inhibition of GSK3β-kinase activity and the adenomatous polyposis coli/axin complex. GSK3β-mediated phosphorylation of β-catenin targets the protein for ubiquitination and subsequent degradation through the proteasome. Nonphosphorylated β-catenin translocates to the nucleus and binds to T cell factor (TCF) transcriptional factors, BCL9, and Pygopus, thereby forming a transcriptional complex that activates expression of downstream target genes such as c-MYC and Cyclin D1 (12, 13). Cells with active β-catenin/TCF-regulated transcription (CRT) are protected against apoptosis; conversely, inhibition of canonical Wnt signaling activates the cell suicide program (14, 15). Activating mutations in CTNNB1 that eliminate N-terminal β-catenin phosphorylation sites, thereby resulting in constitutive transcriptional activity, are commonly found in epithelial tumors. Likewise, inactivating mutations in APC, AXIN, and GSK3β can also deregulate CRT in a variety of cancers (16–18).
A prior study of Wnt activity in MM demonstrated that nearly all primary MM cells and MM cell lines expressed β-catenin, whereas expression was absent in normal PCs (7). The mechanism activating aberrant β-catenin expression in MM cells is unknown; however, expression of β-catenin was associated with transcriptional activity, suggesting that it mediates growth of MM cells as in HSCs. MM cell lines responded to the Wnt ligand Wnt3A, the GSK3β-inhibitor lithium chloride, and an active mutant form of β-catenin with both significantly increased proliferation and higher levels of nonphosphorylated nuclear β-catenin. Furthermore, growth of MM cell lines was blocked on transfection with a dominant-negative form of TCF4. No activating mutations of the β-catenin gene CTNNB1 were found in MM (7). These findings demonstrate that Wnt signaling is active in MM, acts through CRT, and responds to Wnt stimulants and/or inhibitors.
A high-throughput ELISA-based screening of compounds that disrupt the formation of the β-catenin/TCF transcriptional complex identified the small-molecule PKF115–584 as a candidate to block CRT. Further study of PKF115–584 demonstrated the ability of the drug to (i) inhibit immunoprecipitation of β-catenin from GST-tethered TCF4, (ii) block the β-catenin/TCF transcriptional complex DNA binding in an EMSA assay, (iii) specifically reduce Wnt reporter activity, (iv) rescue the phenotype of Xenopus embryos when injected with exogenous β-catenin, and (v) block Cyclin D1 and c-Myc expression as well as reduce growth in colon cancer cell lines (19). Thus, the identification of the Wnt pathway influencing MM proliferation, in conjunction with the availability of small-molecule inhibitors of β-catenin/TCF interaction, set the stage for investigating the therapeutic benefits of targeting the canonical Wnt pathway in MM.
In this study, we show that PKF115–584 significantly reduces proliferation and viability in patient MM cells and MM cell lines, including those resistant to established drug therapies, without a major effect on PCs or BM mononuclear cells. The effect of PKF115–584 on blocking the Wnt pathway was demonstrated by using a reporter assay and expression profiling analysis, documenting down-regulation of Wnt target genes. PKF115–584 overcame the protective/proliferative effects of coculturing cell lines with BMSCs, treatment with Wnt3A, or the prosurvival cytokine IL-6. We also document the in vivo efficacy of the drug in a murine xenograft model of human MM. Taken together, these data provide the preclinical framework for the evaluation of PKF115–584 and other Wnt pathway inhibitors, alone and in combination, to improve patient outcome in MM and other hematologic neoplasms driven by active Wnt signaling.
Results
Expression of Wnt Pathway Genes in MM Cells.
