Abstract
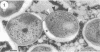
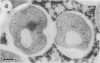
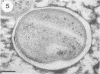
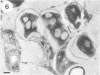
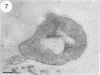
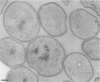
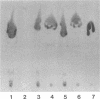
The cell wall architecture of a slowly growing mycobacterium, Mycobacterium kansasii, was examined by freeze-substitution following growth in vitro. Freeze-substituted bacteria were marked by the presence of an electron-translucent space (or electron-transparent zone [ETZ] described by previous workers [T. Yamamoto, M. Nishiura, N. Harada, and T. Imaeda, Int. J. Lepr. 26:111-114, 1958]) surrounding the majority of cells. At least two morphotypes of mycobacteria were revealed by freeze-substitution. In the first, a relatively thin (11 +/- 2.3 to 3.5 +/- 3.1 nm), uniform ETZ surrounded intact cells which contained cytoplasm filled with well-stained ribosomes and a DNA nucleoid distributed throughout the cell. The second morphotype consisted of a small proportion of organisms that were distorted in shape and were surrounded by a much thicker (59 +/- 2.6 to 198 +/- 2.5 nm) ETZ in areas of the cell which appeared to have retracted from the space it had originally occupied, leaving depressions in the ETZ. The lipid nature of the ETZ was demonstrated because cells were devoid of an ETZ when organisms were freeze-substituted in the absence of osmium tetroxide in the substitution medium or treated with neutral lipid solvents (acetone or ethanol) before freeze-substitution. Moreover, thin-layer chromatography of acetone or ethanol extracts obtained from solvent-treated cells identified a lipid component which corresponded to the M. kansasii-specific phenolic glycolipid. In contrast, negligible amounts of glycolipids were detected in extracts obtained from control HEPES (N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid) buffer-treated cells, and these cells retained an ETZ. These results demonstrate that species-specific phenolic glycolipids are essential components in the architecture of the M. kansasii ETZ. Furthermore, we show that freeze-substitution is a reliable technique for the retention and precise preservation of lipid-containing polymers in the mycobacterial cell wall.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrow W. W., Brennan P. J. Immunogenicity of type-specific C-mycoside glycopeptidolipids of mycobacteria. Infect Immun. 1982 May;36(2):678–684. doi: 10.1128/iai.36.2.678-684.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow W. W., Ullom B. P., Brennan P. J. Peptidoglycolipid nature of the superficial cell wall sheath of smooth-colony-forming mycobacteria. J Bacteriol. 1980 Nov;144(2):814–822. doi: 10.1128/jb.144.2.814-822.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belisle J. T., Brennan P. J. Chemical basis of rough and smooth variation in mycobacteria. J Bacteriol. 1989 Jun;171(6):3465–3470. doi: 10.1128/jb.171.6.3465-3470.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddingius J., Dijkman H. Subcellular localization of Mycobacterium leprae-specific phenolic glycolipid (PGL-I) antigen in human leprosy lesions and in M. leprae isolated from armadillo liver. J Gen Microbiol. 1990 Oct;136(10):2001–2012. doi: 10.1099/00221287-136-10-2001. [DOI] [PubMed] [Google Scholar]
- Brennan P. J., Heifets M., Ullom B. P. Thin-layer chromatography of lipid antigens as a means of identifying nontuberculous mycobacteria. J Clin Microbiol. 1982 Mar;15(3):447–455. doi: 10.1128/jcm.15.3.447-455.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P. J., Souhrada M., Ullom B., McClatchy J. K., Goren M. B. Identification of atypical mycobacteria by thin-layer chromatography of their surface antigens. J Clin Microbiol. 1978 Oct;8(4):374–379. doi: 10.1128/jcm.8.4.374-379.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P. J. Structure of mycobacteria: recent developments in defining cell wall carbohydrates and proteins. Rev Infect Dis. 1989 Mar-Apr;11 (Suppl 2):S420–S430. doi: 10.1093/clinids/11.supplement_2.s420. [DOI] [PubMed] [Google Scholar]
- Brudney K., Dobkin J. Resurgent tuberculosis in New York City. Human immunodeficiency virus, homelessness, and the decline of tuberculosis control programs. Am Rev Respir Dis. 1991 Oct;144(4):745–749. doi: 10.1164/ajrccm/144.4.745. [DOI] [PubMed] [Google Scholar]
- CHAPMAN G. B., HANKS J. H., WALLACE J. H. An electron microscope study of the disposition and fine structure of Mycobacterium lepraemurium in mouse spleen. J Bacteriol. 1959 Feb;77(2):205–211. doi: 10.1128/jb.77.2.205-211.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlemalm E., Villiger W., Hobot J. A., Acetarin J. D., Kellenberger E. Low temperature embedding with Lowicryl resins: two new formulations and some applications. J Microsc. 1985 Oct;140(Pt 1):55–63. doi: 10.1111/j.1365-2818.1985.tb02660.x. [DOI] [PubMed] [Google Scholar]
- Culliton B. J. Drug-resistant TB may bring epidemic. Nature. 1992 Apr 9;356(6369):473–473. doi: 10.1038/356473a0. [DOI] [PubMed] [Google Scholar]
