Abstract
Rapid growth could significantly reduce methylmercury (MeHg) concentrations in aquatic organisms by causing a greater than proportional gain in biomass relative to MeHg (somatic growth dilution). We hypothesized that rapid growth from the consumption of high-quality algae, defined by algal nutrient stoichiometry, reduces MeHg concentrations in zooplankton, a major source of MeHg for lake fish. Using a MeHg radiotracer, we measured changes in MeHg concentrations, growth and ingestion rates in juvenile Daphnia pulex fed either high (C:P = 139) or low-quality (C:P = 1317) algae (Ankistrodesmus falcatus) for 5 d. We estimated Daphnia steady-state MeHg concentrations, using a biokinetic model parameterized with experimental rates. Daphnia MeHg assimilation efficiencies (≈95%) and release rates (0.04 d−1) were unaffected by algal nutrient quality. However, Daphnia growth rate was 3.5 times greater when fed high-quality algae, resulting in pronounced somatic growth dilution. Steady-state MeHg concentrations in Daphnia that consumed high-quality algae were one-third those of Daphnia that consumed low-quality algae due to higher growth and slightly lower ingestion rates. Our findings show that rapid growth from high-quality food consumption can significantly reduce the accumulation and trophic transfer of MeHg in freshwater food webs.
Keywords: contaminants, food quality, heavy metals, nutrient stoichiometry, plankton
Methylmercury (MeHg) poses a serious human and wildlife health risk primarily through fish consumption, so understanding the key factors driving MeHg accumulation in fish has become a global priority (1). Rapid somatic growth rates are hypothesized to reduce mass-specific MeHg concentration (burden) in fish and other aquatic organisms (2, 3) by the process of somatic growth dilution (SGD). SGD occurs when rapid growth results in a disproportionate increase in the net rate of biomass gain relative to MeHg gain. The relative quality of food consumed can strongly influence growth rates in aquatic organisms. Moreover, food quality for aquatic consumers varies widely across lakes and seasons (4), thus potentially contributing to the large variation in Hg concentrations observed in lake fish in situ. Despite its potential importance, the influence of food quality on MeHg accumulation has not been well examined.
At present, evidence for somatic growth dilution of MeHg, whether due food quality or other factors, is sparse and somewhat contradictory. Thus far, our understanding of SGD has been limited to inferences drawn from field correlations between somatic growth rates and Hg concentrations in fish. In most of these studies, the many possible factors driving SGD (e.g., temperature, food availability, food quality, activity level, and stress) are not controlled and are often confounded. Negative correlations between somatic growth rate and concentrations of total Hg (5–8) and other contaminants, such as Pb (9), have been found for fish. However, other studies have found no (10), or positive correlations between fish growth and Hg concentrations (11). This discrepancy may be because the effects of growth on Hg accumulation strongly depend on the particular mechanism driving growth, which is often difficult to determine in situ. For example, in the studies by Stafford and Haines (10) and Dutton (11), it is possible that high fish consumption rates increased both net biomass and net Hg gain through increased food-borne Hg ingestion, effectively maintaining, or even increasing mass-specific Hg concentrations. Thus, a complete mass-balance approach that accounts for all inputs, assimilation and outputs of both Hg and total biomass is necessary to identify the conditions under which SGD can occur.
SGD is likely to occur when growth increases with relatively little or no change in Hg gain. A disproportionate increase in biomass gain relative to Hg gain can occur when activity or respiration rates are relatively low (12, 13) or when food quality is high. Organisms consuming high-quality food gain more biomass per unit food consumed, hence per unit Hg consumed, than from low-quality food. Thus, our general hypothesis is that, all else being equal, consumption of high-quality food can cause greater SGD of Hg compared with the consumption of low-quality food.
Somatic growth dilution of Hg in zooplankton due to food quality may explain variation in fish Hg concentrations across lakes that differ in nutrient availability. Recent studies have found a positive correlation between the relative availability of N and P and fish mercury concentrations from hundreds of lakes (14, 15). High availability of P relative to N (i.e., low N:P) results in a low algal N:P ratio (indicative of high algal quality as food), causing rapid growth in zooplankton, particularly Daphnia (4, 16). A large body of work has found that Daphnia consistently grow more efficiently on phytoplankton with high P content relative to N or C (16–18) because of their high P demand for protein synthesis (19). Hence, SGD may cause zooplankton in lakes with low N:P algae to have lower MeHg concentrations. Fish consuming these zooplankton, in turn, should accrue less MeHg, thereby propagating the effects of SGD through the food web. In this way, differences in growth rates of zooplankton due to natural variation in phytoplankton nutrient quality could drive considerable differences in mercury concentrations in fish from different lakes even if mercury concentrations in the water do not differ.