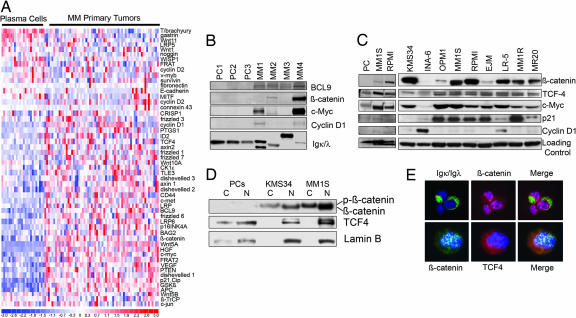
Previous studies have documented activation of the canonical Wnt pathway in MM cells (7, 11). We, therefore, first extended these findings by investigating endogenous expression levels of Wnt pathway genes from a public repository of normal PCs and patient MM tumor gene expression profiles (20). A comparison of 24 PCs against 64 patient MM cells showed many Wnt pathway genes to be significantly up-regulated in tumors (Fig. 1A). These included upstream pathway members frizzled and Wnt ligands, as well as β-catenin, TCF4, and BCL9, which compose the transcriptional complex responsible for Wnt signaling. Notably, a number of downstream Wnt target genes, including c-MYC, Cyclin D1, CD44, and p16INK4, were also up-regulated (Fig. 1A). We confirmed these findings by using an independent set of normal PCs and MM tumors by Western blot (Fig. 1B). These gene expression alterations in patient MM cells were corroborated with immunoblotting results from MM cell lines, which also showed high expression of β-catenin, TCF4, and c-MYC in nearly all cases (Fig. 1C). Because β-catenin must be translocated to the nucleus for its transcriptional activity, we next assessed protein levels in nuclear and cytoplasmic fractions of MM cell lines. Whereas PCs did not express β-catenin, MM cell lines demonstrated phosphorylated β-catenin (inactive) migrating as a higher band in the cytoplasmic fraction, and the nonphosphorylated (active), lower migrating band was in the nuclear fraction (Fig. 1D). The nuclear localization of β-catenin was further confirmed by immunofluorescence studies (Fig. 1E). TCF4 was expressed in the nuclei of both PCs and all MM cell lines studied (Fig. 1 C and D). It colocalized with β-catenin in patient MM cells, suggesting an interaction of the two proteins in the transcriptional complex (Fig. 1E). Indeed, expression of Wnt target oncogene c-MYC was high in nearly all cell lines tested, further validating the gene expression profiling data set. Cyclin D1 expression was minimally expressed in most MM cell lines, and p21Cip levels were not associated with c-MYC, suggesting additional levels of regulation of these genes (Fig. 1C). The prevalence of activated Wnt signaling in MM patient cells and MM cell lines therefore provides a rational basis for targeting this pathway with the small-molecule inhibitor of both β-catenin/TCF4 protein interaction and Wnt signaling, PKF115–584 (19). Of the drug-sensitive MM cell lines expressing the highest levels of β-catenin and c-MYC, the KMS34 and MM1S cell lines were chosen for further in-depth study.
Fig. 1.
Expression of Wnt pathway genes in MM. (A) Gene expression heat map profiles of 24 PCs and 64 primary MM tumors. (B) Immunoblotting for Wnt signaling proteins in CD138+ plasma cells (PC) and MM primary tumors (MM). Loading controls, light chains. (C) MM cell lines. Nonspecific band (Left) and tubulin (Right). (D) Detection of activated, unphosphorylated nuclear β-catenin in MM cell lines. Nuclear (N) and cytoplasmic (C) fractions of MM cell lines. Loading control, Lamin B-nuclear. Incomplete N and C separation in PC sample shows some TCF4 and Lamin B signal in the cytoplasm. (E) Immunofluorescence confirming the nuclear localization of β-catenin (red, Upper; green, Lower) and TCF4 (red) in a representative case of patient MM cells. Igκ and Igλ light chain expression identifies the cytoplasm of primary MM cells, distinct from β-catenin signal. DAPI staining labels nuclei blue. Merged image shows colocalization of both signals in the nucleus.
PKF115–584 Blocks β-Catenin/TCF Transcriptional Activity.
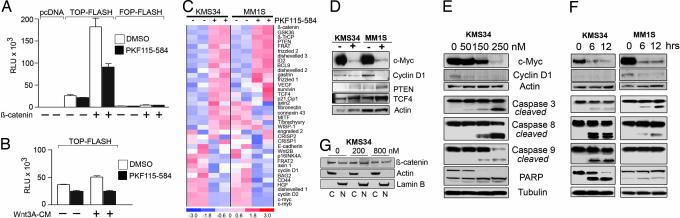
Disruption of the β-catenin/TCF complex and transcriptional activity by PKF115–584 has been shown at the molecular level and in colon cancer cell lines (19). To assess efficacy of PKF115–584 against CRT in the context of MM cells in vitro, we transfected MM cells with TOP-FLASH or FOP-FLASH Wnt reporter plasmids. When treated with PKF115–584, reporter activity was significantly reduced in KMS34 and MM1S cells (Fig. 2A and data not shown). To assess the ability of PKF115–584 to specifically block Wnt pathway activation, we cotransfected MM cells with a β-catenin expression vector, which dramatically increased reporter activity (>5-fold), but this effect was inhibited in response to PKF115–584 (Fig. 2A). Furthermore, treatment with Wnt3A ligand stimulated reporter activity, but was negatively regulated by PKF115–584 (Fig. 2B). Similar results were obtained by using a GFP-based Wnt reporter system [supporting information (SI) Fig. 6]. The effect of PKF115–584 in blocking CRT was further investigated by expression profiling analysis. Comparing MM cell lines treated with PKF115–584 versus vehicle-alone controls revealed contrasting expression patterns for Wnt pathway members. Notably, c-MYC was among the most suppressed genes in KMS34 and MM1S cells (Fig. 2 C and D). Cyclin D1 expression was almost completely abrogated by drug treatment. A number of genes upstream of the β-catenin/TCF transcriptional complex were up-regulated, indicating a possible compensatory mechanism of MM cells reliant on Wnt activity for survival. These elevated expression levels also confirm that observed down-regulation of genes was not due to overall cell death. These findings were validated by immunoblotting for c-MYC and Cyclin D1. The down-regulation of these genes followed a time- and dose-dependent response at intervals from 0 to 12 h and doses of 0–250 nM PKF115–584 (Fig. 2 E and F). We did not detect changes in the localization of β-catenin between nuclear and cytoplasmic fractions by Western blot or immunofluorescence analysis (Fig. 2G and data not shown).
