Abstract
The signaling molecule nitric oxide (NO), first described as endothelium-derived relaxing factor (EDRF), acts as physiological activator of NO-sensitive guanylyl cyclase (NO-GC) in the cardiovascular, gastrointestinal, and nervous systems. Besides NO-GC, other NO targets have been proposed; however, their particular contribution still remains unclear. Here, we generated mice deficient for the β1 subunit of NO-GC, which resulted in complete loss of the enzyme. GC-KO mice have a life span of 3–4 weeks but then die because of intestinal dysmotility; however, they can be rescued by feeding them a fiber-free diet. Apparently, NO-GC is absolutely vital for the maintenance of normal peristalsis of the gut. GC-KO mice show a pronounced increase in blood pressure, underlining the importance of NO in the regulation of smooth muscle tone in vivo. The lack of an NO effect on aortic relaxation and platelet aggregation confirms NO-GC as the only NO target regulating these two functions, excluding cGMP-independent mechanisms. Our knockout model completely disrupts the NO/cGMP signaling cascade and provides evidence for the unique role of NO-GC as NO receptor.
Keywords: cardiovascular, knockout mice, cGMP, platelet aggregation, smooth muscle relaxation
The nitric oxide (NO)/cGMP signaling cascade regulates a plethora of physiological functions in the cardiovascular, neuronal, and gastrointestinal systems (1–3). In the vascular system, NO, first recognized as endothelium-derived relaxing factor (EDRF; ref. 4), has been shown to mediate smooth muscle relaxation and inhibition of platelet aggregation. NO is synthesized by the family of NO synthases which exist in endothelial, neuronal, and inducible forms. The prominent receptor known to date is the enzyme NO-sensitive guanylyl cyclase (NO-GC). Stimulation of NO-GC by NO results in the production of the second messenger, cGMP, which exerts its effects via cGMP-dependent kinases, channels, or phosphodiesterases (5–8). Besides these cGMP-mediated effects, NO is thought to mediate a variety of effects via cGMP-independent mechanisms in the cardiovascular system (for a review, see ref. 9).
To gain further insight into the NO/cGMP signaling cascade, mice deficient in NO synthases (NOS) have been generated (10–14). Although these mouse lines have tremendously helped to understand NO/cGMP signaling, it is still not known which of NO's effects are mediated via NO-GC and thus cGMP, or alternatively, via pathways not involving cGMP.
To address this point and to further investigate the physiological role of the enzyme and of the NO/cGMP signaling cascade in vivo, we generated an NO-GC-deficient mouse line. NO-GC is a heterodimer made up of two subunits, α and β. Two isoforms are known to exist (α1β1 and α2β1; ref. 15) in which the β1 subunit acts as the dimerizing partner for either α subunit. α subunits in the absence of the β1 subunit do not form dimers and are not catalytically active. Thus, deletion of the β1 subunit should completely eliminate NO-GC and yield a mouse line in which NO/cGMP signaling is completely abrogated. With these mice, we should be able to unequivocally answer the question of whether NO signals via NO-GC and/or via alternative targets and thus be able to discern between cGMP-dependent and cGMP-independent effects of NO.
Results
Generation of KO Mice.
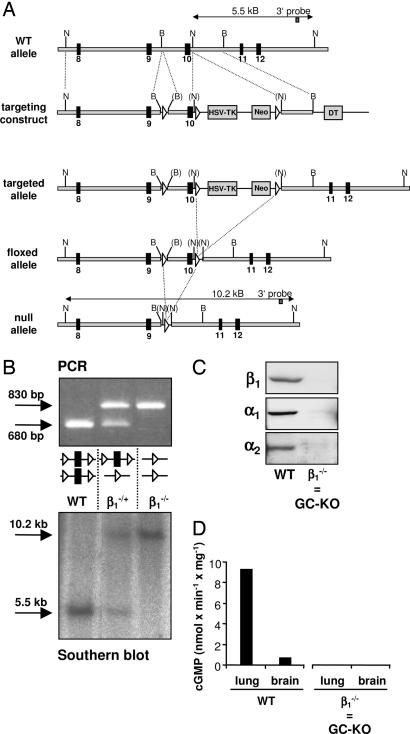
For the generation of KO mice, we used the Cre/loxP system (16). Exon 10 of the β1 subunit was floxed, and after ES transfer, it was deleted by using the EIIa/Cre mouse [ref. 17; Fig. 1A and supporting information (SI)]. The null mutation was confirmed by PCR and Southern blot analysis (Fig. 1B and SI). By using Western blot analysis, neither the β1 subunit nor, surprisingly, the α subunits could be detected on the protein level (Fig. 1C). In line with this finding, NO-induced cGMP synthesis was neither detected in lung nor in brain (Fig. 1D) nor in other GC-rich tissues/cells (aorta and platelets; data not shown). We conclude that deletion of the β1 subunit leads to a complete loss of NO-GC.
Fig. 1.