This study mechanisticly demonstrates somatic growth dilution of mercury in consumers. We experimentally test the general hypothesis that phytoplankton nutrient stoichiometry (C:P) affects MeHg accumulation in juvenile Daphnia because of SGD. We fed juvenile Daphnia either high-quality (HiQ, low C:P) or low-quality (LoQ, high C:P) green algae (Ankistrodesmus falcatus) radiolabeled with Me203Hg. We nondestructively measured Daphnia MeHg assimilation and MeHg release, or efflux rates, as well as growth and ingestion rates over the juvenile growth period and parameterized a highly predictive biokinetic model (20) with these rates to estimate steady-state MeHg concentrations in Daphnia. We predicted that steady-state MeHg concentrations would be significantly lower in Daphnia fed high-quality, low C:P phytoplankton than Daphnia fed low-quality, high C:P phytoplankton because of somatic growth dilution.
Results
Phytoplankton Treatment Conditions.
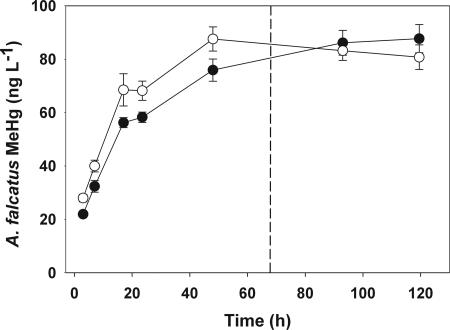
We established A. falcatus treatments with atomic ratios of 139:1 C:P, 15:1 N:P (HiQ), and 1317:1 C:P, 92:1 N:P (LoQ) (“experimental conditions” in Table 1). Me203Hg uptake by A. falcatus cells was similar in both the HiQ and LoQ treatments (Fig. 1). After harvesting radioactively labeled A. falcatus cells and resuspending them in nonradioactive media, algal Me203Hg concentration based on cell dry weight was higher in HiQ cells (114 ng mg−1) than in LoQ cells (62 ng mg−1) (“experimental conditions” in Table 1), because LoQ cell density in the culture flasks (3.13 104 cells ml−1) was higher than HiQ cell density (2.75 104 cells ml−1). These differences in algal Me203Hg concentrations have no effect on Daphnia Me203Hg assimilation and efflux estimates, which are normalized to initial exposure levels.
Table 1.
Experimental treatment conditions and Daphnia physiological responses
| Treatment | Experimental conditions |
Daphnia responses |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C:P, atomic (n = 3) | N:P, atomic (n = 3) | Cell mass, pg d.w. cell−1 (n = 4) | Cell volume, μm3 cell−1 (n = 15) | Algal MeHg concentration, ng Hg mg−1*(n = 3) | MeHg AE, %[n = 6 (HiQ), 5 (LoQ)] | MeHg efflux rate, d−1[n = 6 (HiQ), 5 (LoQ)] | tb½, d[n = 6 (HiQ), 5 (LoQ)] | Specific ingestion rate, mg mg−1 d−1[n = 36 (HiQ), 35 (LoQ)] | Specific growth rate, mg mg−1 d−1(n = 6) | Hg burden at day 5, % | |
| HiQ | 139 ± 14 | 15.25 ± 0.56 | 0.029 ± 0.004 | 150.29 ± 38.43 | 113.54 ± 0.56 | 96.02 ± 11.15 | 0.0415 ± 6 × 10−5 | 16.70 ± 0.03 | 1.296 ± 0.110 | 0.254 ± 0.011 | 16 |
| LoQ | 1,317 ± 174 | 91.84 ± 2.73 | 0.044 ± 0.006 | 156.50 ± 24.56 | 62.48 ± 0.54 | 94.75 ± 12.83 | 0.0413 ± 8 × 10−5 | 16.77 ± 0.03 | 1.694 ± 0.108 | 0.069 ± 0.004 | 34 |
Values are means ± SE.
*Algae MeHg concentrations were based on ng Hg mg−1 dry weight after resuspension.
Fig. 1.
A. falcatus Me203Hg uptake over 5 d. Values are means ± SE (bars) based on ng MeHg associated with cells per liter of culture. Open circles represent LoQ cells; filled circles represent HiQ cells. The dashed line indicates when cells were harvested at 67.5 h.
Daphnia Growth and Me203Hg Dynamics.
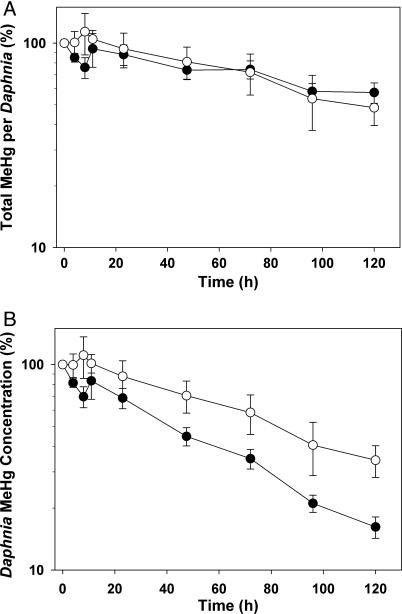
Daphnia specific growth rate on the HiQ diet was ≈3.5 times greater than that on the LoQ diet (t10 = 2.23; P < 0.0001) (“Daphnia responses” in Table 1). As a result, the biomass of individuals fed HiQ algae at day 5 was ≈3 times higher than those fed LoQ algae. Overall, Me203Hg depuration (percent Me203Hg remaining) over 5 d was similar between Daphnia fed high- and low-quality algae (Fig. 2A). There was no significant difference in Daphnia Me203Hg assimilation efficiency (≈95%), efflux rate (≈0.041 d−1), or biological half-life (≈17 d) between HiQ and LoQ treatments (“Daphnia responses” in Table 1). However, at the end of the 5-d depuration period, Daphnia mass-specific Me203Hg concentration (ng Hg mg−1 dry weight) was twice as reduced when consuming HiQ algae (16% remaining) than when consuming LoQ algae (34% remaining) (t10 = 2.23; P = 0.017) (Fig. 2B and “Daphnia responses” in Table 1).