Fig. 2.
PKF115–584 inhibits the activity of the β-catenin/TCF transcriptional complex. (A) KMS34 cells transfected with indicated reporter plasmids either treated or untreated with PKF115–584. Cotransfection with β-catenin expression vector where indicated. Mock-transfected cells with pcDNA3.1 displayed negligible readings. RLU, relative light units. (B) KMS34 endogenous reporter activity is enhanced on addition of Wnt3A, but blocked with PKF115–584. (C) Gene expression profiling of KMS34 and MM1S cell lines treated with PKF115–584. (D) Immunoblot validation of gene expression microarray results shown in C. (E) Increasing concentrations of PKF115–584 with MM cell lines reduced after 6-h treatment. Under the same conditions, apoptotic regulators caspase-3, -8, -9, and PARP were activated. (F) Gene expression changes after treatment for 6 and 12 h with 200 nM PKF115–584. (G) The nuclear and cytoplasmic distributions of β-catenin are unaffected by PKF115–584 treatment in KMS34 cells at indicated concentrations. Loading controls: Actin, cytoplasmic; Lamin B, nuclear.
Activation of Apoptosis Genes and Cell Cycle Arrest.
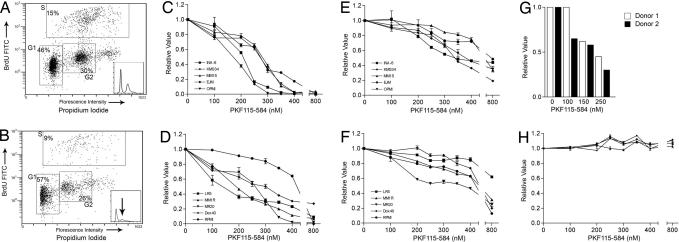
Previous studies have documented that inhibition of the Wnt pathway removes a prosurvival signal and induces apoptosis (14, 15). Therefore, we next investigated the effect of PKF115–584 treatment on the activation of the cell suicide response. Western blot analysis of treated cells documented activation of apoptosis-regulating genes caspase-3, -8, -9, and PARP at the same drug concentrations and time intervals showing down-regulation of c-MYC and Cyclin D1 (Fig. 2 E and F). We further analyzed changes in the cell cycle by using BrdU incorporation and propidium iodide (PI) staining. After PKF115–584 treatment, there was a decrease in KMS34 cells entering the S phase (−6%) and progressing to mitosis in the G2 phase (−4%), accompanied by an increase in subG1 cells (+11%) (Fig. 3 A and B). Similar results were seen in the MM1S cell line (data not shown). These data suggest that PKF115–584 triggers cell cycle arrest with activating apoptotic signaling in MM cells in a dose- and time-dependent manner.
Fig. 3.
Cell cycle arrest and cytotoxic effects of PKF115–584 in MM cell lines. (A and B) Representative examples of FACScan analysis with BrdU incorporation plotted against PI in untreated (A) and treated (B) KMS34 cells. Cell cycle arrest is shown by an increase in the BrdUloPIlo G1 population. (Insets) Histograms of PI staining. (C and D) Proliferation of MM cell lines sensitive (C) and resistant (D) to conventional therapies was inhibited by treatment with increasing concentrations (0–800 nM) of PKF115–584. (E and F) This treatment also decreased cell viability of sensitive (E) and resistant (F) cell lines. (G and H) Dose-dependent cytotoxicity in purified primary patient MM cells (G), but not PCs (H), upon PKF115–584 treatment.
PKF115–584 Inhibits Proliferation and Induces Cytotoxicity in MM Cell Lines.
Our findings described above, coupled with a previous study by Derksen et al. (7) documenting that a dominant-negative form of TCF4 was able to inhibit CRT and proliferation of MM cell lines in vitro, prompted us to investigate the potential therapeutic benefit of PKF115–584 in MM cell lines. PKF115–584 inhibited proliferation in cell lines sensitive (Fig. 3C) and resistant (Fig. 3D) to conventional therapies in a dose-dependent fashion, with IC50 ranging between 150 and 450 nM. Reduction in proliferation correlated with decreased viability in all cell lines and was also triggered in a dose-dependent manner (Fig. 3 E and F). PKF115–584 also had activity against patient MM cells, with an IC50 ≈150 nM (Fig. 3G). In contrast, PKF115–584 did not trigger cytotoxicity in PCs and BM mononuclear cells isolated from four healthy volunteers (Fig. 3H and data not shown). Furthermore, we found synergistic activity with PFK115–584 and established anti-MM therapies dexamethasone and Bortezomib (SI Fig. 7). These results indicate that MM cells are dependent on Wnt activation for growth relative to PCs, suggesting a favorable therapeutic index for PKF115–584.
