Abstract
Emergence of bacterial resistance is a major issue for all classes of antibiotics; therefore, the identification of new classes is critically needed. Recently we reported the discovery of platensimycin by screening natural product extracts using a target-based whole-cell strategy with antisense silencing technology in concert with cell free biochemical validations. Continued screening efforts led to the discovery of platencin, a novel natural product that is chemically and biologically related but different from platensimycin. Platencin exhibits a broad-spectrum Gram-positive antibacterial activity through inhibition of fatty acid biosynthesis. It does not exhibit cross-resistance to key antibiotic resistant strains tested, including methicillin-resistant Staphylococcus aureus, vancomycin-intermediate S. aureus, and vancomycin-resistant Enterococci. Platencin shows potent in vivo efficacy without any observed toxicity. It targets two essential proteins, β-ketoacyl-[acyl carrier protein (ACP)] synthase II (FabF) and III (FabH) with IC50 values of 1.95 and 3.91 μg/ml, respectively, whereas platensimycin targets only FabF (IC50 = 0.13 μg/ml) in S. aureus, emphasizing the fact that more antibiotics with novel structures and new modes of action can be discovered by using this antisense differential sensitivity whole-cell screening paradigm.
Keywords: platensimycin, natural product, thiolactomycin, antisense, fatty acid synthesis
Resistance to existing antibiotics is a serious threat to human health worldwide, but efforts since the early 1960s to discover a new class of antibiotics have met with limited success (1). Although hundreds of essential proteins have been identified in bacteria as potential drug targets (2–5) only a few are targets of therapeutically useful drugs. In past decades, chemical modification of existing scaffolds has afforded antibiotics with incrementally improved activity against their targets. This strategy served well to develop new and effective antibiotics; however, such modifications are becoming increasingly challenging. To meet critical clinical needs, especially to overcome the emerging drug resistance, the discovery of novel antibiotic chemical scaffolds with new modes of action is crucial for saving lives.
Type II fatty acid synthesis (FASII) is essential to bacterial cell viability and therefore is a potential target for new antibiotics. Significant differences between bacterial and human fatty acid synthesis systems exist that include the organization and structure of enzymes and the specific roles played by fatty acids making this system an attractive target for antibacterial drug discovery (6–11). One of the essential enzymes, FabI, in the fatty acid biosynthetic pathway is a proven drug target with two marketed antibacterial agents, triclosan (antiseptic) and isoniazid (an anti-Mycobacterium tuberculosis agent) (12, 13). The initiation condensing enzyme, FabH, and elongation condensing enzymes, FabF/B, are essential components of this biosynthetic pathway (14–17) and consequently are highly conserved among key pathogens. Although no drugs targeting condensing enzymes are used in the clinic, two inhibitors, cerulenin (18) and thiolactomycin (19), with poor antibacterial activities were discovered more than two decades ago. Cerulenin selectively targets FabF/B forming a covalent bond with the catalytic cysteine in the active site with its tail occupying the long hydrophobic cavity that normally contains the growing acyl chain of the natural substrate (20, 21). Thiolactomycin and its analogs (22, 23) target the malonyl binding site of both FabH and FabF/B. Several other inhibitors targeting the bacterial condensing enzymes have been reported, but none were suitable drug candidates because of poor penetration and/or lack of target selectivity in whole cells and/or in vivo toxicity. In previous studies we have described the discovery and full characterization of platensimycin (24), a novel class highly selective FabF inhibitor with potent antibiotic properties, by screening >250,000 natural product extracts using a target-based whole-cell strategy with antisense silencing technology (25) in concert with biochemical validation (26). In the present study we report the discovery of platencin, a natural product from a strain of Streptomyces platensis MA7339 recovered from a soil sample collected in Spain. The chemical structure of platencin contains a unique ketolide portion that is different from platensimycin. Platencin showed broad-spectrum Gram-positive antibacterial activity and in vivo efficacy in a mouse model. Characterization of platencin in biochemical assays showed that platencin targets both FabH and FabF enzymes.