Fig. 2.
Depuration of the Me203Hg pulse over 5 d, normalized to T = 0 after pulse (%). (A) Total MeHg (ng Hg) remaining in individual Daphnia. (B) MeHg concentration (ng Hg per mg of dry weight) remaining in Daphnia. Filled circles represent the HiQ treatment; open circles represent the LoQ treatment. Values are means ± SE (bars).
Daphnia Ingestion Rates.
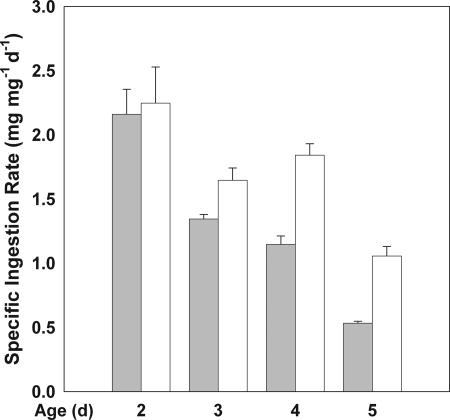
Overall, Daphnia specific ingestion rates were higher on LoQ algae than on HiQ algae (F1,15 = 24.45; P = 0.0002) (Fig. 3 and “Daphnia responses” in Table 1). However, differences in ingestion between treatments were smaller than differences in growth rate (“Daphnia responses” in Table 1). The repeated measures analysis also revealed significant differences in specific ingestion rate over time (F1.18, 17.72 = 28.25; P < 0.0001). There was no significant time-by-treatment interaction.
Fig. 3.
Specific ingestion rates in 2–5 d old Daphnia fed HiQ (shaded) and LoQ (white) A. falcatus cells. Values are means + SE (bars).
Steady-State MeHg Concentrations Based on Experimental Conditions.
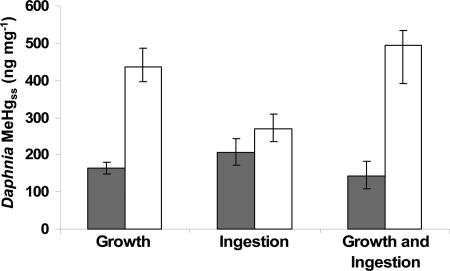
The biokinetic model indicated that differences in ingestion and growth rate result in a steady-state MeHg concentration (MeHgss) that is 3.5 times higher in Daphnia feeding on LoQ algae than in Daphnia consuming HiQ algae (Fig. 4). Due to the reduced growth rate alone, MeHgss in Daphnia fed LoQ algae is 2.5 times higher than MeHgss in Daphnia fed HiQ algae (Fig. 4). Similarly, because of increased ingestion rate alone, MeHgss in Daphnia fed LoQ algae is 1.3 times higher than the MeHg concentration in Daphnia fed HiQ algae (Fig. 4). Thus, growth rate differences between HiQ and LoQ algae consumption are predicted to have a larger effect on MeHgss than ingestion rate differences at these nutrient levels.
Fig. 4.
Daphnia steady-state MeHg concentration based on response to experimentally measured rates (growth, ingestion, growth and ingestion) from the consumption of HiQ (shaded) and LoQ (white) algae. Error bars represent the range of values (minimum, mean, maximum) estimated based on the observed confidence limits of growth and ingestion rates.
Discussion
This study shows that the consumption of high-quality food reduces MeHg accumulation in consumers. Specifically, we showed that consumption of high-quality phytoplankton, defined by nutrient stoichiometry, reduces MeHg accumulation in Daphnia. This result has several implications. First, even though Daphnia have little regulatory control over the tendency of MeHg to persist in somatic tissue, consumption of high-quality, low C:P phytoplankton can considerably reduce Daphnia MeHg concentrations by somatic growth dilution. Second, reduced consumption rates, which can accompany feeding on high-quality, low C:P algae, may further reduce MeHg accumulation. Together, the effects of SGD and reduced consumption of high-quality food are likely to propagate through the food web, reducing Hg concentrations in fish. This expectation is consistent with widespread observations of lower fish Hg concentrations in relatively productive aquatic systems that have relatively high P availability (14, 15).