PKF115–584 Overcomes Protective Effects from BMSCs, Wnt3A, and IL-6 Stimulation.
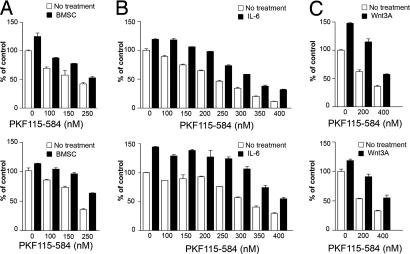
Cytokines and/or growth factors produced by BMSCs have a profound effect on the growth and survival of MM cells, as well as resistance to dexamethasone (3, 21, 22). In particular, the secretion of Wnt ligands by BMSCs may activate Wnt signaling in MM. Moreover, MM cells secrete Wnt ligands (7, 11), suggesting an autoregulatory loop. Proliferation of KMS34 and MM1S cells cocultured with BMSCs was blocked in a dose-dependent fashion by PKF115–584, indicating the drug can overcome the stimulatory and protective effects of the BM microenvironment (Fig. 4A). Similarly, proliferation triggered by Wnt3A ligand or IL-6 was also inhibited by PKF115–584 in both KMS34 and MM1S cells (Fig. 4 B and C).
Fig. 4.
PKF115–584 inhibits KMS34 and MM1S cell growth and synergizes with conventional therapies. (A) Proliferation in cocultures with BMSCs is inhibited by increasing PKF115–584 concentration. (B and C) Increased MM cell proliferation triggered by IL-6 (B) and Wnt3A (C) conditional media were blocked by PKF115–584.
In Vivo Efficacy of PKF115–584 in a Murine Xenograft Model of Human MM.
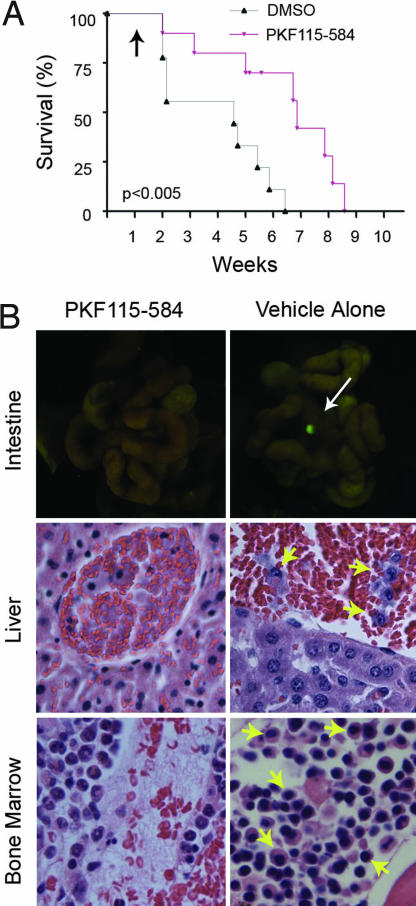
The data described above suggest that PKF115–584 possesses significant anti-MM activity in vitro. We next sought to assess its efficacy against MM cells in vivo by using a xenograft mouse model of human MM. SCID mice were transplanted (i.v.) with KMS34 cells stably expressing GFP (KMS34-GFP+). After 1 week, mice were treated with PKF115–584 or vehicle alone every other day. Beginning ≈2 weeks post-MM cell line transplantation, when mice began to appear moribund, they were killed for necropsy, UV light examination, and histopathologic analysis; Kaplan–Meier survival curves were prepared (Fig. 5A). On gross examination under filtered UV light, we noted the formation of small, GFP+ tumor nodules along the gastrointestinal track of mice receiving vehicle alone. PKF115–584-treated mice, however, rarely developed metastatic tumors. Representative examples are shown in Fig. 5B. Pathological analysis of affected control mice revealed leukemic KMS34-GFP+ MM cells circulating within the bloodstream and BM (Fig. 5B, yellow arrows). In contrast, tumor cells were found less frequently, if at all, in PKF115–584-treated mice. Furthermore, survival of the PKF115–584-treated animals was significantly prolonged compared with controls (P < 0.005). Notably, BM hematopoiesis was adversely affected in PKF115–584-treated mice, leading to some degree of BM hypoplasia, anemia, and generalized wasting in some animals (Fig. 5B). This observation may be related to the inhibition of Wnt signaling in HSCs, which is vital for their self-renewal and proliferative capacity, thereby leading to depleted stem cell populations and impaired regeneration of the BM stroma (9).
Fig. 5.
In vivo efficacy of PKF115–584. (A) KMS34-GFP+ cells were injected into SCID mice. Kaplan–Meier survival curve of control and PKF115–584-treated animals is shown. Black arrow indicates initiation of drug regimen. (B) Gross anatomical and histopathological analysis of animal tissues upon necroscopy. UV excitation showed GFP+ tumor nodules along the gastrointestinal tract of untreated mice. H&E stain of liver capillaries revealed leukemic-circulating KMS34-GFP+ cells and BM populations (yellow arrows).