Results and Discussion
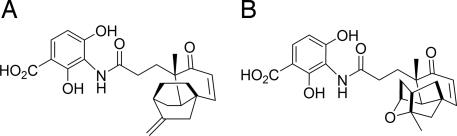
Systematic screening of natural product extracts, using a combination of target-based whole-cell antisense and biochemical assays, led to the identification of an antibacterial agent, platensimycin, from a strain of S. platensis, MA7327, collected in South Africa (24, 27). Continued screening efforts to identify new antibiotics (24–26, 28) led to the discovery of another antibacterial agent, named platencin (C24H25NO6, molecular weight 425.47), from a strain of S. platensis, MA7339, recovered from a soil sample collected in Minut II (Mallorca) in Spain. Although the 3-amino-2,4-dihydroxy benzoic acid units of platencin and platensimycin are common, the ketolide portions are different. Platensimycin contains a pentacyclic motif with a cyclic ether ring, whereas platencin contains a unique tetracyclic motif without the ether ring (Fig. 1).
Fig. 1.
Structures of platencin (A) and platensimycin (B).
Like platensimycin, platencin shows potent, broad-spectrum Gram-positive in vitro activity (Table 1) including activity against key antibiotic-resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-intermediate S. aureus, and vancomycin-resistant Enterococci, linezolid-resistant and macrolide-resistant pathogens. Platencin is more potent than platensimycin against vancomycin-resistance Enterococcus faecium and efflux-negative Escherichia coli (tolC), exhibiting minimum inhibitory concentrations (MIC) of <0.06 μg/ml and 2 μg/ml, respectively. Low mammalian cell toxicity [IC50 > 100 μg/ml and minimum lytic concentration (MLC) >67 μg/ml in HeLa cytotoxicity (MTT) and in RBC lysis assays, respectively] and lack of antifungal activity (Candida albicans MIC > 64 μg/ml) further suggest that platencin acts selectively in inhibiting bacterial growth. Platencin is 4-fold less effective against Streptococcus pneumoniae (MIC = 4 μg/ml) compared with platensimycin whereas it showed comparable MIC values of 0.5–2 μg/ml against other pathogens tested (Table 1). The frequency of resistance of platensimycin is quite variable and is obviously dependent on cell lines and concentration of drug used for selection. The frequency of resistance is 9 × 10−8 at 2 μg/ml against S. aureus MRSA Col strain and <10−9 at 16 μg/ml against S. aureus Smith strain.
Table 1.
Profiles of antibiotics
| Organism and genotype | Platensimycin* | Platencin | Linezolid*† |
|---|---|---|---|
| MIC, μg/ml | |||
| S. aureus (MSSA) | 0.5 | 0.5 | 4 |
| S. aureus plus serum | 2 | 8 | 4 |
| S. aureus (MRSA) | 0.5 | 1 | 2 |
| S. aureus (MRSA, macrolideR) | 0.5 | 1 | 2 |
| S. aureus (MRSA, linezolidR) | 1 | 1 | 32 |
| S. aureus (VISA, vancomycinI) | 0.5 | 0.5 | 2 |
| E. faecalis (macrolideR) | 1 | 2 | 1 |
| E. faecium (vancomycinR) | 0.1 | <0.06 | 2 |
| S. pneumoniae‡ | 1 | 4 | 1 |
| E. coli (WT) | >64 | >64 | >64 |
| E. coli (tolC) | 16 | 2 | 32 |
| S. aureus (AS-fabF) (MDC,§ μg) | 0.004 | 0.002 | ND |
| Toxicity, μg/ml | |||
| HeLa MTT (IC50) | >1,000 | >100 | >100 |
| RBC lysis (MLC)¶ | >67 | >67 | >67 |
| C. albicans (MIC) | >64 | >64 | >64 |
| Whole-cell activity, IC50, μg/ml | |||
| Fatty acid synthesis (S. aureus) | 0.1 | 0.19 | ND |
| Fatty acid synthesis (S. pneumoniae) | 0.8 | 2.7 | ND |
The MIC and toxicity assays were described previously (28). ND, not determined.
*Data were published previously (24) except for MLC from RBC lysis.
†Synthetically derived agent in clinical use since 2000.
‡Cells were inoculated at 105 cfu followed by incubation at 37° C with a serial dilution of compounds in Todd–Hewitt broth overnight.
§MDC of compound that exhibits a >5-mm differential of zone of inhibition on the plate seeded with S. aureus strain expressed fabF antisense compared with the plate seeded with the control strain (25).
¶MLC is defined as the lowest concentration of a test compound that visibly produces complete or partial lysis of RBCs.