Gene inactivation of the β1 subunit of NO-sensitive GC. (A) Targeted disruption of the β1 subunit of NO-sensitive GC. loxP sites are depicted as white triangles, and exons (black squares) are numbered from the first coding exon. TK/Neo, thymidine kinase/neomycin cassette; DT, diphteria toxin gene; WT, wild-type loci of the β1 subunit; S, SpeI; N, NheI; B, BamHI; brackets indicate destroyed restriction sites, and newly created ones are in italics. For more information, see SI. (B) Genotyping of mice. PCR and Southern blotting were performed as described in Materials and Methods. In the Southern blot, the β1-KO band is larger than that of the WT because the NheI site in intron 10 was lost by the insertion of the TK/Neo cassette. (C) Western blot analysis of the expression of the β1, α1, and α2 subunits of NO-GC in lung homogenates. (D) NO-stimulated cGMP-forming activity in brain and lung homogenates in the presence of diethylamine-NO (100 μM). Data are from one representative experiment. The cGMP-forming activity in KO tissues was zero under nonstimulated and NO-stimulated conditions. Because the deletion of the β1 subunit results in deletion of NO-GC, we will henceforward use the term “GC-KO.”
Mortality of GC-KO Mice.
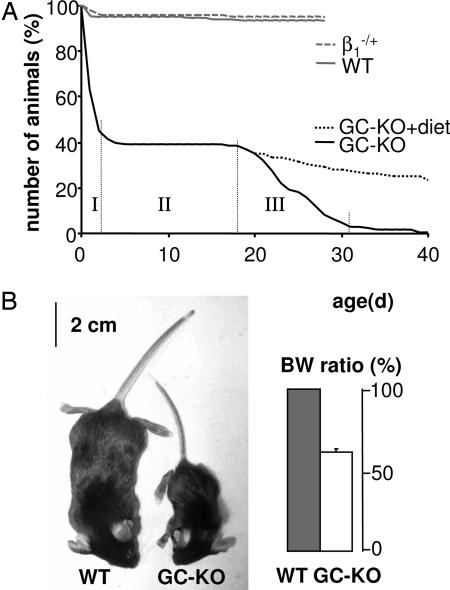
Whereas mice heterozygous for the β1 subunit of NO-GC were phenotypically undistinguishable from WT, homozygous GC-KO mice died prematurely and revealed two phases of mortality (Fig. 2A): 60% of born KO mice died within the first 2 days (indicated by I in Fig. 2A) whereas most (90%) of the remaining mice died between day 18 and 31 (indicated by III). To our surprise, in the 2 weeks between these two phases, practically all mice survived (indicated by II). The first period of lethality is indicative of maladaptation to the neonatal situation (18). As weaning and thus a change in diet takes place around day 18, we suspected gastrointestinal complications to underlie the second phase of mortality (see below).
Fig. 2.
Postnatal survival and appearance of mice deficient in NO-GC. (A) Postnatal survival of WT, heterozygous, and GC-KO mice. WT (n = 243) and heterozygous (β1−/+; n = 483) mice were observed for ≈4 weeks. The survival of GC-KO mice (n = 126) was monitored until death. Feeding with fiber-free diet improved the survival of GC-KO mice (GC-KO+diet; n = 58). (B) WT and GC-KO siblings at ≈21 days of age. Body weight (BW) ratio of WT (100%; n = 24) and GC-KO (n = 28) animals are shown (Right). Three-week-old siblings of the same sex were compared for BW determination.
When performing Caesarean section between prenatal days 17.5 and 18.5, mice fetuses were found to be phenotypically indistinguishable from WT or heterozygote siblings. Transmission of the mutation at the Mendelian ratio was found in prenatal offspring (n = 94 pups from eight litters) but was <25% in mice 1 day after birth (see numbers in Fig. 2). We assume that KO mice are born but die soon after birth because of reasons yet to be identified. Thus, the numbers in Fig. 2A underestimate the initial death rate of the KO mice by ≈50%.
Gastrointestinal Phenotype of GC-KO Mice.
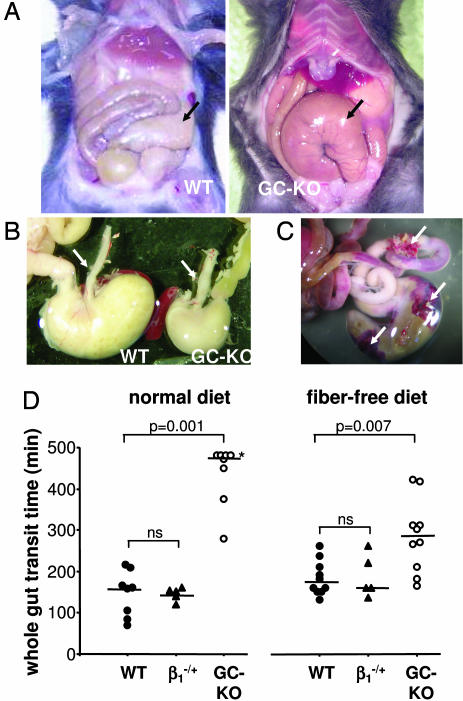
The overall appearance of a 3-week-old GC-KO mouse and its WT sibling is shown in Fig. 2B; homozygous GC-KO mice exhibit considerable growth retardation, shown by a 40% reduction in body weight. Because this growth retardation may be explained by malnutrition, we therefore focused on the gastrointestinal tract: the vast majority of the GC-KO mice had a grossly enlarged and dilated caecum which, in most cases, was displaced from its normal position (Fig. 3A). To individually different degrees, these mice showed additional morphological changes such as an enlarged esophagus (Fig. 3B), a dilated stomach, a bloated gall bladder, and an enlarged colon (data not shown). To assess the functional relevance, we measured whole-gut transit time as a marker of gastrointestinal motility. After application of the red dye carmine into the stomach, the time until excretion was monitored (19). The whole-gut transit time in GC-KO mice was increased approximately three times compared with WT and heterozygote siblings (median of >475 min versus 160 and 151 min, respectively; Fig. 3D). This reduced gut transport may be the basis for further attendant symptoms in KO mice like ileus, peritonitis, intestinal necrosis (data not shown), and, in some cases, the perforation of the intestinal wall (Fig. 3C). Apparently, GC is indispensable for the physiological coordination of contraction/relaxation that is required for the segmental and propulsive movements of the gut, and the dysfunctional peristalsis due to lack of GC may well be the cause of death.