Discussion
The activity of the canonical Wnt signaling pathway, mediated through β-catenin, plays a significant oncogenic role in many epithelial cancers and some hematologic neoplasms (8). Although β-catenin expression is lost in normal differentiated B-lineage PCs, β-catenin expression, CRT, and Wnt ligand expression are gained through an unknown mechanism in MM cells. Our independent analysis of the Wnt pathway in the transcriptome of patient MM tumors versus PCs, as well as tumor-associated stromal cells (data not shown), confirmed an up-regulation of nearly all Wnt signaling pathway members. Furthermore, expression of the central Wnt signaling molecule β-catenin was seen predominantly in the nonphosphorylated, active form and translocated to the nucleus in the majority of MM cells.
We hypothesized that targeting this pathway may represent a novel therapeutic approach to treat MM. The identification of molecular inhibitors of the β-catenin/TCF4 complex provided an opportunity for investigating the biological sequelae of inhibiting Wnt signaling in MM. We have shown here that Wnt transcriptional activity was specifically blocked in MM cell lines in response to treatment with PKF115–584, evidenced by reduced Wnt-responsive luciferase reporter expression, even with exogenous β-catenin or Wnt3A signal, and corresponded with down-regulation of CRT target genes c-MYC and Cyclin D1. However, some upstream Wnt pathway components as well as some downstream target genes were up-regulated in response to PKF115–584, which may reflect differences in the regulation of Wnt genes according to cell type (lymphoid vs. epithelial tissues). Moreover, some target genes displayed contrasting patterns of expression in response to drug treatment. The mechanisms of activating and regulating Wnt pathway genes in MM are currently under investigation.
Importantly, PKF115–584 induced cell cycle arrest and apoptosis in MM cells through activation of apoptotic regulators. The proliferation and viability of MM cell lines were significantly inhibited in a time- and dose-dependent manner. IC50 values ranged from 150 to 450 nM, which are clinically achievable. Similar cytotoxic effects were also seen in patient MM cells, but not in normal PCs. Although BMSCs confer protection against conventional therapies, PKF115–584 maintained MM cell cytotoxicity even in the presence of BMSCs or other prosurvival factors. Finally, we observed synergistic anti-MM activity in combinational experiments with Bortezomib and dexamethasone. Altogether, we believe our data strongly support the evaluation of a Wnt pathway antagonist, such as PKF115–584, in combinational clinical protocols.
Although transplanted human MM cells in SCID mice were effectively treated by PKF115–584, the role of the Wnt pathway in the BM HSCs (9) likely accounts for hypoplastic BM in treated mice. This apparent deleterious effect of the drug in the stem cell compartment supports preclinical studies by using Wnt inhibitors in the treatment of myeloid and lymphoid leukemia, particularly in patients with chronic myeloid leukemia in blast crisis (23) and pre-B-ALL associated with E2A-Pbx1 transcription factor and enhanced Wnt signaling (5).
However, Wnt signaling within the BM is essential for osteoblast differentiation and maintaining bone density (24). Dikkopf-1, an inhibitor of Wnt signaling and expressed by primary MM cells, uncouples bone absorption by promoting osteoclast activity and blocking osteogenesis, leading to the development of bone lytic lesions (25). Therefore, the inhibitory effect of PKF115–584 against Wnt signaling may exacerbate bone disease in some MM patients. Collectively, these considerations highlight the need for additional preclinical studies to investigate possible toxicities to define optimal dosage and schedules of drug administration, both alone and in combination with other therapies.
Our studies further provide the rationale for synthesizing molecules targeting Wnt signaling for high-throughput screening, facilitated by the use of Wnt reporter systems in MM cells. Finally, our studies highlight the need to identify the specific ligands produced in the BM microenvironment, as well as the corresponding receptor on MM cells, to enhance our understanding of the role of Wnt signaling in MM pathogenesis and develop novel and more specific therapies such as monoclonal antibodies to further target this pathway.
Materials and Methods
Cell Lines and Reagents.
