Abstract
Objective
Electroconvulsive therapy (ECT) is a widely used and effective treatment for mood disorders and appears to have positive effects on the motor symptoms of Parkinson's disease (PD), improving motor function for several weeks. Because repeated electroconvulsive shock (ECS) in normal animals enhances striatal dopamine (DA) D1 and D3 receptor binding, we hypothesized that upregulation of D1 and D3 receptors may also be occurring in the parkinsonian brain after repeated ECS treatment.
Methods
Rats were rendered hemiparkinsonian through unilateral infusion of the DA-specific neurotoxin 6-hydroxydopamine into the medial forebrain bundle and substantia nigra. The animals were tested for hindlimb and forelimb function before and 48 hours after the last of 10 daily treatments with ECS or sham. After sacrifice, DA receptor binding was determined autoradiographically.
Results
While there was no increase in forelimb use in the cylinder test, ECS treatment significantly improved hindlimb motor performance on a tapered beam-walking test and enhanced striatal D1 and D3 receptor binding, without affecting D2 receptor binding.
Conclusion
This study suggests that at least part of the mechanism of action of ECT in PD may be enhanced DA function within the direct pathway of the basal ganglia and may support the further study and use of ECT as a potential adjunct treatment for PD.
Medical subject headings: dopamine receptors, electroconvulsive therapy, motor skills, Parkinson disease
Abstract
Objectif
L'électrochoc est un traitement très répandu et efficace contre les troubles de l'humeur et il semble avoir des effets positifs sur les symptômes de la maladie de Parkinson (MP) qui touchent la motricité, car il améliore la fonction motrice pendant plusieurs semaines. Parce que l'électrochoc à répétition chez des animaux normaux améliore la fixation aux récepteurs D1 et D3 de la dopamine striatale, nous avons posé en hypothèse qu'il peut y avoir aussi régulation à la hausse des récepteurs D1 et D3 dans un cerveau parkinsonien après des électrochocs à répétition.
Méthodes
On a rendu des rats hémiparkinsoniens par perfusion unilatérale de neurotoxine 6-hydroxydopamine spécifique à la dopamine dans le faisceau médian du cerveau antérieur et les substances noires. On a vérifié le fonctionnement des pattes arrière et avant des animaux avant et 48 heures après 10 traitements quotidiens d'électrochoc ou traitements factices. Après le sacrifice, on a déterminé par autoradiographie qu'il y avait fixation aux récepteurs de la dopamine.
Résultats
Même si les sujets n'ont pas utilisé davantage leurs pattes avant au cours du test du cylindre, l'électrochoc a amélioré considérablement la performance motrice des pattes arrière au cours du test de la poutre en queue de billard et a amélioré la fixation aux récepteurs D1 et D3 de la dopamine striatale sans avoir d'effet sur la fixation aux récepteurs D2.
Conclusion
Cette étude indique que le mécanisme d'action de l'électrochoc dans les cas de MP peut consister en partie à améliorer la fonction de la dopamine par la voie directe des noyaux gris centraux et pourrait appuyer une étude plus poussée et l'utilisation de l'électrochoc comme traitement d'appoint éventuel contre la MP.
Introduction
Parkinson's disease (PD) is a neurodegenerative movement disorder characterized by tremor, bradykinesia, rigidity and postural disturbances. The primary pathological features of PD are the loss of dopamine (DA)-producing cell bodies in the substantia nigra pars compacta of the midbrain, and the concomitant loss of striatal DA.1 Therapeutic options for PD attempt to either replace the lost DA tone, for example, by treating patients with L-DOPA (the biochemical precursor to DA) or DA agonists or both, or, in later stages of the disease, to inhibit the basal ganglia output structures that become overactive in PD via surgical intervention, as in the case of pallidotomy or deep brain stimulation (DBS). Most patients, however, experience negative side effects or a loss of efficacy after prolonged L-DOPA treatment (reviewed in Jankovic2), and many patients are poor candidates for brain surgery. Further, many PD patients also experience symptoms of major depression, the treatment of which is often rendered difficult by negative side effects or interactions with antiparkinsonian drugs in this elderly population.3 The development of adjunctive or alternative therapeutic options for PD is essential to improve the quality of life of patients with this disease.