Fig. 3.
Gastrointestinal tract and total gut transit time of WT and GC-KO mice. (A) The gastrointestinal tract of 3-week-old WT and GC-KO mice on regular diet. Arrows indicate the caecum, which in the GC-KO mouse is enlarged and displaced. (B) Comparison of esophagus (arrows) and stomach. (C) Lower gastrointestinal tract (caecum and parts of colon) of a 3-week-old GC-KO mouse immediately after dying. The arrows indicate perforations with adjoining hemorrhages. (D) Total gut transit time (see Materials and Methods). After weaning, mice were either fed fiber-containing standard diet (Left) or fiber-free diet (Right). In the case of standard diet, the measurement was discontinued for four KO mice (indicated by the asterisk) that did not produce red-colored feces even after 8 h. The lines indicate the respective median value. Statistical analysis is described in detail in Materials and Methods. Kruskal–Wallis global test was followed by Mann–Whitney U test for the comparison of two individual groups. The P values for the Mann–Whitney U test are given. ns, not significant.
Rescue of GC-KO Mice.
To circumvent the second phase of mortality starting around day 18, we fed mice with fiber-free diet after weaning. As shown in Fig. 2A, KO mice could be rescued and survival improved immensely. Accordingly, the whole-gut transit time increased although it still did not equal that of WT or heterozygous mice (KO mice on fiber-free diet: median of 285 min vs. 170 and 160 min, respectively; see Fig. 3D). Despite the special diet, mice lacking NO-GC still appear to be susceptible to gastrointestinal complications because they still died prematurely. Taken together, the rescue of GC-KO mice indicates that mortality is indeed caused by severe gastrointestinal obstruction in animals on normal diet.
Elevated Blood Pressure and Reduced Heart Rate in GC-KO Mice.
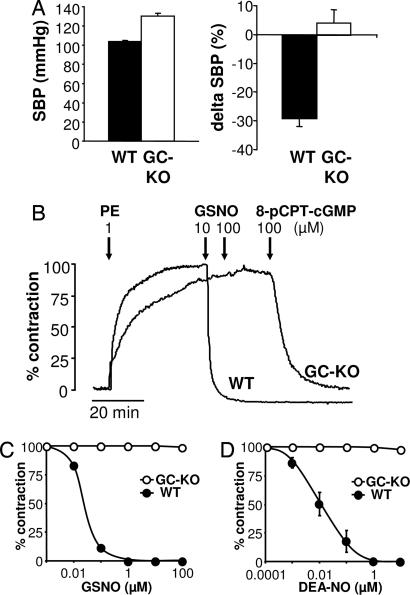
The physiological role of NO as EDRF has been established in a series of elegant experiments leading to the award of the Nobel prize (20). To estimate the impact of the lack of the most important NO receptor, we measured blood pressure using a noninvasive tail-cuff system. Fig. 4A shows that GC-KO mice have a pronouncedly elevated systolic blood pressure that surmounts that of WT siblings by 26 mmHg [130 ± 2 mmHg (KO) vs. 104 ± 1 mmHg (WT)].
Fig. 4.
Cardiovascular effects in mice lacking NO-GC. (A) Systolic blood pressure (SBP) was measured in conscious WT and GC-KO mice by tail-cuff plethysmography as described in Materials and Methods (Left, n = 7–8 for each genotype). Blood pressure data are given as mean ± SD. In a different set of experiments, mice (nine WT and six GC-KO of either sex) were anesthetized with ketamine/hydrazine. After i.p. injection of 5 mg/kg GTN, the subsequent change in blood pressure was recorded. Data represent mean ± SEM. (B) Representative original registration of aortic rings from WT and GC-KO mice. Rings were precontracted with 1 μM phenylephrine (PE). After reaching steady state, relaxation was induced by GSNO (10 and 100 μM). The membrane-permeable 8-pCPT-cGMP was added to confirm the integrity of the GC-KO rings and to show intact signaling beyond NO-GC. 8-pCPT-cGMP induced a similar relaxing response in WT rings (data not shown in this trace). (C) Relaxation curves for GSNO in aortic rings from WT and GC-KO mice (n = 5–8 per genotype). Error bars are smaller than the size of the symbols. (D) Relaxation curves for diethylamine-NO in aortic rings from WT and GC-KO mice (n = 5–8 per genotype).