Cultured MM cell lines included MM1S (Dex-sensitive) and MM1R (Dex-resistant) from Steven Rosen (Northwestern University, Evanston, IL); RPMI8226, Dox40 (doxorubicin-resistant), and LR5 (melphalan-resistant) from William Dalton (Moffit Cancer Center, Tampa, FL); OPM2 and KMS34 from Leif Bergsagel (Mayo Clinic, Scottsdale, AZ); OPM1 from Edward Thompson (University of Texas Medical Branch, Galveston, TX); and IL-6-dependent INA-6 cells from Renate Burger (University of Erlangen-Nuernberg, Germany). With the exception of Wnt3A stimulation experiments, cell lines were maintained in RMPI 1640 media containing 10% FBS (Sigma–Aldrich, St. Louis, MO), 2 μmol/liter l-glutamine, 100 units/penicillin, and 100 μg/ml streptomycin (Life Technologies, Paisley, U.K.). Samples for expression profiling shown in Fig. 1A were obtained and described previously (20). BM specimens were obtained from MM patients after informed consent under the auspices of a Dana–Farber Cancer Institute Institutional Review Board-approved protocol. Mononuclear cells (Cambrex, Hopkinton, MA) separated by Ficoll–Hypaque density sedimentation were used to establish long-term BMSC cultures as described previously (26, 27). Plasma cells (CD138+) from healthy donors were purified by using magnetic beads (Miltenyi Biotec, Auburn, CA). Wnt3A-conditioned media were collected from cultured L cell (kindly provided by Stuart Rudikoff, National Cancer Institute/National Institutes of Health, Bethesda, MD) supernatants as previously described (11). Lyophilized PKF115–584 was acquired from Novartis (Basel, Switzerland), resuspended in DMSO (10 mM), and frozen at −20°C until use. Dexamethasone (Sigma–Aldrich) and Bortezomib were used as previously described (28).
Gene Expression Profiling.
The gene expression data for results shown in Fig. 1A were obtained from the National Center for Biotechnology Information Gene Expression Omnibus (GEO; www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO series accession no. GSE4452 (20). For drug treatment, KMS34 and MM1S cells (5 × 106) were cultured with 100 nM PKF115–584 for 16 h, washed in PBS, and resuspended in TRIzol Reagent (Invitrogen, Carlsbad, CA). RNA was extracted according to the manufacturer's protocol, purified by using an RNeasy kit (Qiagen, Valencia, CA), and run on an Affymetrix (Santa Clara, CA) U133A 2.0 array chip as per the manufacturer's instructions. Expression data were analyzed in DChip Analyzer 4.0 (29) by using invariant-set normalization (perfect match only) and model-based expression index to summarize the expression value for each probe set; a ≥2-fold change (90% confidence interval) between treated and untreated samples was identified as significant (30). Hierarchical clustering was performed by using one Pearson correlation distance and average linkage.
TOP-/FOP-FLASH Wnt Reporter.
MM cells (5.0 × 105) were transfected with TOP-FLASH or FOP-FLASH Wnt reporter plasmids (Millipore Corporation, Billerica, MA) containing wild-type or mutant TCF DNA binding sites or empty vector pcDNA3.1(+) by using FuGENE 6 (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's protocol. Cells were also cotransfected with the hRL-Null Renilla plasmid. To determine Wnt activity in response to stimulation, TOP-/FOP-FLASH-transfected cells were either (i) cotransfected with wild-type β-catenin (generously provided by Hans Clevers, Hubrecht Laboratory, the Netherlands) or pcDNA3.1, or (ii) treated with control or Wnt3A-conditioned medium for 12 h before treatment with 200 nM PKF115–584 for an additional 12 h. Reporter activity was assayed by using the Dual Luciferase Assay System (Promega, Madison, WI). Results were normalized to total protein amounts and Renilla values of each sample. The reporter assay results represent the average of three independent transfection experiments. The TOP-/FOP-GFP Wnt reporter (9) is described in SI Fig. 6.
Immunoblotting.
Total-cell lysates for Western blots were prepared as previously described (27). Immunoblotting was done by using antibodies against β-catenin (CAT5-H10; Zymed Laboratories, South San Francisco, CA), TCF4 (6H5–3; Upstate Biotechnology, Lake Placid, NY), c-MYC (OP10; Calbiochem, San Diego, CA), as well as caspase-3, -8 (IC12), -9, PARP, Cyclin D1 (DSS6), and p-Akt (Ser-473) (Cell Signaling Technology, Danvers, MA). Anti-PTEN, p-STAT3 (B-7), p-ERK (E-4), ERK2 (D-2), and tubulin (D-10) primary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The BCL9 antibody was from polyclonal rabbit serum (D.R.C., unpublished data). Secondary antibodies conjugated to horseradish peroxidase, as well as loading control antibody pan-actin-HRP (C-11), were acquired from Santa Cruz Biotechnology. Nuclear and cytoplasmic fractions were prepared as previously described (31).
Immunofluorescence.
Cytospin samples of cultured MM cell lines were prepared by using a cytocentrifuge (Thermo Shandon, Waltham, MA). Cells spun onto cytoslides (Thermo Shandon) were fixed and stained as described previously (32). Anti-κ and -λ light chain antibodies conjugated to FITC were obtained from DakoCytomation (Carpinteria, CA). β-catenin (CAT5-H10; Zymed Laboratories) and TCF4 antibodies (6H5–3; Upstate Biotechnology) were added for 2 h at room temperature and then washed three times in PBS. Primary antibodies were visualized with secondary antibodies conjugated to FITC or rhodamine (5 μg/ml; Southern Biotechnology Associates, Birmingham, AL).
β-Catenin Localization.