Our primary result is that somatic growth driven by food quality can strongly influence MeHg accumulation. In our study, Daphnia MeHg assimilation and efflux rates were unaffected by food quality. However, Daphnia grew faster on high-quality, low C:P algae (“Daphnia responses” in Table 1) resulting in a significant reduction in the concentration of MeHg in their tissues. Daphnia MeHg efflux rates (≈0.041 d−1) were consistent with those found in other studies (21, 22) and were lower than the efflux rates of other heavy metals, such as Cd, Cr, Se, Zn (23), and inorganic Hg (24). Low MeHg efflux rates result in the buildup of high levels of MeHg in somatic tissue. Thus, SGD may be a particularly important process by which organisms abate the accumulation of MeHg and other biologically persistent substances such as other metals (25) and chlorinated hydrocarbon compounds (26).
Unlike MeHg assimilation and retention, daphnid growth varies greatly with algal nutrient stoichiometry. Moreover, the effect of algal stoichiometry on growth is greater than the effect of the quantity of algae consumed (Fig. 3), as shown in other studies (27–29). This is largely because Daphnia consuming high-nutrient-quality algal food (low C:P) incorporate a greater fraction of ingested C into tissue (thus increasing net biomass gain) than do Daphnia consuming low nutrient quality algae (high C:P) (30). This can occur through differences in C assimilation through the gut (29) or C respiration (31). Either way, the consumption of high nutrient quality algae can increase Daphnia growth without a concomitant increase in the quantity of MeHg ingested. The trend of faster growth from high-quality, low C:P algae has been found for numerous daphnid and algal species (16, 17, 32). Therefore, stoichiometric controls of SGD may occur in many other Daphnia species that commonly dominate aquatic food webs, making SGD an important, yet generally overlooked factor capable of explaining much variation in zooplankton mercury concentrations across lakes.
Reduced consumption of high-quality algae can act in concert with rapid growth to further dilute Daphnia MeHg concentrations. Because of lower ingestion rates, Daphnia consuming high-quality algae may reduce their intake of food-borne MeHg. Together, lower ingestion and increased growth from a high-quality diet reduced steady-state Hg concentration to a value one-third that of Daphnia fed the LoQ diet. Similarly, when compensatory feeding of low-quality food occurs, higher ingestion and slower growth would both increase MeHg accumulation. Compensatory feeding has been found in other studies of Daphnia (33, 34) although it is not universal. For example, some studies have found no effect of P limitation on Daphnia ingestion rates (18, 31) or lower ingestion of low-quality food (27, 35). Nevertheless, the effect of ingestion rate on Hg accumulation was minimal compared with the growth dilution effect in our study. This suggests that even a reversal of ingestion rates, i.e., higher consumption of high-quality food, would only slightly reduce the net dilution of Hg concentration. Moreover, compensatory feeding has been found in a variety of organisms (36–38) and its potential to enhance the accumulation of MeHg and other contaminants merits further study.
In summary, our results clearly demonstrate that a low algal C:P ratio substantially reduces the trophic transfer of MeHg to Daphnia through somatic growth dilution and, to a lesser extent, reduced consumption rates. A reduction in MeHg in Daphnia is most likely to reduce fish MeHg concentrations in lakes where Daphnia are a primary food source for fish. SGD of mercury in Daphnia has the potential to reduce mercury accumulation in food webs of relatively productive lakes with relatively high P availability. Additionally, when high nutrient availability stimulates rapid population growth of algae (39) and zooplankton (28), the processes of algal bloom dilution (40–42) and zooplankton density dilution (43), respectively, may further reduce zooplankton MeHg concentrations. Consistent with our findings for MeHg, studies of other biologically persistent contaminants have found negative relationships between total P and fish concentrations for PCBs (44, 45) and other chlorinated hydrocarbons (46). Thus, low C:P stoichiometry may cause a combination of multiple dilution processes that influences the accumulation of MeHg and other persistent contaminants in freshwater organisms. Other, system-specific metrics of food quality or nutrient limitation may influence the accumulation of MeHg and other biologically persistent contaminants in any organism through SGD. Hence, exploring the prevalence of SGD under a range of field conditions could help predict conditions leading to high or low contaminant concentrations in organisms across a broad spectrum of ecosystems.
Methods
Algae and Zooplankton Culturing.
Cultures of the chlorophyte, A. falcatus var. acicularis (UTEX clone 101; University of Texas, Austin, TX) were maintained under two different nutrient conditions, a high nutrient quality treatment (HiQ, 15:1 atomic N:P) and a low nutrient quality treatment (LoQ, 110:1 atomic N:P). These nutrient levels are known to have contrasting effects on Daphnia growth and reproduction (27, 28) and are well within the range typically found in lakes throughout the northeastern United States (14). Algae were cultured according to treatment by using a modified Woods Hole MBA medium without buffer (47) enriched with a vitamin mixture (48) [see supporting information (SI) Table 2 for media composition]. All culture flasks, tubing, filters, and other culturing supplies were autoclaved. Culturing media were filtered through a sterilized 0.22-μm filter into five replicate flasks for each treatment. A sterile inoculum of A. falcatus was added to each flask. Cultures were continually aerated through a 0.22 μm membrane filter and incubated under continuous light at 20°C. After ≈8 d, algal cultures were in log-phase growth, at which time cells were harvested for experimental feeding. For each treatment, harvested cells were filtered, rinsed, and resuspended in fresh media and stored in a common flask. This algae storage medium was the same as the Woods Hole medium without EDTA, vitamins, or trace elements. At this time, algal cells were sampled for nutrient concentrations, cell density, and cell volume.