Electroconvulsive therapy (ECT) is a widely used treatment for psychiatric disorders, and several recent meta-analyses have shown that it is the most effective antidepressant treatment available.4,5 Not only is ECT an effective treatment for depression, but it also appears to have positive effects on the motor symptoms of PD patients, regardless of whether they have depression. Over 200 PD patients treated with ECT have been reported in the literature,6 with most showing dramatic improvement in their motor symptoms. Case reports, open trials and double-blind placebo-controlled studies have all shown that ECT treatment significantly improves a wide range of motor symptoms, including rigidity, bradykinesia, and “on–off” phenomenon, and that the improvements last from several weeks to months after the last treatment.7–9 Although additional well-designed clinical trials with large numbers of subjects are needed to determine the most appropriate parameters to achieve optimal impact on the motor symptoms of PD, this treatment holds great promise as a potential adjunct treatment.
In animals, repeated treatment with electroconvulsive shock (ECS) has been shown to have specific effects in limbic brain regions such as the frontal cortex and hippocampus. One of the most consistent effects of repeated ECS on the normal rodent limbic system is an enhancement of serotonin (5-HT) neurotransmission, as evidenced by increased 5-HT-mediated behaviours,10,11 increased interstitial 5-HT metabolites12 and upregulation of the 5-HT2 receptor.10,13 Taken together with increases in neurotrophic factors14,15 and cell growth,16,17 the enhancement of 5-HT neurotransmission after repeated ECS is part of a cascade of cellular events thought to underlie the mechanism of action of ECT in mood disorders.18
DA receptors belong to the superfamily of G-protein coupled receptors, having 7 transmembrane domains. Five different subtypes of DA receptors have been cloned (D1–D5), and these are broadly categorized into either D1-like receptors (D1 and D5) or D2-like receptors (D2, D3, D4) (reviewed in Jackson and Westlind-Danielsson),19 classically distinguished by their ability or inability to activate the enzyme adenyl cyclase, respectively.20 In the striatum, D1 and D2 receptors are further distinguished by their localization in the direct or indirect output pathways of the basal ganglia;21 thus, the different receptor subtypes play a critical role in PD. Current pharmacological interventions for PD are aimed at enhancing the activity of the direct pathway and reducing the activity in the indirect pathway, ultimately leading to enhanced motor output.
Previous studies have shown that, in experimental models of brain injury, both seizures22,23 and the application of DA-releasing agents such as amphetamine24,25 can enhance the recovery of motor function. We suggest that seizures may also have positive effects in a rodent model of PD, and more specifically, that the effects of repeated ECS treatment on the DA system of the parkinsonian striatum may be similar to those observed on the 5-HT system of the hippocampus. In this study, we hypothesized that repeated ECS treatment in unilateral 6-hydroxydopamine (6-OHDA)-lesioned rats would improve motor function and increase binding to DA receptors of the direct pathway of the basal ganglia.
Methods
Subjects
Adult male Sprague-Dawley rats (bred at the University of British Columbia animal facility from Charles River Canada [Montréal, Que.] stock), weighing 250 g at the start of the experiment were housed on a 12:12 light:dark schedule (with lights off at 12:00 pm), at constant temperature and humidity (21°C, 55%). The animals had access to food and water ad libitum and were housed in pairs. A total of 40 animals were used in the experiment: 20 were used for D1 receptor binding (n = 10 sham and 10 ECS) and 20 were used for D2 and D3 receptor binding. Behavioural data were pooled from the entire group of animals. All procedures were approved by the University of British Columbia Committee on Animal Care.
6-OHDA lesioning
The animals were allowed to habituate and were handled for at least 3 days before receiving a right unilateral 6-OHDA-induced lesion of the DA nigrostriatal pathway following our previously published procedures.26 Desipramine hydrochloride (25 mg/kg given intraperitoneally [i.p]; Sigma-Aldrich Canada, Oakville, Ont.) was administered 30–60 minutes before 6-OHDA infusion to protect noradrenergic terminals. Animals were anesthetized with isoflurane in O2 (4% for induction, 1% for maintenance), given atropine sulfate (0.05 mg/kg subcutaneous [s.c.]), and placed into a stereotaxic frame (David Kopf Instruments, Tujunga, Calif.). With the skull flat between lambda and Bregma, a 2% solution of 6-OHDA hydrobromide (8 mg in 4 mL 0.05% ascorbic acid in saline; Sigma) was infused at 2 sites along the medial forebrain bundle (site 1: anterior-posterior [AP] 2.8 mm, medio-lateral [ML] 1.8 mm, dorso-ventral [DV] 8.0 [all from Bregma]; site 2: AP 4.7 mm [Bregma], ML 1.5 mm [midline], DV 7.9 mm [hole]) according to Paxinos and Watson.27 The infusion rate was 1 mL per minute, and the cannula was left in place an additional 4 minutes to allow diffusion of the 6-OHDA solution. After surgery, the animals received subcutaneous saline, antibiotics (Duplocillin 0.1 mL/kg given intramuscularly [i.m]), and analgesia (Anafen 2 mg/kg s.c.), and were kept warm in an incubator until fully recovered from anesthesia. The animals were allowed to recover for at least 2 weeks after surgery before being treated with ECS.