Lack of NO-Induced Relaxation in Aortae from GC-KO Mice.
The impact of GC deficiency was further investigated in aortic smooth muscle. To date, it is still unclear whether NO induces relaxation of vascular smooth muscle solely via GC and/or via alternative mechanisms. To investigate this issue, we performed organ bath experiments (Fig. 4 B–D). The NO donor S-nitrosoglutathione (GSNO) led to relaxation of phenylephrine-precontracted WT aortic rings (EC50 ≈0.04 μM; Fig. 4B). No relaxation was seen in aortic rings from GC-KO mice even at GSNO concentrations three orders of magnitude above those fully relaxing the WT aorta. Similarly, another NO donor, diethylamine-NO, failed to relax aortae from KO animals (Fig. 4D). In line with these data, carbachol did not induce a relaxing response in the KO animals (data not shown). Yet, in the GC-KO mice, the signaling cascade downstream of GC functioned normally because relaxation was induced by stimulation of cGMP-dependent protein kinase (PKG) using the membrane-permeable cGMP analogue 8-pCPT-cGMP (see Fig. 4A). In addition to GSNO and diethylamine-NO, we tested other NO donors [Proli-NO, Angeli salt, and SNP (all up to 100 μM)] that liberate either NO radical, NO−, or NO+ species, and they all failed to relax GC-deficient aortic rings (data not shown). Thus, in our aortic model, the relaxing effect of NO is solely conveyed by NO-sensitive GC.
Lack of Glycerol Trinitrate (GTN)-Induced Decrease in Systolic Blood Pressure from GC-KO Mice in Vivo.
The impact of GC deficiency was further investigated in vivo by testing the blood pressure-lowering effect of GTN (Fig. 4A). Anesthetized WT and GC-KO animals were injected with 5 mg/kg GTN, and the subsequent decrease in systolic blood pressure was recorded by using tail-cuff plethysmography. In WT animals, GTN induced a decrease in systolic blood pressure of ≈30 mmHg. In GC-KO animals, GTN did not decrease the systolic blood pressure.
Lack of NO-Induced Inhibition in Platelets from GC-KO Mice.
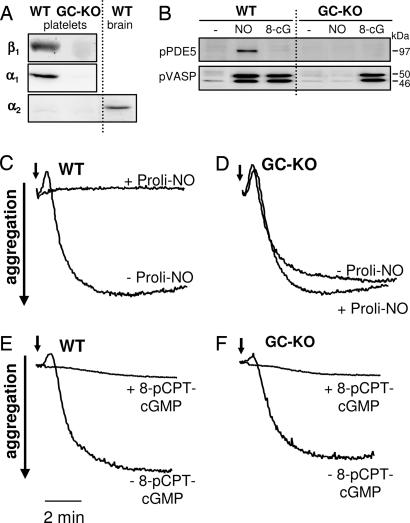
Similar to its role in smooth muscle, NO has been postulated to mediate platelet inhibition by cGMP-dependent as well as cGMP-independent mechanisms (9, 21–23). The Western blot in Fig. 5A shows that both α1 and β1 subunits are absent in GC-KO platelets and that the α2 subunit is neither expressed in WT nor in KO platelets. Next, we studied the physiological impact of NO on platelet aggregation. Collagen (4 μg/ml) induced aggregation of both WT and GC-KO platelets (Fig. 5 C–F). Collagen-induced aggregation was inhibited by NO in WT platelets whereas NO had no inhibitory effect in KO platelets (Fig. 4D). Even with millimolar concentrations of Proli-NO (and also a variety of other NO donors including GSNO, diethylamine-NO, and SNP; data not shown), we were unable to induce inhibition of aggregation. In addition, aggregation of KO platelets by other agonists (thrombin or U46619, a thromboxane A2 mimetic) was also not inhibited by NO (data not shown).
Fig. 5.
NO does not inhibit aggregation of platelets from GC-KO mice. (A) Western blot analysis of the expression of the β1, α1, and α2 subunits of NO-GC in WT and GC-KO platelets and, as positive control for the α2 subunit, in WT brain. (B) Phosphorylation of PDE5 and vasodilator-associated phosphoprotein in intact WT and GC-KO platelets upon stimulation with GSNO (NO; 100 μM) and 8-pCPT-cGMP (8-cG; 40 μM). The lack of PDE5 phosphorylation by 8-pCPT-cGMP-activated PKG is explained by the fact that PDE5 requires bound cGMP to change its conformation for being phosphorylated. (C–F) Aggregation of platelets. Platelets from WT (C and E) and GC-KO mice (D and F) were stimulated with collagen (4 μg/ml; indicated by the arrow). To inhibit aggregation, the NO donor Proli-NO (4 mM; C and D) was administered simultaneously with collagen, and 8-pCPT-cGMP (40 μM; E and F) was added 20 min before collagen.