Before the experiments, cultures of a clonal isolate of a Daphnia pulex/Daphnia pulicaria hybrid (log52 clone; Indiana University, Bloomington, IN) had been maintained in modified Daphnia COMBO media (49) (see SI Table 3 for media composition) and fed on HiQ A. falcatus for 65 generations. Same-age neonates (<24 h old) were isolated from maintenance cultures one generation before experiments. These “brood females” provided neonates for experiments. For both the radiolabel feeding-depuration and ingestion rate experiments, <24-h-old neonates from the third brood clutch were isolated and alternately assigned to either the HiQ or LoQ treatment. Experimental animals were kept in fresh COMBO media without P (P-free media) to ensure that algae were the only source of phosphorus to the animals. Experimental neonates were fed HiQ or LoQ algae according to treatment for 24 h before the experiments to allow individuals to acclimate to their food.
Algae Me203Hg Radiolabeling.
To track Daphnia assimilation and depuration of mercury, we used an organic, methylated form of the γ-emitting radioisotope 203Hg. We chose to examine the trophic transfer of methylmercury (CH3203Hg+, or MeHg), because this particular form of mercury is known to biomagnify through food webs (50), and therefore has a greater potential for toxicity through food consumption than inorganic mercury. MeHg was synthesized from 203Hg according to methods described (ref. 51 and references therein). The specific activity of the resulting Me203Hg was 127 kBq μg−1.
In labeling A. falcatus cells with Me203Hg, our goal was to minimize differences in MeHg uptake between HiQ and LoQ algae to isolate the effects of algal nutrient stoichiometry on Daphnia SGD of Me203Hg. We did not test for the effects of nutrient stoichiometry on A. falcatus Me203Hg uptake. For each treatment, A. falcatus cells were added to six replicate flasks of 250 ml of HiQ or LoQ algae storage media to a density of 0.1 mg of dry weight liter−1. Three control flasks per treatment contained only media to control for the adsorption of the Me203Hg label to the sampling filters. Each replicate flask received Me203Hg to give an aqueous concentration of 0.58 nM (≈115 ng/liter) at the initial time point. Whereas this concentration is higher than those typical of unpolluted lakes (1–2 ng liter−1, 50), it allowed us to monitor the Me203Hg over a number of days and is not known to cause toxic effects over short-term exposure (52, 53). To minimize differences in algal Me203Hg uptake and cell concentrations between treatments, cell growth was minimized by holding the cells in darkness (flasks were wrapped in foil). The cultures were incubated at 17°C for 5 d.
A. falcatus Me203Hg uptake was monitored at multiple time points over 5 d. At each time point, radioactivity associated with the algal cells was assessed by filtering 10-ml aliquots from each flask onto 1-μm polycarbonate membranes, following the method of Fisher et al. (54). After 67.5 h, ≈72% of the label had been taken up by HiQ and LoQ algae. To expose the Daphnia to radiolabeled algae without aqueous exposure to Me203Hg, the labeled algal cells were separated from their radioactive water by filtering 20 ml of labeled A. falcatus cell suspension from each flask onto polycarbonate membranes and resuspending them into a common flask with fresh, unlabeled algal storage media for each treatment. Samples of resuspended algae were analyzed for radioactivity (see Radioassays), and cell density was determined by using a hemocytometer.
Daphnia MeHg Exposure and Bioenergetics.
Radiolabeled algae of different nutrient qualities (HiQ or LoQ) were pulse-fed to Daphnia after which Daphnia were fed unlabeled HiQ or LoQ algae for 5 d during the juvenile growth period. The depuration of the label was followed in live Daphnia over the 5 d to quantify Me203Hg assimilation efficiency and efflux rates. Daphnia of the same size and age (48-h-old) were added to each of six replicate borosilicate containers per treatment with 17 individuals per container. This density of individuals was sufficient for radioactivity measurements in the Daphnia while remaining below crowding conditions (55). Each replicate contained 100 ml of P-free Daphnia culturing media. Additional 48-h-old Daphnia were measured for initial dry weights to quantify growth rate. Daphnia were allowed to clear their guts in the absence of food for 2 h. Then, 2.2 × 106 labeled HiQ or LoQ cells, corresponding to 3.3 × 107 μm3, were added to each replicate. To minimize cross-contamination between treatments, animals from the LoQ replicates were removed from their feeding chambers before HiQ animals. As a result, Daphnia in the LoQ treatment fed on radiolabeled algae for a shorter time (35–78 min) than those in the HiQ treatment (86–128 min). Both exposure times were comparable with the gut passage time of these organisms (56) to minimize recycling of the radiolabel.