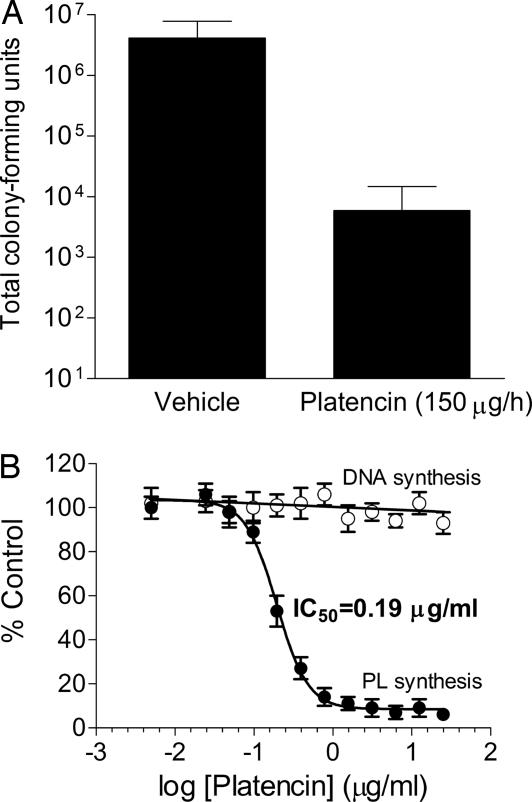
Platencin showed in vivo efficacy in a continuous-infusion mouse model of a disseminated S. aureus (MSSA) infection (24) with 3-log reduction of S. aureus cfu in kidney over a 24-h treatment period (Fig. 2A) at 150 μg/h (144 mg/kg total) and with no indication of overt toxicity (data not shown). In vivo effectiveness of platencin dosing at 150 μg/h equals that of platensimycin at ≈90 μg/h, which is consistent with the relative in vitro antibacterial activities of the two drugs in the presence of serum [MIC = 8 μg/ml for platencin (Table 1) and 2 μg/ml for platensimycin (24)], suggesting that the MIC in the presence of serum mimics the animal test condition.
Fig. 2.
Characterization of platencin. (A) In vivo studies on platencin. Dosing at 150 μg/h showed a 3-log reduction of viable S. aureus cell from the infected kidney compared with the control (vehicle). Error bars indicate standard deviation observed with seven infected mice upon treatment with platencin (treatment group) and five without treatment (control group). S. aureus Smith strain 4 × 103 cfu/ml in 5% hog gastric mucin, and 0.5 ml was used to infect i.p., and 30 min after infection the drug was delivered by continuous infusion. After the 24-h period, the mice were killed immediately and the kidneys were aseptically removed and homogenized. Serial dilutions of the homogenates were plated on Mannitol plates and incubated overnight at 37°C. Bacterial counts were enumerated. (B) Whole-cell labeling assay with platencin. The assay was performed by using a serial dilution of platencin starting at 50 μg/ml. Platencin showed no inhibition of DNA synthesis (○) and a significant inhibition of phospholipid synthesis (●) with an IC50 value of 0.19 μg/ml. Error bars show the standard deviation of two individual experiments.
Platencin inhibited phospholipid biosynthesis in whole-cell labeling experiments in S. aureus (Fig. 2B) and S. pneumoniae with IC50 values of 0.19 μg/ml (0.45 μM) and 2.7 μg/ml (6.3 μM) (Table 1), respectively. Similar to platensimycin, it did not inhibit DNA, RNA, protein, or cell wall biosynthesis at concentrations up to 50 μg/ml (Fig. 2B and data not shown), thereby suggesting that the bacterial growth inhibition is due to selective inhibition of fatty acid biosynthesis. In the agar-diffusion two-plate differential sensitivity antisense assay, platencin exhibited a larger zone of inhibition on the FabF antisense plate compared with the control plate and exhibited a minimum detection concentration (MDC) of 2 ng compared with platensimycin, which showed MDC of 4 ng (Table 1). In contrast, the overexpression of FabF protein in S. aureus showed resistance to platencin [supporting information (SI) Text and SI Fig. 4], thus indicating similar overall whole-cell target selectivity and in vitro antibacterial activities. The correspondence between MICs (0.5 and 4 μg/ml against S. aureus and S. pneumoniae, respectively) and cellular phospholipid biosynthesis IC50 values (0.19 and 2.7 μg/ml against S. aureus and S. pneumoniae, respectively) of platencin (Table 1) further demonstrates that platencin kills bacteria specifically/selectively through inhibition of fatty-acid biosynthesis.