MM cells (5.0 × 106) were cultured with 0, 200, and 800 nM concentrations of PKF115–584 for 12 h under standard conditions. Cells were pelleted and washed with 1× PBS before nuclear and cytoplasmic fractions were separated for immunoblotting.
Proliferation and Cell Viability Assays.
DNA synthesis was measured by incorporation of [3H]thymidine (Amersham Biosciences, Piscataway, NJ) as described previously (33). Briefly, 3 × 104 cells were incubated in a 96-well plate (Costar, Cambridge, MA) with or without BMSC, IL-6, or Wnt3A in the presence of PKF115–584 for 40 h. Cells were pulsed with 0.5 μCi 3TdR per well for the last 8 h, and scintillation counts were measured by using LKB Betaplate (Wallac, Gaithersburg, MD). Human IL-6 (R&D Systems, Minneapolis, MN) was used at a concentration of 10 ng/ml for stimulation assays. Proliferation with Wnt3A conditional media was performed in serum-free media as described previously (7, 11). Experiments were performed in triplicate and repeated three times.
Colormetric assays were used to assess cell viability after treatment with PKF115–584 at indicated concentrations for 40 h. WST-1 reagent (Roche) was then added, with metabolic conversion of tetrazolium WST-1 salt to formazan by mitochondrial enzymes. Absorbance readings were measured after 4 h on a spectrophotometer (Molecular Devices, Sunnyvale, CA) at a 450-nm wavelength and referenced against 650-nm readings.
Cell Cycle Analysis.
Cell cycle analysis was assessed by using BrdU incorporation and PI staining by using standard procedures (34). KMS34 or MM1S cells (1 × 106) were incubated with 200 nM PKF115–584 for 12 h and then analyzed on a FACScan cytometer.
In Vivo Experimentation.
To generate stable GFP-expressing KMS34 cells, we first created lentiviral particles containing a vector with GFP under the constitutively active cytomegalovirus promoter by cotransfecting 293T cells by using the five plasmid methodology (a generous gift of Richard Mulligan, Children's Hospital, Boston, MA) (35, 36). FACScan analysis confirmed that KMS34-GFP+ cells highly expressed GFP. We then injected (i.v.) 10-week-old NOD.CB17-PrkdcSCID/J mice (The Jackson Laboratory, Bar Harbor, ME) with 1 × 106 KMS34-GFP+ cells; treatment began 1 week later. Control mice (n = 9) received DMSO, whereas treated mice (n = 10) received 0.16 mg/kg PKF115–584 every other day via i.p. injections. The cohorts were monitored daily. Severely morbid animals were killed, and autopsies were performed. GFP tumor nodules were visualized by using the LT-9500 florescent light box (Lighttools Research, Encintas, CA). All tissues were formalin-fixed and embedded in paraffin, sectioned, and stained with H&E. All animal experiments were approved by and conform to the standards of the Institutional Animal Care and Use Committee at the Dana–Farber Cancer Institute.
Supplementary Material
Acknowledgments
We thank Dr. Teru Hideshima for his valuable comments and assistance with the manuscript, Dr. Hans Clevers (Hubrecht Laboratory, The Netherlands) for providing the wild-type β-catenin plasmid, and Dr. Irving Weissman (Stanford University, Palo Alto, CA) for the GFP reporter lentiviral vectors used in this study. This work was funded by a K08 SPORE Mentored Scientist Award. D.R.C. is a Sydney Kimmel Foundation Scholar.
Abbreviations
- BM
bone marrow
- BMSC
bone marrow stromal cell
- CRT
β-catenin/TCF-regulated transcription
- HSC
hematopoietic stem cell
- MM
multiple myeloma
- PCs
plasma cells
- PI
propidium iodide.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610299104/DC1.