- David H. L., Lévy-Frébault V., Thorel M. F. Characterization of distinct layers of the Mycobacterium avium envelope in respect of their composition by fatty acids, proteins, oligosaccharides and antigens. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Apr;268(2):193–208. doi: 10.1016/s0176-6724(88)80003-8. [DOI] [PubMed] [Google Scholar]
- Draper P., Rees R. J. Electron-transparent zone of mycobacteria may be a defence mechanism. Nature. 1970 Nov 28;228(5274):860–861. doi: 10.1038/228860a0. [DOI] [PubMed] [Google Scholar]
- Draper P., Rees R. J. The nature of the electron-transparent zone that surrounds Mycobacterium lepraemurium inside host cells. J Gen Microbiol. 1973 Jul;77(1):79–87. doi: 10.1099/00221287-77-1-79. [DOI] [PubMed] [Google Scholar]
- Draper P. The mycoside capsule of Mycobacterium Avium 357. J Gen Microbiol. 1974 Aug;83(2):431–433. doi: 10.1099/00221287-83-2-431. [DOI] [PubMed] [Google Scholar]
- Ellner J. J., Daniel T. M. Immunosuppression by mycobacterial arabinomannan. Clin Exp Immunol. 1979 Feb;35(2):250–257. [PMC free article] [PubMed] [Google Scholar]
- Frehel C., Rastogi N. Mycobacterium leprae surface components intervene in the early phagosome-lysosome fusion inhibition event. Infect Immun. 1987 Dec;55(12):2916–2921. doi: 10.1128/iai.55.12.2916-2921.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham L. L., Beveridge T. J. Evaluation of freeze-substitution and conventional embedding protocols for routine electron microscopic processing of eubacteria. J Bacteriol. 1990 Apr;172(4):2141–2149. doi: 10.1128/jb.172.4.2141-2149.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., McNeil M., Modlin R. L., Mehra V., Bloom B. R., Brennan P. J. Isolation and characterization of the highly immunogenic cell wall-associated protein of Mycobacterium leprae. J Immunol. 1989 Apr 15;142(8):2864–2872. [PubMed] [Google Scholar]
- Moreno C., Mehlert A., Lamb J. The inhibitory effects of mycobacterial lipoarabinomannan and polysaccharides upon polyclonal and monoclonal human T cell proliferation. Clin Exp Immunol. 1988 Nov;74(2):206–210. [PMC free article] [PubMed] [Google Scholar]
- Nishiura M., Izumi S., Mori T., Takeo K., Nonaka T. Freeze-etching study of human and murine leprosy bacilli. Int J Lepr Other Mycobact Dis. 1977 Jul-Sep;45(3):248–254. [PubMed] [Google Scholar]
- Paul T. R., Beveridge T. J. Reevaluation of envelope profiles and cytoplasmic ultrastructure of mycobacteria processed by conventional embedding and freeze-substitution protocols. J Bacteriol. 1992 Oct;174(20):6508–6517. doi: 10.1128/jb.174.20.6508-6517.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul T. R., Beveridge T. J. Ultrastructure of mycobacterial surfaces by freeze-substitution. Zentralbl Bakteriol. 1993 Nov;279(4):450–457. doi: 10.1016/s0934-8840(11)80416-0. [DOI] [PubMed] [Google Scholar]
- Rastogi N., David H. L. Mechanisms of pathogenicity in mycobacteria. Biochimie. 1988 Aug;70(8):1101–1120. doi: 10.1016/0300-9084(88)90272-6. [DOI] [PubMed] [Google Scholar]
- Rastogi N., Hellio R. Evidence that the capsule around mycobacteria grown in axenic media contains mycobacterial antigens: implications at the level of cell envelope architecture. FEMS Microbiol Lett. 1990 Jul;58(2):161–166. doi: 10.1111/j.1574-6968.1990.tb13971.x. [DOI] [PubMed] [Google Scholar]
- Rulong S., Aguas A. P., da Silva P. P., Silva M. T. Intramacrophagic Mycobacterium avium bacilli are coated by a multiple lamellar structure: freeze fracture analysis of infected mouse liver. Infect Immun. 1991 Nov;59(11):3895–3902. doi: 10.1128/iai.59.11.3895-3902.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH D. W., RANDALL H. M., MACLENNAN A. P., LEDERER E. Mycosides: a new class of type-specific glycolipids of Mycobacteria. Nature. 1960 Jun 11;186:887–888. doi: 10.1038/186887a0. [DOI] [PubMed] [Google Scholar]
- Shafer R. W., Sierra M. F. Mycobacterium xenopi, Mycobacterium fortuitum, Mycobacterium kansasii, and other nontuberculous mycobacteria in an area of endemicity for AIDS. Clin Infect Dis. 1992 Jul;15(1):161–162. doi: 10.1093/clinids/15.1.161. [DOI] [PubMed] [Google Scholar]
- Snider D. E., Jr, Roper W. L. The new tuberculosis. N Engl J Med. 1992 Mar 5;326(10):703–705. doi: 10.1056/NEJM199203053261011. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Takeo K., Kimura K., Kuze F., Nakai E., Nonaka T., Nishiura M. Freeze-fracture observations of the cell walls and peribacillary substances of various mycobacteria. J Gen Microbiol. 1984 May;130(5):1151–1159. doi: 10.1099/00221287-130-5-1151. [DOI] [PubMed] [Google Scholar]
- Tereletsky M. J., Barrow W. W. Postphagocytic detection of glycopeptidolipids associated with the superficial L1 layer of Mycobacterium intracellulare. Infect Immun. 1983 Sep;41(3):1312–1321. doi: 10.1128/iai.41.3.1312-1321.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne L. G., Sramek H. A. Agents of newly recognized or infrequently encountered mycobacterial diseases. Clin Microbiol Rev. 1992 Jan;5(1):1–25. doi: 10.1128/cmr.5.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAMOTO T., NISHIURA M., HARADA N., IMAEDA T. Electron microscopy of Mycobacterium leprae murium in ultra-thin sections of murine leprosy lesions. Int J Lepr. 1958 Apr-Jun;26(2):111–114. [PubMed] [Google Scholar]