In S. aureus, fabH is upstream of fabF and both genes are members of the same operon. Therefore, overexpression of fabF antisense RNA, targeting fabF mRNA, causes a complete degradation of the 5′ portion of the transcript, which in turn causes down-regulation of both FabF and FabH expression (25). Inhibition of phospholipid biosynthesis by platencin demonstrated that it targets fatty acid synthesis pathway. The activity and selectivity of platencin in the two-plate antisense assay indicated that platencin targeted FabF, FabH, or both enzymes. To determine the target of platencin, first we performed a direct binding assay (24) with purified FabF. In this assay platencin showed direct binding to acyl-enzyme intermediate; however, its binding affinity was 5.9-fold lower (IC50 = 0.113 ± 0.089 μM) than platensimycin. We have already reported that platensimycin poorly inhibited the FabH enzyme (IC50 = 67 μM) in a purified FabH single-enzyme assay (24). In this assay, platencin exhibited 4.1-fold higher inhibitory activity (IC50 = 16.2 ± 4.2 μM) compared with platensimycin. These results suggested that platencin tends to inhibit both condensing enzymes whereas platensimycin selectively targets FabF. The differences between the IC50 values of the single-enzyme assay (IC50 = 0.048 μM) and whole-cell labeling [IC50 = 0.1 μg/ml (0.23 μM)] remained unexplained (24). This could be because of poor cell uptake including membrane penetration (29) and active efflux. Another possibility is that the in vitro single-enzyme assay does not represent the measurement of the precise reaction inside the cell.
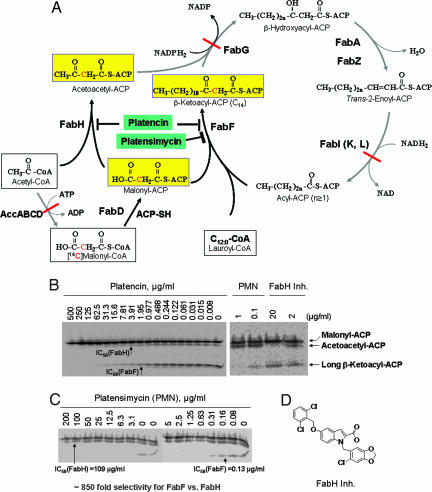
As shown in Fig. 3A, fatty acid biosynthesis enzymes in bacteria work sequentially as a biofactory. A product from one individual enzyme becomes a substrate to the next enzyme, which requires that all enzymes work closely, possibly forming a complex through protein–protein interactions. To address this question in as precise a way as possible, we developed a FabH/FabF PAGE elongation assay, which allowed us not only to test but also to quantitatively differentiate inhibitors against FabF and/or FabH simultaneously. This assay directly uses the crude S. aureus cytosolic proteins, which should provide an enzyme complex similar to what exists in the cell in the natural state. The schematic description of the assay is detailed in Fig. 3A. The basic idea is that, when the assay is performed with all natural enzymes, but without addition of ATP and NADPH2/NADH2, both the AccABCD reaction and the elongation cycle cannot proceed. As a result, addition of radiolabeled malonyl-CoA, lauryl-CoA, and the natural substrate acetyl-CoA produced only three radiolabeled protein [acyl carrier protein (ACP)] products (shown in yellow boxes in Fig. 3A). These are identified as malonyl-ACP (FabD product), acetoacetyl-ACP (FabH products), and long-chain β-ketoacyl-ACP (C14, FabF products) and can be separated on 0.5 M urea PAGE. In a control experiment, when a known FabH inhibitor (Fig. 3D) (30) was tested in this assay it blocked FabH activity, leading to loss of accumulation of acetoacetyl-ACP [IC50 = 2 μg/ml (3.96 μM) for FabH] (Fig. 3B and data not shown). Platensimycin, a selective FabF inhibitor, led to the significant reduction of accumulation of long-chain β-ketoacyl-ACP [IC50 = 0.13 μg/ml (0.29 μM) for FabF] and loss of accumulation of acetoacetyl-ACP [IC50 = 109 μg/ml (247 μM) for FabH] (Fig. 3 B and C). In a similar experiment platencin blocked both FabF and FabH and reduced the level of accumulated acetoacetyl ACP and β-ketoacyl-ACP with IC50 values of 1.95 μg/ml (4.58 μM) and 3.91 μg/ml (9.17 μM), respectively (Fig. 3B), suggesting that platencin is a dual inhibitor with similar inhibition efficiency for both condensing enzymes. Platencin and platensimycin did not inhibit FabD, as its product (malonyl-ACP) did not decrease, but increased slightly because of inhibition of the downstream FabH and/or FabF enzymes that consume malonyl-ACP. This further demonstrates that the compounds specifically targeted the condensing enzymes. Interestingly, the FabF IC50 value (0.13 μg/ml) of platensimycin in the FabH/FabF PAGE elongation assay is similar to the IC50 value (0.1 μg/ml) (Table 1) obtained from the whole-cell labeling assay, emphasizing the fact that (i) the FabF enzyme in S. aureus is the target that platensimycin selectively inhibits and (ii) the FabH/FabF PAGE elongation assay with the crude S. aureus cytosolic proteins indeed closely mimics what takes place inside living cells. Compared with platensimycin, both IC50 values of platencin (FabF IC50 = 1.95 μg/ml and FabH IC50 = 3.91 μg/ml) are >10-fold higher than that obtained from the whole-cell labeling assay (0.19 μg/ml) (Fig. 2B), suggesting that platencin inhibits fatty acid synthesis in S. aureus through a synergistic effect by targeting both condensing enzymes.