References
- 1.Rajkumar SV, Kyle RA. Mayo Clin Proc. 2005;80:1371–1382. doi: 10.4065/80.10.1371. [DOI] [PubMed] [Google Scholar]
- 2.Mitsiades CS, Mitsiades N, Munshi NC, Anderson KC. Cancer Cell. 2004;6:439–444. doi: 10.1016/j.ccr.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Chauhan D, Uchiyama H, Akbarali Y, Urashima M, Yamamoto K, Libermann TA, Anderson KC. Blood. 1996;87:1104–1112. [PubMed] [Google Scholar]
- 4.Vacca A, Ria R, Semeraro F, Merchionne F, Coluccia M, Boccarelli A, Scavelli C, Nico B, Gernone A, Battelli F, et al. Blood. 2003;102:3340–3348. doi: 10.1182/blood-2003-04-1338. [DOI] [PubMed] [Google Scholar]
- 5.McWhirter JR, Neuteboom ST, Wancewicz EV, Monia BP, Downing JR, Murre C. Proc Natl Acad Sci USA. 1999;96:11464–11469. doi: 10.1073/pnas.96.20.11464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Den Berg DJ, Sharma AK, Bruno E, Hoffman R. Blood. 1998;92:3189–3202. [PubMed] [Google Scholar]
- 7.Derksen PW, Tjin E, Meijer HP, Klok MD, MacGillavry HD, van Oers MH, Lokhorst HM, Bloem AC, Clevers H, Nusse R, et al. Proc Natl Acad Sci USA. 2004;101:6122–6127. doi: 10.1073/pnas.0305855101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reya T, Clevers H. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 9.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 10.Staal FJ, Clevers HC. Nat Rev Immunol. 2005;5:21–30. doi: 10.1038/nri1529. [DOI] [PubMed] [Google Scholar]
- 11.Qiang YW, Endo Y, Rubin JS, Rudikoff S. Oncogene. 2003;22:1536–1545. doi: 10.1038/sj.onc.1206239. [DOI] [PubMed] [Google Scholar]
- 12.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 13.Tetsu O, McCormick F. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 14.Chen S, Guttridge DC, You Z, Zhang Z, Fribley A, Mayo MW, Kitajewski J, Wang CY. J Cell Biol. 2001;152:87–96. doi: 10.1083/jcb.152.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang M, Wang Y, Sun D, Zhu H, Yin Y, Zhang W, Yang S, Quan L, Bai J, Wang S, et al. BMC Cancer. 2006;6:221–231. doi: 10.1186/1471-2407-6-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bienz M, Clevers H. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 17.Kinzler KW, Vogelstein B. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 18.Polakis P. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 19.Lepourcelet M, Chen YN, France DS, Wang H, Crews P, Petersen F, Bruseo C, Wood AW, Shivdasani RA. Cancer Cell. 2004;5:91–102. doi: 10.1016/s1535-6108(03)00334-9. [DOI] [PubMed] [Google Scholar]
- 20.Carrasco DR, Tonon G, Huang Y, Zhang Y, Sinha R, Feng B, Stewart JP, Zhan F, Khatry D, Protopopova M, et al. Cancer Cell. 2006;9:313–325. doi: 10.1016/j.ccr.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 21.Gupta D, Treon SP, Shima Y, Hideshima T, Podar K, Tai YT, Lin B, Lentzsch S, Davies FE, Chauhan D, et al. Leukemia. 2001;15:1950–1961. doi: 10.1038/sj.leu.2402295. [DOI] [PubMed] [Google Scholar]
- 22.Hideshima T, Nakamura N, Chauhan D, Anderson KC. Oncogene. 2001;20:5991–6000. doi: 10.1038/sj.onc.1204833. [DOI] [PubMed] [Google Scholar]
- 23.Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, Gotlib J, Li K, Manz MG, Keating A, et al. N Engl J Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 24.Krishnan V, Bryant HU, Macdougald OA. J Clin Invest. 2006;116:1202–1209. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD., Jr Blood. 2006 doi: 10.1182/blood-2006-09-047712. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hideshima T, Chauhan D, Richardson P, Mitsiades C, Mitsiades N, Hayashi T, Munshi N, Dang L, Castro A, Palombella V, et al. J Biol Chem. 2002;277:16639–16647. doi: 10.1074/jbc.M200360200. [DOI] [PubMed] [Google Scholar]
- 27.Raje N, Kumar S, Hideshima T, Ishitsuka K, Yasui H, Chhetri S, Vallet S, Vonescu E, Shiraishi N, Kiziltepe T, et al. Br J Haematol. 2006;135:52–61. doi: 10.1111/j.1365-2141.2006.06261.x. [DOI] [PubMed] [Google Scholar]
- 28.Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, Anderson KC. Cancer Res. 2001;61:3071–3076. [PubMed] [Google Scholar]
- 29.Li C, Hung Wong W. Genome Biol. 2001;2:RESEARCH0032. doi: 10.1186/gb-2001-2-8-research0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davies FE, Dring AM, Li C, Rawstron AC, Shammas MA, O'Connor SM, Fenton JA, Hideshima T, Chauhan D, Tai IT, et al. Blood. 2003;102:4504–4511. doi: 10.1182/blood-2003-01-0016. [DOI] [PubMed] [Google Scholar]
- 31.Schreiber E, Matthias P, Muller MM, Schaffner W. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stegh AH, Herrmann H, Lampel S, Weisenberger D, Andra K, Seper M, Wiche G, Krammer PH, Peter ME. Mol Cell Biol. 2000;20:5665–5679. doi: 10.1128/mcb.20.15.5665-5679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raje N, Kumar S, Hideshima T, Ishitsuka K, Chauhan D, Mitsiades C, Podar K, Le Gouill S, Richardson P, Munshi NC, et al. Blood. 2004;104:4188–4193. doi: 10.1182/blood-2004-06-2281. [DOI] [PubMed] [Google Scholar]
- 34.Cormier SA, Mello MA, Kappen C. BMC Dev Biol. 2003;3:4. doi: 10.1186/1471-213X-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 36.Mostoslavsky G, Kotton DN, Fabian AJ, Gray JT, Lee JS, Mulligan RC. Mol Ther. 2005;11:932–940. doi: 10.1016/j.ymthe.2005.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.