The effect of NO-induced cGMP synthesis in platelets can be monitored by the activity of PKG, e.g., by the phosphorylation of substrate proteins such as vasodilator-associated phosphoprotein and phosphodiesterase type 5 (PDE5) (24, 25). The Western blot in Fig. 4B shows that, in contrast to WT, NO does not induce activation of PKG in KO platelets, as shown by the lack of vasodilator-associated phosphoprotein or PDE5 phosphorylation. However, similar to aortic tissue, the signaling cascade beyond NO-GC is still intact because stimulation of PKG using 8-pCPT-cGMP results in phosphorylation of vasodilator-associated phosphoprotein as well as in the inhibition of aggregation of both collagen-stimulated WT and KO platelets (Fig. 5 B, E, and F). Thus, we conclude that NO induces the inhibition of platelet aggregation solely via cGMP.
Discussion
Two isoforms of NO-GC are known, α1β1 and α2β1. Because the β1 subunit is the common dimerizing subunit for the two α subunits, we assumed the deletion of the β1 subunit would result in a loss of both isoforms. In fact, we were unable to detect either of the α subunits on the protein level in mice lacking the β1 subunit of NO-GC, indicating instability of the α subunits when expressed without the dimerizing partner. A reduction in the respective dimerizing partner was also detected in mice lacking either α1 or α2 subunit of NO-GC (26). The reason for this down-regulation is unknown, however, there has to be some posttranscriptional regulatory mechanism because mRNA levels of the α subunits were similar in KO and WT animals (data not shown). The lack of GC expression is corroborated by a total lack of NO-stimulated cGMP production in several tissues. Thus, by deleting the common dimerizing β1 subunit, we have generated a mouse line that lacks NO-GC.
Sixty percent of these mice die within the first 2 days, most of them on the day of birth. We assume that these mice are born at the Mendelian ratio because we detect 25% KO fetuses when performing Caesarean section. When controlling genotypes 1 day after birth, we do not find this percentage of KO animals, which points to a high postnatal death rate and subsequent removal of dead cubs by the mother. However, animals that survived the first 3 days lived for at least 18 days and died within the next 2 weeks. We assumed dysmotility of the gastrointestinal tract as cause of death, because several of the KO mice showed perforations of various parts of the gut when examined postmortem. The dysfunctional smooth muscle relaxation was confirmed by the functional constipation because total gut transit time was extremely prolonged in the absence of NO-GC. In mice lacking neuronal NOS (27) or acetylcholinesterase (28), the lethal consequences of GI dysmotility could be avoided by feeding a standard liquid rodent diet; this was not the case in NO-GC KO mice. Yet, by feeding a low-residue diet, we were able to rescue our mice, which indicates an impairment in the intestinal fiber transport in our KO mice. Taken together, the overall gastrointestinal phenotype emphasizes the prominent role of NO-GC and thus cGMP in the regulation of intestinal smooth muscle tone.
Since the discovery of NO as paracrine mediator, the identification of receptor molecules has been of fundamental interest. NO-GC has been accepted as the most important mediator of NO effects; however, apart from the toxicological effects of very high NO concentrations, receptors other than NO-GC have been published to mediate effects of “signaling” concentrations of NO (for a review, see ref. 9). Using our GC-KO mouse line, we are now able to discern between NO effects mediated by NO-GC and those mediated by other molecules. We investigated the two most important physiological effects of NO, aortic relaxation and platelet inhibition. Very clearly, lack of NO-GC totally abrogates the vasodilatory and platelet-inhibitory effects of NO. Thus, regarding these two functions, at least under our conditions, no other mediator of the relaxing and anti-inhibitory effect of NO exists besides NO-GC.
KO mice deficient in either of the α subunits exist (26). α1-KO (retaining the α2-GC) as well as α2-KO mice (retaining the α1-GC) are viable and have a normal life expectancy. Thus, mice can live with only a fractional amount of GC (e.g., α1-KO retaining only 6% aortic GC activity) but develop the life-limiting phenotype only when NO-GC is totally abolished. Interestingly, α1-KO mice, in which >90% of GC is lacking, develop only a moderately elevated systolic blood pressure (increase of 7 mmHg compared with WT) in contrast to total GC-KO mice, which reveal a drastic increase of 26 mmHg. Although only a fraction of normal GC content in the vessel wall is sufficient to control blood pressure, a total lack of NO-GC cannot be compensated for. This finding underlines the important role of constitutive NO production in blood pressure regulation.
Comparing the symptoms of our mice with those of mice lacking PKGs or NOSs reveals different degrees of overlap. Closest in phenotype are mice lacking PKGI, which also show a reduced life span (although less severe, as 50% die before 5–6 weeks of age) and very similar disturbances in the gastrointestinal tract (29–30). PKGI-deficient mice have an increased blood pressure as well as similar defects in the relaxation of vascular smooth muscle and platelet function (31). Vascular symptoms such as an increase in systolic blood pressure, reduced heart rate, and a lack of acetylcholine-induced relaxation of aortic rings can be seen in mice deficient in endothelial NOS (10–12).