Forelimb use asymmetry test (cylinder test)
The animals were evaluated in the forelimb use asymmetry test (cylinder test)28,29 at 3 time points: before lesion, after lesion and 48 hours after ECS or sham treatment. At each time point, the animals were placed in a plexiglas cylinder (20-cm diameter × 30-cm high) elevated on a glass plate for a 3-minute period on 2 consecutive days. Testing was done during the dark phase of the cycle and under red lighting. The trials were videotaped from below and scored at a later date by an investigator blind to the animals' treatment. Forelimb placements on the walls of the cylinder were categorized as left independent, right independent or simultaneous movements, and a forelimb use asymmetry score was calculated as:
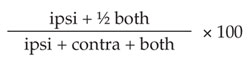 |
where ipsi and contra refer to the forelimbs ipsilateral and contralateral to the 6-OHDA-induced lesion, respectively.29 Animals had to make greater than 20 movements at any given time point for their data to be included in the analysis, and data from 3 animals were excluded based on this criterion.
Tapered beam (TB) walking test
Animals were also evaluated before lesion, after lesion and 48 hours after ECS or sham treatment on a TB walking test, adapted with slight modifications from Schallert and Woodlee29 and Zhao and colleagues.30 The test has been described in detail.26 Briefly, the animals are trained to walk across a 165-cm long beam that progressively narrows as they approach the goal (their darkened home cage). Beneath the surface of the beam is a ledge, which allows the animals to make slips with their feet off the main surface of the beam without falling off and therefore prevents postural compensation in lesioned animals. Taking a step with only 1 or 2 toes on the main surface of the beam (and the other 4 or 3 toes overhanging the ledge) is scored as a half footfault, while stepping with the entire foot on the ledge rather than on the main surface of the beam is scored as a full footfault. In between trials, the animals remained in their home cage with the lights in the room turned off for 1 minute for reinforcement. Normal animals make very few errors (footfaults), and those only occur on the narrowest section of the beam.
On a testing day, the animals were brought to the behavioural room at the start of the dark cycle and are allowed to habituate for 15–30 minutes. Before testing, each animal was allowed one “refresher” trial, which is not videotaped. One TB test is made up of 5 consecutive trials, and each trial is videotaped from the rear to allow a clear observation of the hindlimbs and scored at a later date by an investigator blind to the animal's condition. For each hindlimb, the number of steps taken and the number of full and half footfaults in each of the 3 sections (wide, medium and narrow portions of the beam) in the 5 trials is determined and summed to obtain a single composite score per time point. Because the scores represent mainly large or small proportions (no. of errors per step), transforming the data via an arcsine transformation (Eq. 2) normalizes the data.31 Unilaterally 6-OHDA-lesioned rats primarily make mistakes on the narrow section of the beam, so the TB test scores consisted of the arcsine transformed data from the narrow section of the beam at each time point. Animals who had TB scores > 40 (the typical score in the hindlimb contralateral to a 6-OHDA lesion) with either hindlimb before lesioning, indicating that they had not completely learned the task, were not included in the analysis (n = 5).
where X = the number of errors and n = the number of steps.
Electroconvulsive shock treatment
Animals were assigned randomly to the ECS or sham treatment groups and were treated daily for 10 days between 8 and 11 am. Atropine sulfate (0.2 mg/kg s.c.) was administered, followed 30 minutes later by ketamine hydrochloride (80 mg/kg i.p.). After induction of ketamine anesthesia, animals were given either sham treatment (electrodes placed, but no current administered), or bilateral ECS (80–99 mA, 5–9.9 s, 70 pulse/s, 0.5 ms pulse width) via earclip electrodes coated with electroconductive gel using a small animal ECS machine (Model 57800, Ugo Basile, Italy). In our early pilot studies, we decided to administer ECS under anesthesia to more closely model the human situation. Ketamine was chosen as our anesthetic for all future studies based on its ease of use, its relevance to the clinic (ketamine is used in patients who are allergic to barbiturates or who have high seizure thresholds32) and because our pilot data showed that 2 of the most commonly reported effects of ECS in rats, upregulation of cortical 5-HT2 receptor binding,10,13 and increased piriform cortex brain-derived neurotrophic factor (BDNF) mRNA14,33 both also occurred when ECS was given under ketamine anesthesia (Strome and others, unpublished data, 2006). All animals received the same initial current dose, based on our previous experience with ECS in rats under ketamine anesthesia, and current doses during subsequent treatments were modified based on the nature of the previous seizure. All ECS-treated animals experienced seizures of 13–19 seconds in duration. All animals in this study consistently showed tonic hind limb extension. One sham-treated animal from each of the D1 and D2/D3 binding groups was lost to ketamine anesthesia.