After radioactive feeding, Daphnia were rinsed twice in fresh P-free media, and five individuals from each replicate were analyzed for initial radioactivity. To monitor depuration of the label, all individuals were placed into new replicate containers with fresh P-free media and fed nonradioactive HiQ or LoQ algae at a daily ration of 1.6 × 106 μm3 per Daphnia for 5 d. Radioactivity in Daphnia was measured nondestructively at multiple time points, and media was renewed every 24 h. At each time point, five Daphnia from each replicate were placed into counting vials, measured for radioactivity and returned to their experimental containers. At the final time point, Daphnia were measured for dry weights to calculate somatic growth rates.
In a parallel experiment, we monitored Daphnia ingestion rates of HiQ or LoQ algae. Daphnia were fed nonradioactive algae according to the same design used for the Me203Hg depuration period. In addition, we monitored changes in algal density in three control containers with no Daphnia for each treatment. Daphnia were transferred daily to new containers with fresh P-free media and algae according to treatment. Algal cell densities were measured in each container before Daphnia were added and 24 h later, after individuals were transferred to a new container. Thus, ingestion rate was measured every 24 h for 5 d.
Radioassays.
Radioactivity of Me203Hg in all samples was determined by using an LKB Amersham Pharmacia Wallac (Gaithersburg, MD) 1282 Compugamma with a NaI(T1) well detector. Gamma-emissions were assayed at 279 keV and counting times were 10 min, yielding typical propagated counting errors of ≤5%. All counts were corrected for decay and background radioactivity, using appropriate standards and blanks.
Calculations and Statistical Analyses.
The assimilation efficiency of MeHg (AE, the proportion of ingested Me203Hg assimilated into tissue) was calculated as the y-intercept of the regression between the natural log of the percent Me203Hg retained in Daphnia and time for the slowly exchanging pool during the 5-d depuration period (57). The efflux rate (Ke, the physiological loss of assimilated Me203Hg) was calculated as the slope of the regression (57). The biological half-life (tb½) of Me203Hg was calculated as tb½ = (Ln 2)/Ke. Estimates of AE, Ke, and tb½ were made for each replicate and averaged for each treatment.
Differences in resuspended algae Me203Hg concentrations, Daphnia Me203Hg AE, Ke, tb½, and percent Me203Hg retained at day 5 between HiQ and LoQ treatments were tested by ANOVA. Daphnia specific growth rate was calculated as (Ln(final weight) − Ln(initial weight) time−1) for each replicate separately, and compared between treatments by ANOVA. Significant differences in Daphnia ingestion rates between treatments and over time were tested with multivariate ANOVA-repeated measures. To meet the assumptions of the multivariate ANOVA-repeated measures approach, a significant lack of sphericity in the variance-covariance matrix indicated by a χ2 test was accounted for by reporting the Geisser–Greenhouse F test correction value (58). All statistical tests were conducted by using JMP 5.01.
Modeling Steady-State MeHg Concentrations in Daphnia.
We calculated steady-state MeHg concentrations in Daphnia (MeHgss, ng g−1 dry weight), using a biokinetic model fit with experimentally measured rates given by the equation
(57, 59) where AE = the assimilation efficiency of MeHg (%), SIR is the specific ingestion rate (mg mg−1 d−1), Cf is the MeHg concentration in the algal food (ng g−1), Ke is the efflux loss rate constant (d−1), and g is the specific growth rate of the animal (mg mg−1 d−1). MeHg accumulation from the aqueous phase is assumed to be negligible (60). Site-specific predictions of steady-state concentrations of numerous metals in diverse aquatic animals, using this model and lab-derived kinetic rates have closely matched independent field measurements for a variety of organisms and ecosystems (20), including crustacean zooplankton (61). This match suggests that we can account for the major factors governing metal concentrations in aquatic animals and that the kinetic parameters quantified in lab experiments are applicable to natural waters.
To model the effects of HiQ and LoQ algal nutrient quality on Daphnia MeHg concentrations, we compared the response of Daphnia MeHgss with the observed variation in Daphnia ingestion and growth rates from HiQ and LoQ treatments. We used the grand mean of AE and Ke for these analyses, because these values were similar between treatments. For Cf, we used the average MeHg concentration of phytoplankton typical of unpolluted freshwater lakes (Cf = 34 ng g−1 dry weight) (ref. 50) to apply model predictions to natural systems. To compare the magnitude of response in MeHgss with differences in growth and ingestion between HiQ and LoQ algae consumption, we used the mean, upper, and lower confidence limits of specific ingestion rate and growth (averaged over the 5 d) for each treatment.
Supplementary Material
Acknowledgments
We thank J. Shaw, S. Glaholt, B. Mayes, L. Keyes, S. Baines and S. Palma for lab assistance. We also gratefully acknowledge K. Cottingham, M. Ayres, S. Kilham, R. Sterner, and an anonymous reviewer for helpful comments. This research was supported by National Institutes of Health Grant P42 ESO7373–7 (to C.L.F. and C.Y.C.), the National Institute of Environmental Health Sciences, National Science Foundation Grant CHE-0221934, and CALFED 03WRAG0038 (to N.S.F.).