There have been two different approaches to knocking out nNOS. In the first strain, exon 2 containing the start codon has been deleted. These mice reveal a comparatively mild gastrointestinal phenotype with enlargement of the stomach and hypertrophy of the pyloric sphincter (13). However, a splice variant, nNOSβ, which lacks exon 2, has been shown to be synthesized and to be catalytically active in these nNOS-KO mice (32, 33). Accordingly, expression of nNOSβ has been shown to explain the puzzling finding that male nNOS-KO mice are still fertile (33). A second nNOS-KO line has been generated by the deletion of exon 6 (27). These mice are not fertile and reveal pyloric stenosis.
The most interesting comparison can be made between our GC-KO mice and a triple-KO strain in which seemingly all three NOS isoforms have been deleted (14). Similar to our mice, triple-NOS-KO mice have an elevated systolic blood pressure of ≈25 mmHg. These triple-NOS-KO mice, similar to the first nNOS-KO line (13), are fertile and appear to exhibit characteristics of nephrogenic diabetes insipidus. Reduction in life expectancy with an 80% survival rate >4–5 months was reported for the triple-NOS-KO mice which, however, is far less pronounced than in GC-KO mice. It has to be noted, however, that this “triple-KO” was derived from the original nNOS-KO generated by Huang et al. (13), which still contains the β splice variant. Thus, it is very likely that the phenotype of these mice is still influenced by the residual NOS, which is expressed in brain, penis, and also in many parts of the GI tract (34). In sum, we believe that the massive phenotype that we observe in our GC-KO mice is due to the fact that the signaling pathway from NOS to GC and further beyond is totally abrogated. The discovery of further phenotypical similarities/differences between our GC-KO mice and those lacking other members of the NO/cGMP cascade will help to refine our knowledge on the diverse functions of these signaling molecules.
By deleting NO-GC, we have developed a mouse model in which NO production is entirely disconnected from downstream signaling to the PKGs and other cGMP effectors. Because KO mice are capable of living up to 4 weeks, we state that lack of NO-GC does not impair the overall fetal and embryonic development. However, our data show that GC-mediated control of gastrointestinal contractility is absolutely vital. The established role of NO in the cardiovascular system as an important vasodilator and vasoprotective agent is further corroborated: Clearly, NO released constitutively from the endothelium has an important impact on blood pressure. The absolute failure of NO to influence aortic tone and platelet aggregation, the two best-studied physiological systems regulated by the NO/cGMP cascade, reveals the unique role of NO-sensitive GC as receptor for NO in our mice.
Materials and Methods
All experiments were conducted in accordance with the German legislation on protection of animals and approved by the local animal care committee.
Generation of β1-KO Mice.
Murine genomic DNA of the β1 subunit was derived from BAC clones (Incyte Genomics, St. Louis, MO) containing genomic DNA of ES cells of 129/SvJ mice. The targeting vector (Fig. 1A) was constructed such that exon 10 of the β1 subunit was flanked by a loxP-Tk/Neo-loxP cassette and a single loxP site using a triple loxP vector set (16). Chimaeras were bred with C57BL/6 mice, and the resulting heterozygotes (β1+/flox) were crossed with the general deleter mouse Ella-Cre (17), resulting in the deletion of the floxed exon 10 (β1+/−). To remove the Cre recombinase gene, the heterozygous KO mice were crossed with WT mice (C57BL/6). Finally, the generated heterozygotes (β1+/−) were intercrossed to yield homozygous mice (β1−/− = β1-KO). Further information on the cloning strategy as well as on the genotyping can be found in the SI and can also be obtained from the corresponding author.
Genotyping of Mice.
Genotyping was performed by PCR analysis and Southern blotting of tail DNA (Fig. 1B). The WT (680 bp) and β1-KO (830 bp) fragments were amplified in a multiplex PCR using the primer lox-β1-U1 (AAG ATG CTG AAG GGA AGG ATG C) versus lox-β1-L1 (CAG CCC AAA GAA ACA AGA AGA AAG) and del-β1-L1 (GAT GTG GGA TTG TTT CTG AGG A). NheI digests of genomic DNA and a 536-bp β1-genomic probe (corresponding to the DNA sequence from exon 12 to exon 13) were used for Southern blots. This probe identifies a 5.5-kb fragment from the endogenous allele and a 10.2-kb fragment from the β1-KO allele. Studies were performed with ≈3-week-old mice of either sex with a hybrid background (129/SvJ × C57BL/6, F3–F9 generation). In each experiment, littermates of the same sex were used wherever feasible.
Analysis of Murine Tissues.
The preparation of tissues, Western blot analysis, determination of GC activity, and cGMP-RIA were performed as described (24).
Total Gut Transit Test.
Carmine (50 μl; 3 mg of carmine in 0.5% methylcellulose or, in the case of animals on fiber-free diet, in tap water) was orally administered to each mouse. Mice were then returned to individual cages and placed on a white sheet of paper. The time taken for excretion of the first colored feces was recorded. Gut transit time was measured in 5–10 animals of each genotype.
Measurement of Blood Pressure.