After the posttreatment behavioural testing was performed (48 h after the last ECS or sham treatment), animals were decapitated and the brains were removed and quickly frozen in isopentane cooled with dry ice and stored at -80°C until sectioning. This time point was chosen for the sacrifice based on our pilot studies, which suggested there may be residual effects of ketamine anesthesia or ECS treatment (or both) on motor function on these behavioural tasks 24 hours after the last treatment but that these effects had resolved by 48 hours after the last treatment. Twenty micron coronal sections were cut at –18°C on a cryostat (Leica) and thaw-mounted onto glass microscope slides (Superfrost Plus, Fisher Scientific, Ont.). The slides were stored at -80°C until the receptor binding assays were performed.
Vesicular monoamine transporter-2 binding
For verification of the extent of lesion, coronal sections through the striatum were incubated with [11C](±)dihydrotetrabenazine, a marker for DA terminals, which binds to the vesicular monoamine transporter-2.34 The details of the autoradiographic technique have been described in detail elsewhere.26
D1 receptor binding
The slides were warmed up to room temperature and were washed for 15 minutes in Tris-HCl buffer. Incubation was in 2 nM [3H]SCH 23390 (Perkin Elmer, Que.; specific activity 81 Ci/mmol) plus 30 nM ritanserin (to block 5-HT2 receptors; Sigma), in the same buffer at 20°C for 45 minutes. Nonspecific binding was determined by incubating adjacent slices with an additional 10 mM (+)-butaclamol (Sigma). At the end of the incubation, the slides were washed for two 3-minute washes in fresh buffer at 4°C, dipped briefly in cold distilled water, and allowed to dry on the bench top overnight. After postfixation in paraformaldehyde vapour under vacuum in a dessicator for 24 hours,35 the slides were apposed to pre-erased tritium-sensitive phosphor screens (Fuji Medical Systems Inc., Stamford, Conn.) in standard film cassettes with [3H] microscales (Amersham, UK) for 3 days. On the third day, the screens were removed from the cassettes and scanned in a Cyclone phosphor imager (Perkin Elmer, Que.) at 600 dpi resolution.
D2 receptor binding
The slides were warmed up to room temperature and pre-washed for 15 minutes in Tris-HCl buffer (50 mM Tris-HCl, 120 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, room temperature, pH 7.4, all from Sigma). The slides were incubated for 45 minute in 3 nM [11C]raclopride (specific activity 959 or 1975 Ci/mmol at the start of incubation) in the same buffer at room temperature. Nonspecific binding was determined by incubating adjacent slices with an additional 10 mM (+)-butaclamol. At the end of the incubation, the slides were washed for 3 × 1 minutes in fresh buffer at 4°C, dipped briefly in cold distilled water and allowed to dry in a fumehood for 20 minute. They were then apposed to Multisensitive storage phosphor screens (Perkin-Elmer) along with 11C standards prepared as previously described.36 The screens were scanned as described above after 2 hours of exposure.
D3 receptor binding
D3 receptor binding was performed with R-(+)-7-hydroxy-[3H]di-n-propyl-2-aminotetralin ([3H]7-OH-DPAT) as described by Levesque and colleagues37 with minor modifications. The slides were warmed to room temperature and were prewashed for three 5-minute washings in an N-(2-hydroxyethyl)-piperazine-N'-2-ethanesulfonic acid (HEPES) buffer (50 mM HEPES, 1 mM EDTA, 0.1% bovine serum albumen, 120 mM NaCl, all from Sigma). Incubation was in 1 nM [3H]7-OH-DPAT (Amersham, specific activity 115 Ci/mmol) in the same buffer at room temperature for 90 minutes. Nonspecific binding was determined by incubating adjacent slices with an additional 10 mM (+)-butaclamol. At the end of the incubation, the slides were washed for 3, 1-minute washes in fresh buffer at 4°C, dipped briefly in cold distilled water, and allowed to dry on the bench top overnight. Postfixation, exposure and plate scanning were identical to that described for D1 binding, except exposure time was 7 days.