Fig. 3.
Determination of target selectivity using FabH/FabF PAGE elongation assay. (A) Schematic description of FabH/FabF PAGE elongation assay. Bacterial fatty acid biosynthesis was initiated with acetyl-CoA, which is carboxylated by AccABCD in the presence of ATP to form malonyl-CoA, which is, in turn, transferred to ACP by FabD. Fatty acid synthesis is initiated by FabH supplying substrates (acetoacetyl-ACP) to the fatty acid elongation cycle, which includes FabG, FabA/Z, FabI (L/K), and FabF/B enzymes. In the cycle, the keto group of β-ketoacyl-ACP is reduced to a hydroxyl group by NADPH2-dependent reductase FabG. β-Hydroxyacyl-ACP is dehydrated by dehydratase FabA or FabZ. The double bond of trans2-enoyl-ACP is reduced by NADPH2-dependent reductase FabI(K/L), which feeds the substrate back to FabF/B, which in turn adds an additional acetate unit (two carbons), and the cycle iterates. When the assay is performed with all natural enzymes without addition of ATP and NADPH2/NADH2, both AccABCD reaction and the elongation cycle are unable to proceed. By addition of radiolabeled malonyl-CoA and lauryl-CoA, as well as the natural substrate acetyl-CoA, only three radiolabeled protein (ACP) products (shown in yellow boxes) are obtained: malonyl-ACP (FabD product), acetoacetyl-ACP (FabH products), and long-chain β-ketoacyl-ACP (C14, FabF products). These products can be separated by urea PAGE (26). (B and C) FabH/FabF PAGE elongation assay was performed as described in Materials and Methods. Three substrates, acetyl-CoA, lauryl-CoA (C12:0), and [14C]malonyl-CoA, were added without addition of ATP, NADH2, and NADPH2, and only three products were obtained: malonyl-ACP (FabD product), acetoacetyl-ACP (FabH product), and long β-ketoacyl-ACP (C14, FabF product). These products can be separated with 0.5 M urea PAGE. (B) A FabH inhibitor (FabH Inh.) did not inhibit FabF activity but blocked FabH activity with 90% and 50% inhibition of the accumulation of acetoacetyl-ACP at 20 and 2 μg/ml, respectively, whereas platensimycin selectively inhibited FabF and accumulated long-chain β-ketoacyl-ACP without inhibition of FabH activity. In contrast, platencin (tested with a serial dilution) inhibited both FabF and FabH, providing IC50 values of 1.95 and 3.91 μg/ml, respectively. (C) The assay was performed with a serial dilution of platensimycin, giving an 838-fold selectivity for FabF over FabH. The IC50 values are 0.13 μg/ml for FabF and 109 μg/ml for FabH. Both platencin and platensimycin did not show any inhibition against FabD protein. (D) Chemical structures of FabH inhibitor (FabH Inh.).
Generally, for antibacterial drugs, targeting two essential proteins is advantageous over targeting only one because the odds of targeting at least one of these conserved proteins in different bacterial species would be increased. Most importantly, this should lead to lowered resistance potential (31). Platencin showed increased antibacterial activity against vancomycin-resistant E. faecium and efflux-negative E. coli (tolC) and decreased activity against S. pneumoniae when compared with platensimycin. This is presumably because of differing target sensitivity of FabF and FabH in these specific strains to platencin.