Systolic blood pressure was measured in conscious and anesthetized mice by tail-cuff plethysmography (BP-98A; Softron, Tokyo, Japan). Measurements of blood pressure in conscious mice were taken from a total of seven WT mice (five females and two males) and eight β1-KO mice (six females and two males). For habituation, the mice were trained daily (3–7 days). After the training period, 7–10 measurements per mouse were recorded daily for 5 days. In a different set of experiments, mice (nine WT and six GC-KO of either sex) were anesthetized with ketamine/hydrazine. In these animals, the change in blood pressure subsequent to i.p. injection of 5 mg/kg GTN was recorded.
Isolation of Platelets from Mice and Aggregation Experiments.
WT and β1-KO mice were anesthetized by ether inhalation. Blood (≈800 μl) was collected from the orbital sinus and was drawn into 200 μl of heparin solution (50 units/ml). After dilution (1 ml of PBS, Ca2+- and Mg2+-free) and centrifugation (15 min at 90 × g and 20°C), the upper phase [platelet-rich plasma (PRP)] was collected. The lower phase was washed with a volume of PBS similar to that of the removed plasma and then recentrifuged; the upper phase was mixed with the PRP. Optical aggregation of PRP (2–3 × 107 platelets per 200 μl) was performed in a four-channel aggregometer (Chronolog, Havertown, PA) after the addition of collagen (4 μg/ml; Chronolog). Proli-NO (4 mM) was administered simultaneously with collagen, and other NO donors or 8-pCPT-cGMP (40 μM) were administered 3–20 min before addition of collagen.
Organ Bath Experiments.
Thoracic aortae were cut into rings and mounted on fixed segment support pins in two four-chamber myographs (Myograph 610; Danish Myo Technology, Aarhus, Denmark) containing 5 ml of Krebs–Henseleit solution (118 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO3, 7.5 mM glucose, pH 7.4) and gassed with 95% O2 and 5% CO2. Diclofenac (3 μM) and N-nitro-l-arginine methyl ester (200 μM) were present. Resting tension was set to 2.5 mN. After equilibration (at least 60 min at 37°C), rings were precontracted with phenylephrine (1 μM; Sigma, Taufkirchen, Germany), and the vasorelaxing effects of Proli-NO (4 mM; Alexis, Lausen, Switzerland) and 8-pCPT-cGMP (40 μM; Biolog, Bremen, Germany) were assessed. Each experiment was performed in parallel with two to four aortic rings derived from KO and WT mice.
Statistics.
If not stated otherwise, data are expressed as mean ± SEM (n = number of animals). To compare gut transit time results from KO, heterozygote, and WT animals, all groups of the experiment were compared by Kruskal–Wallis test. If the resulting P was <0.05, two individual groups were compared by Mann–Whitney tests in a hierarchical order. Specifically, we first tested GC-KO animals versus WT animals, then heterozygote animals versus WT animals, and then heterozygote versus GC-KO animals. The comparison was rated significant if P < 0.05, and hierarchical testing of individual groups was stopped if P > 0.05 to correct for multiple testing.
Supplementary Material
Acknowledgments
We thank Stephan Teglund (Karolinska Institutet, Sweden) for electroporation and blastocyst injection and Michael Russwurm for critical reading of the manuscript. The excellent technical help of Ulla Krabbe, Friedrich Eichhorst, Erika Mannheim, and Arkadius Pacha is acknowledged. We also thank Inke and Peter König for help with the statistical analysis. The work was supported by the Deutsche Forschungsgemeinschaft (A.F. and D.K.).
Abbreviations
- GSNO
S-nitrosoglutathione
- NO-GC
NO-sensitive guanylyl cyclase
- NOS
NO synthase
- PKG
cGMP-dependent protein kinase
- GTN
glycerol trinitrate
- PDE5
phosphodiesterase type 5
- KO
knockout.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0609778104/DC1.