Abbreviations
- HiQ
high-quality
- LoQ
low-quality
- MeHg
methylmercury
- SGD
somatic growth dilution.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.United Nations Environment Programme. Global Mercury Assessment. New York: Inter-Organization Programme for the Sound Management of Chemicals; 2002. [Google Scholar]
- 2.Stow CA, Carpenter SR. Environ Sci Technol. 1994;28:1543–1549. doi: 10.1021/es00057a026. [DOI] [PubMed] [Google Scholar]
- 3.Sunda WG, Huntsman SA. Sci Total Environ. 1998;219:165–181. [Google Scholar]
- 4.Brett MT, Muller-Navarra DC, Park SK. Limnol Oceanogr. 2000;45:1564–1575. [Google Scholar]
- 5.Schindler DW, Kidd KA, Muir DCG, Lockhart WL. Sci Total Environ. 1995;161:1–17. [Google Scholar]
- 6.Doyon JF, Schetagne R, Verdon R. Biogeochemistry. 1998;40:203–216. [Google Scholar]
- 7.Stafford CP, Hansen B, Stanford JA. Transactions of the American Fisheries Society. 2004;133:349–357. [Google Scholar]
- 8.Simoneau M, Lucotte M, Garceau S, Laliberte D. Environ Res. 2005;98:73–82. doi: 10.1016/j.envres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Hogan LS, Marshall E, Folt CL, Stein RA. J Great Lakes Res. 2007;33:46–61. [Google Scholar]
- 10.Stafford CP, Haines TA. Environ Toxicol Chem. 2001;20:2099–2101. [PubMed] [Google Scholar]
- 11.Dutton MD. Biology. Waterloo, ON, Canada: University of Waterloo; 1997. p. 77. [Google Scholar]
- 12.Essington TE, Houser JN. Transactions of the American Fisheries Society. 2003;132:57–68. [Google Scholar]
- 13.Trudel M, Rasmussen JB. Can J Fish Aquat Sci. 2006;63:1890–1902. [Google Scholar]
- 14.Stemberger RS, Miller EK. Environ Monit Assess. 1998;51:29–51. [Google Scholar]
- 15.Chen CY, Stemberger RS, Kamman NC, Mayes BM, Folt CL. Ecotoxicology. 2005;14:135–147. doi: 10.1007/s10646-004-6265-y. [DOI] [PubMed] [Google Scholar]
- 16.Sterner RW, Hessen DO. Annu Rev Ecol Syst. 1994;25:1–29. [Google Scholar]
- 17.Urabe J, Clasen J, Sterner RW. Limnol Oceanogr. 1997;42:1436–1443. [Google Scholar]
- 18.Hessen DO, Faerovig PJ, Andersen T. Ecology. 2002;83:1886–1898. [Google Scholar]
- 19.Elser JJ, Sterner RW, Gorokhova E, Fagan WF, Markow TA, Cotner JB, Harrison JF, Hobbie SE, Odell GM, Weider LJ. Ecol Lett. 2000;3:540–550. [Google Scholar]
- 20.Luoma SN, Rainbow PS. Environ Sci Technol. 2005;39:1921–1931. doi: 10.1021/es048947e. [DOI] [PubMed] [Google Scholar]
- 21.Tsui MTK, Wang WX. Environ Toxicol Chem. 2004;23:1504–1511. doi: 10.1897/03-310. [DOI] [PubMed] [Google Scholar]
- 22.Tsui MTK, Wang WX. Aquat Toxicol. 2004;70:245–256. doi: 10.1016/j.aquatox.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Yu RQ, Wang WX. Limnol Oceanogr. 2002;47:495–504. [Google Scholar]
- 24.Tsui MTK, Wang WX. Environ Sci Technol. 2004;38:808–816. doi: 10.1021/es034638x. [DOI] [PubMed] [Google Scholar]
- 25.Karimi RK, Folt CL. Ecol Lett. 2006;9:1273–1283. doi: 10.1111/j.1461-0248.2006.00979.x. [DOI] [PubMed] [Google Scholar]
- 26.Jorgenson JL. Environ Health Perspect. 2001;109:113–139. doi: 10.1289/ehp.01109s1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sterner RW, Hagemeier DD, Smith WL. Limnol Oceanogr. 1993a;38:857–871. [Google Scholar]
- 28.Kilham SS, Kreeger DA, Goulden CE, Lynn SG. Freshw Biol. 1997;38:639–647. [Google Scholar]
- 29.DeMott WR, Gulati RD, Siewertsen K. Limnol Oceanogr. 1998;43:1147–1161. [Google Scholar]
- 30.Hessen DO, Agren GI, Anderson TR, Elser JJ, De Ruiter PC. Ecology. 2004;85:1179–1192. [Google Scholar]
- 31.Darchambeau F, Faerovig PJ, Hessen DO. Oecologia. 2003;136:336–346. doi: 10.1007/s00442-003-1283-7. [DOI] [PubMed] [Google Scholar]
- 32.DeMott WR, Pape BJ. Oecologia. 2005;142:20–27. doi: 10.1007/s00442-004-1716-y. [DOI] [PubMed] [Google Scholar]
- 33.Plath K, Boersma M. Ecology. 2001;82:1260–1269. [Google Scholar]