The discovery of a second structure from natural products with dual mode of action, similar to thiolactomycin but with >200-fold higher antibacterial activity against S. aureus, emphasizes the applicability of the target-based whole-cell antisense screening approach on FabF/FabH targets. This universal approach could be applied to other targets in bacteria, particularly the ≈90% of essential proteins that remain unexplored. The antisense strategy provides a great opportunity for the discovery of novel antibiotic chemical structures from natural products. In addition, the structure of platencin provides more insight for the structure–function relationship of FASII inhibitors and for the possible development of critically needed antibacterial agents.
Materials and Methods
Reagents.
All reagents were obtained from Sigma–Aldrich (St. Louis, MO) unless otherwise indicated.
Preparation of Total FASII Enzymes.
The details of the preparation of FASII enzymes from S. aureus were described previously (26). Briefly, cells were grown to stationary phase and centrifuged at 8,000 rpm for 10 min with a Beckman JA-10 rotor. The pellets were washed twice with ice-cold buffer A (0.1 M sodium phosphate, pH 7/1 mM EDTA/5 mM 2-mercaptoethanol) and resuspended in the same buffer. The cells were lysed in a cold microfluidizer (M-110EH; Microfluidics) at 18,000 lbs/in2 and centrifuged at 20,000 rpm for 15 min at 4°C. The supernatant was collected, and ammonium sulfate was added into the supernatant with low-speed stirring at 4°C to reach 45% of ammonium sulfate concentration. The mixture was centrifuged at 10,000 rpm for 5 min, the supernatant was collected, and ammonium sulfate was added again to reach 80% of ammonium sulfate. The mixture was centrifuged again, and the pellet, containing all necessary fatty acid synthesis (FASII) enzymes, was dissolved in buffer A, dialyzed at 4°C against four changes of buffer A using 10-kDa molecular mass cutoff dialysis tubing (15961-022; Invitrogen), and then concentrated. The protein concentration was determined by using the standard Bio-Rad protocol. The protein was aliquoted, flash-frozen with liquid nitrogen, and stored at −80°C.
FabH/FabF PAGE Elongation Assay.
The assay was performed by preincubating 0.5 μg of the S. aureus FASII enzymes with a serial dilution of the inhibitors at room temperature for 20 min in 50 μl of buffer containing 100 mM sodium phosphate (pH 7.0), 10 μM lauroyl-CoA, 10 μM acetyl-CoA, 1% Me2SO, and 5 μM S. aureus ACP pretreated with 3 mM DTT. The reaction was initiated by addition of 5 μM [14C]malonyl-CoA (60 mCi/mmol, NEC612, labeled at C-2 of the malonyl group). The reaction was incubated at 30°C for 10 min, terminated by adding 10 μl of native sample buffer, and kept on ice or kept at −20°C. The samples were applied to and resolved by a 20% polyacrylamide gel containing 0.5 M urea. The gel was blotted to a polyvinylidene difluoride membrane and visualized by using a PhosphorImager.
RBC Assays.
Human erythrocytes were collected (at least 5 ml of whole blood) in a vacutainer tube with EDTA from a single human donor on the day of the experiment. Two milliliters of the freshly drawn whole blood was added to 6 ml of sterile saline and gently mixed, then centrifuged at 5°C for 5 min at 2,000 rpm. The supernatant was discarded, and the human RBC were resuspended with 6 ml of sterile saline and centrifuged again at 5°C for 5 min at 2,000 rpm. This procedure was repeated two times. Three percent suspension of the washed RBCs was prepared by adding 0.3 ml of the washed RBCs to 9.7 ml of sterile saline. The final concentration of test compounds ranging from 64 μg/ml to 0.06 μg/ml in 100 μl of sterile saline containing 3.2% Me2SO was prepared in 96-well Microtest U-bottom plates (BD-353227; Becton Dickinson). The RBC lysis assay (32) was initiated by addition of 5 μl of 3% washed human RBC suspension to the each well in the plates. The plates were then immediately shaken for 1–2 min at 550 rpm to disperse the RBCs and incubated at room temperature for 60 min. The plates were centrifuged at 2,000 rpm for 5 min and read immediately. Hemolysis of RBCs is indicated by complete or partial clearing (lysis) of the supernatants with either no or a rough RBC pellet in the bottom of the wells. No hemolysis is judged by clear supernatant with a smooth RBC pellet in the bottom of the well. The MLC is defined as the lowest concentration of a test compound to visibly produce complete or partial lysis of RBCs. Amphotericin B was used as a positive control (MLC = 2–4 μg/ml), and chloramphenicol was used as a negative control (MLC > 64 μg/ml).