References
- 1.Waldman SA, Murad F. Pharmacol Rev. 1987;39:163–196. [PubMed] [Google Scholar]
- 2.Moncada S, Higgs EA. FASEB J. 1995;9:1319–1330. [PubMed] [Google Scholar]
- 3.Ignarro LJ. J Physiol Pharmacol. 2002;53:503–514. [PubMed] [Google Scholar]
- 4.Furchgott RF, Zawadzki JV. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 5.Friebe A, Koesling D. Circ Res. 2003;93:96–105. doi: 10.1161/01.RES.0000082524.34487.31. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann F. J Biol Chem. 2005;280:1–4. doi: 10.1074/jbc.R400035200. [DOI] [PubMed] [Google Scholar]
- 7.Kaupp UB, Seifert R. Physiol Rev. 2002;82:769–824. doi: 10.1152/physrev.00008.2002. [DOI] [PubMed] [Google Scholar]
- 8.Rybalkin SD, Yan C, Bornfeldt KE, Beavo JA. Circ Res. 2003;93:280–291. doi: 10.1161/01.RES.0000087541.15600.2B. [DOI] [PubMed] [Google Scholar]
- 9.Wanstall JC, Homer KL, Doggrell SA. Curr Vasc Pharmacol. 2005;3:41–53. doi: 10.2174/1570161052773933. [DOI] [PubMed] [Google Scholar]
- 10.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 11.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PE, Sessa WC, Smithies O. Proc Natl Acad Sci USA. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godecke A, Decking UK, Ding Z, Hirchenhain J, Bidmon HJ, Godecke S, Schrader J. Circ Res. 1998;82:186–194. doi: 10.1161/01.res.82.2.186. [DOI] [PubMed] [Google Scholar]
- 13.Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- 14.Morishita T, Tsutsui M, Shimokawa H, Sabanai K, Tasaki H, Suda O, Nakata S, Tanimoto A, Wang KY, Ueta Y, et al. Proc Natl Acad Sci USA. 2005;102:10616–10621. doi: 10.1073/pnas.0502236102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russwurm M, Koesling D. Mol Cell Biochem. 2002;230:159–164. [PubMed] [Google Scholar]
- 16.Gainetdinov RR, Bohn LM, Sotnikova TD, Cyr M, Laakso A, Macrae AD, Torres GE, Kim KM, Lefkowitz RJ, Caron MG, Premont RT. Neuron. 2003;38:291–303. doi: 10.1016/s0896-6273(03)00192-2. [DOI] [PubMed] [Google Scholar]
- 17.Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, Westphal H. Proc Natl Acad Sci USA. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng Q, Song W, Lu X, Hamilton JA, Lei M, Peng T, Yee SP. Circulation. 2002;106:873–879. doi: 10.1161/01.cir.0000024114.82981.ea. [DOI] [PubMed] [Google Scholar]
- 19.Nagakura Y, Naitoh Y, Kamato T, Yamano M, Miyata K. Eur J Pharmacol. 1996;311:67–72. doi: 10.1016/0014-2999(96)00403-7. [DOI] [PubMed] [Google Scholar]
- 20.Howlett R. Nature. 1998;395:625–626. doi: 10.1038/27019. [DOI] [PubMed] [Google Scholar]
- 21.Moncada S, Radomski MW, Palmer RM. Biochem Pharmacol. 1998;37:2495–2501. doi: 10.1016/0006-2952(88)90236-5. [DOI] [PubMed] [Google Scholar]
- 22.Crane MS, Rossi AG, Megson IL. Br J Pharmacol. 2005;144:849–859. doi: 10.1038/sj.bjp.0706110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sogo N, Magid KS, Shaw CA, Webb DJ, Megson IL. Biochem Biophys Res Commun. 2000;279:412–419. doi: 10.1006/bbrc.2000.3976. [DOI] [PubMed] [Google Scholar]
- 24.Mullershausen F, Russwurm M, Thompson WJ, Liu L, Koesling D, Friebe A. J Cell Biol. 2001;155:271–278. doi: 10.1083/jcb.200107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smolenski A, Bachmann C, Reinhard K, Honig-Liedl P, Jarchau T, Hoschuetzky H, Walter U. J Biol Chem. 1998;273:20029–20035. doi: 10.1074/jbc.273.32.20029. [DOI] [PubMed] [Google Scholar]
- 26.Mergia E, Friebe A, Dangel O, Russwurm M, Koesling D. J Clin Invest. 2006;116:1731–1737. doi: 10.1172/JCI27657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gyurko R, Leupen S, Huang PL. Endocrinology. 2002;143:2767–2774. doi: 10.1210/endo.143.7.8921. [DOI] [PubMed] [Google Scholar]
- 28.Duysen EG, Stribley JA, Fry DL, Hinrichs SH, Lockridge O. Brain Res Dev Brain Res. 2002;137:43–54. doi: 10.1016/s0165-3806(02)00367-x. [DOI] [PubMed] [Google Scholar]
- 29.Pfeifer A, Klatt P, Massberg S, Ny L, Sausbier M, Hirneiss C, Wang GX, Korth M, Aszodi A, Andersson KE, et al. EMBO J. 1998;17:3045–3051. doi: 10.1093/emboj/17.11.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ny L, Pfeifer A, Aszodi A, Ahmad M, Alm P, Hedlund P, Fassler R, Andersson KE. Br J Pharmacol. 2000;129:395–401. doi: 10.1038/sj.bjp.0703061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Massberg S, Sausbier M, Klatt P, Bauer M, Pfeifer A, Siess W, Fassler R, Ruth P, Krombach F, Hofmann F. J Exp Med. 1999;189:1255–1264. doi: 10.1084/jem.189.8.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eliasson MJ, Blackshaw S, Schell MJ, Snyder SH. Proc Natl Acad Sci USA. 1997;94:3396–3401. doi: 10.1073/pnas.94.7.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hurt KJ, Sezen SF, Champion HC, Crone JK, Palese MA, Huang PL, Sawa A, Luo X, Musicki B, Snyder SH, Burnett AL. Proc Natl Acad Sci USA. 2006;103:3440–3443. doi: 10.1073/pnas.0511326103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saur D, Neuhuber WL, Gengenbach B, Huber A, Schusdziarra V, Allescher HD. Am J Physiol. 2002;282:G349–G358. doi: 10.1152/ajpgi.00226.2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.