- 34.Darchambeau F, Thys I. J Plankton Res. 2005;27:227–236. [Google Scholar]
- 35.Sterner RW, Smith RF. Bull Marine Sci. 1993b;53:228–239. [Google Scholar]
- 36.Sibley RM. In: Physiological Ecology: an Evolutionary Approach to Resource Use. Townsend CR, Callow P, editors. Oxford: Blackwell Scientific; 1981. pp. 109–139. [Google Scholar]
- 37.Chen CY, Folt CL. J Plankton Res. 1993;15:1247–1261. [Google Scholar]
- 38.Yearsley J, Tolkamp BJ, Illius AW. P Nutr Soc. 2001;60:145–156. doi: 10.1079/pns200062. [DOI] [PubMed] [Google Scholar]
- 39.Sterner RW. Ecology. 1993c;74:2351–2360. [Google Scholar]
- 40.Pickhardt PC, Folt CL, Chen CY, Klaue B, Blum JD. Proc Natl Acad Sci USA. 2002;99:4419–4423. doi: 10.1073/pnas.072531099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Folt CL, Chen CY, Pickhardt PC. In: Biomarkers of Environmentally Associated Disease: Technologies, Concepts, and Perspectives. Wilson SH, Suk WA, editors. Boca Raton, FL: CRC/Lewis; 2002. pp. 287–304. [Google Scholar]
- 42.Pickhardt PC, Folt CL, Chen CY, Klaue B, Blum JD. Sci Total Environ. 2005;339:89–101. doi: 10.1016/j.scitotenv.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 43.Chen CY, Folt CL. Environ Sci Technol. 2005;39:115–121. [PubMed] [Google Scholar]
- 44.Larsson P. Environ Sci Technol. 1992;26:346–352. [Google Scholar]
- 45.Berglund O, Larsson P, Ewald G, Okla L. Ecology. 2001;82:1078–1088. doi: 10.1016/s0269-7491(00)00166-4. [DOI] [PubMed] [Google Scholar]
- 46.Taylor WD, Carey JH, Lean DRS, McQueen DJ. Can J Fish Aquat Sci. 1991;48:1960–1966. [Google Scholar]
- 47.Nichols HW. In: Handbook of Phycological Methods. Stein JR, editor. Cambridge, England: Cambridge Univ Press; 1973. pp. 7–24. [Google Scholar]
- 48.Goulden CE, Comotto RM, Hendrickson JA, Jr, Hornig LL, Johnson KL. In: Aquatic Toxicology and Hazard Assessment: Fifth Conference, American Society for Testing and Materials Special Technical Publication 766. Pearson JG, Foster RB, Bishop WE, editors. Philadelphia, PA: American Society for Testing and Materials; 1982. pp. 139–160. [Google Scholar]
- 49.Kilham SS, Kreeger DA, Lynn SG, Goulden CE, Herrera L. Hydrobiologia. 1998;377:147–159. [Google Scholar]
- 50.Watras CJ, Back RC, Halvorsen S, Hudson RJM, Morrison KA, Wente SP. Sci Total Environ. 1998;219:183–208. doi: 10.1016/s0048-9697(98)00228-9. [DOI] [PubMed] [Google Scholar]
- 51.Pickhardt PC, Stepanova M, Fisher NS. Environ Toxicol Chem. 2006;25:2132–2142. doi: 10.1897/05-595r.1. [DOI] [PubMed] [Google Scholar]
- 52.Tsui MTK, Wang WX. Environ Toxicol Chem. 2005;24:2927–2933. doi: 10.1897/05-085r.1. [DOI] [PubMed] [Google Scholar]
- 53.Tsui MTK, Wang WX. Environ Sci Technol. 2006;40:4025–4030. doi: 10.1021/es052377g. [DOI] [PubMed] [Google Scholar]
- 54.Fisher NS, Bjerregaard P, Fowler SW. Limnol Oceanogr. 1983;28:432–447. [Google Scholar]
- 55.Burns CW. Oecologia. 1995;101:234–244. doi: 10.1007/BF00317289. [DOI] [PubMed] [Google Scholar]
- 56.Peters RH. In: A Manual on Methods for the Assessment of Secondary Productivity in Fresh Waters. Downing JA, Rigler RH, editors. Oxford: Blackwell Scientific; 1984. pp. 336–412. [Google Scholar]
- 57.Wang WX, Fisher NS. Environ Toxicol Chem. 1999;18:2034–2045. [Google Scholar]
- 58.Geisser S, Greenhouse SW. Ann Math Stat. 1958;29:885–891. [Google Scholar]
- 59.Reinfelder JR, Fisher NS, Luoma SN, Nichols JW, Wang WX. Sci Total Environ. 1998;219:117–135. doi: 10.1016/s0048-9697(98)00225-3. [DOI] [PubMed] [Google Scholar]
- 60.Hall BD, Bodaly RA, Fudge RJP, Rudd JWM, Rosenberg DM. Water Air Soil Pollut. 1997;100:13–24. [Google Scholar]
- 61.Fisher NS, Stupakoff I, Sanudo-Wilhelmy S, Wang WX, Teyssie JL, Fowler SW, Crusius J. Mar Ecol Prog Ser. 2000;194:211–218. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.