Other Assays.
Screening of natural products (25), in vivo studies (24), whole-cell labeling assay (26), agar-diffusion two-plate differential sensitivity assay (two-plate assay) (25), and determination of antibacterial activity (MIC) (28) were described previously.
Isolation of Platencin.
Platencin was isolated from a fermentation broth of S. platensis (MA7339) by extraction with acetone followed by successive reversed-phase chromatographies on amberchrome followed by HPLC using aqueous methanol and aqueous acetonitrile gradient as elution solvents, respectively. The production yield of platencin was ≈1 mg/liter (33).
Supplementary Material
Acknowledgments
We are grateful to Dr. Lynn Silver for her overall help and critical reading of the manuscript.
Abbreviations
- MRSA
methicillin-resistant S. aureus
- ACP
acyl carrier protein
- MIC
minimum inhibitory concentration
- MDC
minimum detection concentration
- FASII
type II fatty acid synthesis
- MLC
minimum lytic concentration.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700746104/DC1.
References
- 1.Singh SB, Barrett JF. Biochem Pharmacol. 2006;71:1006–1015. doi: 10.1016/j.bcp.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Ji Y, Zhang B, Van SF, Horn, Warren P, Woodnutt G, Burnham MK, Rosenberg M. Science. 2001;293:2266–2269. doi: 10.1126/science.1063566. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi K, Ehrlich SD, Albertini A, Amati G, Andersen KK, Arnaud M, Asai K, Ashikaga S, Aymerich S, Bessieres P, et al. Proc Natl Acad Sci USA. 2003;100:4678–4683. doi: 10.1073/pnas.0730515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hutchison CA, Peterson SN, Gill SR, Cline RT, White O, Fraser CM, Smith HO, Venter JC. Science. 1999;286:2165–2169. doi: 10.1126/science.286.5447.2165. [DOI] [PubMed] [Google Scholar]
- 5.Akerley BJ, Rubin EJ, Novick VL, Amaya K, Judson N, Mekalanos JJ. Proc Natl Acad Sci USA. 2002;99:966–971. doi: 10.1073/pnas.012602299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell JW, Cronan JE. Annu Rev Microbiol. 2001;55:305–332. doi: 10.1146/annurev.micro.55.1.305. [DOI] [PubMed] [Google Scholar]
- 7.Heath R, White S, Rock C. Prog Lipid Res. 2001;40:467–497. doi: 10.1016/s0163-7827(01)00012-1. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y-M, Marrakchi H, White SW, Rock CO. J Lipid Res. 2003;44:1–10. doi: 10.1194/jlr.r200016-jlr200. [DOI] [PubMed] [Google Scholar]
- 9.Heath RJ, Rock CO. Curr Opin Investig Drugs. 2004;5:146–153. [PMC free article] [PubMed] [Google Scholar]
- 10.Smith S, Witkowski A, Joshi AK. Prog Lipid Res. 2003;42:289–317. doi: 10.1016/s0163-7827(02)00067-x. [DOI] [PubMed] [Google Scholar]
- 11.White SW, Zheng J, Zhang Y-M, Rock CO. Annu Rev Biochem. 2005;74:791–831. doi: 10.1146/annurev.biochem.74.082803.133524. [DOI] [PubMed] [Google Scholar]
- 12.Heath RJ, Yu Y-T, Shapiro MA, Olson E, Rock CO. J Biol Chem. 1998;273:30316–30320. doi: 10.1074/jbc.273.46.30316. [DOI] [PubMed] [Google Scholar]
- 13.Banerjee A, Dubnau E, Quemard A, Balasubramanian V, Um K, Wilson T, Collins D, de Lisle G, Jacobs WJ. Science. 1994;263:227–230. doi: 10.1126/science.8284673. [DOI] [PubMed] [Google Scholar]
- 14.Revill WP, Bibb MJ, Scheu A-K, Kieser HJ, Hopwood DA. J Bacteriol. 2001;183:3526–3530. doi: 10.1128/JB.183.11.3526-3530.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai C-Y, Cronan JE. J Biol Chem. 2003;278:51494–51503. doi: 10.1074/jbc.M308638200. [DOI] [PubMed] [Google Scholar]
- 16.Tsay J, Rock C, Jackowski S. J Bacteriol. 1992;174:508–513. doi: 10.1128/jb.174.2.508-513.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schujman GE, Choi K-H, Altabe S, Rock CO, de Mendoza D. J Bacteriol. 2001;183:3032–3040. doi: 10.1128/JB.183.10.3032-3040.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumae A, Nomura S, Hata T. J Antibiot (Tokyo) 1964;17:1–7. [PubMed] [Google Scholar]
- 19.Noto T, Miyakawa S, Oishi H, Endo H, Okazaki H. J Antibiot (Tokyo) 1982;35:401–410. doi: 10.7164/antibiotics.35.401. [DOI] [PubMed] [Google Scholar]
- 20.Kauppinen S, Siggaard-Andersen M, von Wettstein-Knowles P. Carlsberg Res Commun. 1988;53:357–370. doi: 10.1007/BF02983311. [DOI] [PubMed] [Google Scholar]
- 21.Price AC, Choi K-H, Heath RJ, Li Z, White SW, Rock CO. J Biol Chem. 2001;276:6551–6559. doi: 10.1074/jbc.M007101200. [DOI] [PubMed] [Google Scholar]
- 22.Dolak L, Castle T, Truesdell S, Sebek O. J Antibiot (Tokyo) 1986;39:26–31. doi: 10.7164/antibiotics.39.26. [DOI] [PubMed] [Google Scholar]
- 23.Omura S, Iwai Y, Nakagawa A, Iwata R, Takahashi Y, Shimizu H, Tanaka H. J Antibiot (Tokyo) 1983;36:109–114. doi: 10.7164/antibiotics.36.109. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Soisson SM, Young K, Shoop W, Kodali S, Galgoci A, Painter R, Parthasarathy G, Tang YS, Cummings R, et al. Nature. 2006;441:358–361. doi: 10.1038/nature04784. [DOI] [PubMed] [Google Scholar]
- 25.Young K, Jayasuriya H, Ondeyka JG, Herath K, Zhang C, Kodali S, Galgoci A, Painter R, Brown-Driver V, Yamamoto R, et al. Antimicrob Agents Chemother. 2006;50:519–526. doi: 10.1128/AAC.50.2.519-526.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kodali S, Galgoci A, Young K, Painter R, Silver LL, Herath KB, Singh SB, Cully D, Barrett JF, Schmatz D, Wang J. J Biol Chem. 2005;280:1669–1677. doi: 10.1074/jbc.M406848200. [DOI] [PubMed] [Google Scholar]
- 27.Singh SB, Jayasuriya H, Ondeyka JG, Herath KB, Zhang C, Zink DL, Tsou NN, Ball RG, Basilio A, Genilloud O, et al. J Am Chem Soc. 2006;128:11916–11920. doi: 10.1021/ja062232p. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Galgoci A, Kodali S, Herath KB, Jayasuriya H, Dorso K, Vicente F, Gonzalez A, Cully D, Bramhill D, Singh S. J Biol Chem. 2003;278:44424–44428. doi: 10.1074/jbc.M307625200. [DOI] [PubMed] [Google Scholar]
- 29.Young K, Silver L. J Bacteriol. 1991;173:3609–3614. doi: 10.1128/jb.173.12.3609-3614.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daines RA, Pendrak I, Sham K, Van Aller GS, Konstantinidis AK, Lonsdale JT, Janson CA, Qiu X, Brandt M, Khandekar SS, et al. J Med Chem. 2003;46:5–8. doi: 10.1021/jm025571b. [DOI] [PubMed] [Google Scholar]
- 31.Silver LL. Nat Rev Drug Discov. 2007;6:41–55. doi: 10.1038/nrd2202. [DOI] [PubMed] [Google Scholar]
- 32.Kurtz MB, Douglas C, Marrinan J, Nollstadt K, Onishi J, Dreikorn S, Milligan J, Mandala S, Thompson J, Balkovec JM, et al. Antimicrob Agents Chemother. 1994;38:2750–2757. doi: 10.1128/aac.38.12.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jayasuriya H, Herath KB, Zhang C, Zink D, Basilio A, Genilloud O, Diez MT, Vicente F, Gonzalez I, Salazar O, et al. Angew Chem Int Ed. 2007 doi: 10.1002/anie.200701058. in press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.