Densitometry
Optical density analysis was performed with the inherent software on the phosphor imager (Optiquant v4.00, Perkin-Elmer). DA receptor binding was measured in the ventral striatum (nucleus accumbens shell) and in the dorsal striatum (approximately + 1.70 mm from Bregma according to Paxinos and Watson.27 D1 receptor binding was also measured in cortical regions at the same level (fronto-parietal cortex and cingulate cortex). Small regions of interest (ROIs) were placed bilaterally in at least 4 total binding and 2 adjacent nonspecific binding sections for each animal in each region. The optical density data were converted to nCi/mg tissue using a standard curve derived from the [3H] or [11C] microscales. For each animal, nonspecific binding was subtracted from total binding to get a measure of specific radiotracer binding.
Statistical analysis
Repeated-measures analysis of variance (ANOVA; treatment × time) was used for the analysis of the behavioural data because multiple measurements were made in the same animals. The effects of ECS treatment on DA receptor binding were evaluated with 2-way (treatment × hemisphere) ANOVA. Post-hoc testing of significant main effects was performed with Tukey's honest significant difference test for unequal n. All statistical analyses were performed with the software program StatSoft Statistica '98 v5.1 (Tulsa, Okla.).
Results
Cylinder test
Figure 1 shows asymmetry scores from the cylinder test before lesioning and before and 48 hours after repeated ECS or sham treatment. Prior to lesioning, all animals showed symmetric use of their forelimbs in exploring the walls of the cylinder, as indicated by asymmetry scores of approximately 50%. Two-way repeated-measures ANOVA comparing the pre-and posttreatment scores indicated that there were no significant main effects of treatment or time and no significant treatment × time interaction effect, indicating no significant effect of ECS treatment on forelimb use asymmetry.
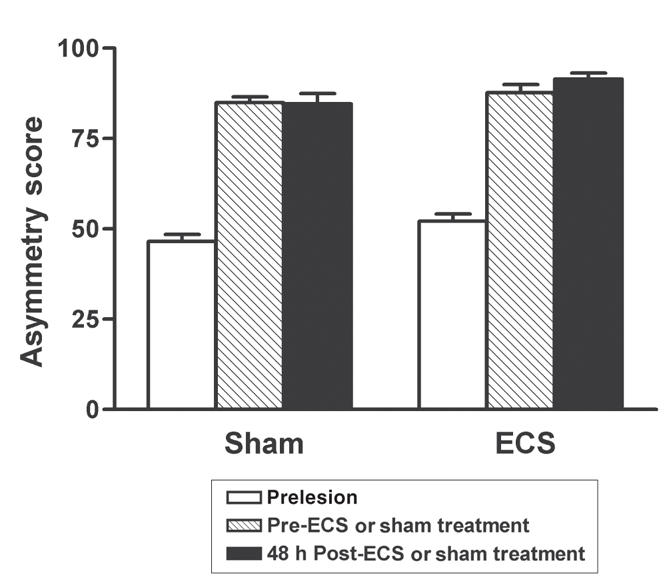
Fig. 1: Forelimb use asymmetry test scores before 6-OHDA lesioning, and before and after repeated electroconvulsive therapy (ECS) or sham treatment. There was no significant effect of ECS treatment. Values are mean ± SEM, n = 17 animals per group. SEM = standard error of the mean.
Tapered beam walking test
Prior to lesioning, the animals in both groups made equal numbers of errors with their left and right hindlimbs on the narrow section of the TB test (2-way ANOVA, p > 0.32; mean [standard deviation {SD}] TB score for the group = 24.27 [SD 8.34]). Figure 2 (white bars) shows data for the hindlimb contralateral to the lesion as well as the effects of ECS or sham treatment on TB test scores. As previously reported,26 severely unilaterally lesioned animals mainly make mistakes in the narrow section of the beam with their hindlimb contralateral to the lesion, so only these data are shown. Two-way repeated-measures ANOVA comparing the pre-and posttreatment scores indicates that there was a significant interaction effect between treatment and time (F1,30 = 5.97, p < 0.02), and visual inspection of the data indicates that the ECS-treated group had lower scores after treatment than the sham-treated group (Fig. 2). There was no effect of repeated ECS or sham treatment on the TB scores of the hindlimb ipsilateral to the lesion (data not shown).

Fig. 2: Tapered beam (TB) test scores for the hindlimb contralateral to the lesion in electroconvulsive therapy (ECT)- and sham-treated rats before 6-OHDA lesioning and before and after repeated ECS or sham treatment. There is a significant treatment × time interaction (*p < 0.02), with ECS-treated rats showing lower scores after treatment, compared with sham-treated controls. Values are mean ± SEM, n = 16 animals per group. SEM = standard error of the mean.
Vesicular monoamine transporter-2 binding
All animals showed > 90% depletion of vesicular monoamine transporter-2 binding in the lesioned, compared with intact dorsal striatum (mean ± SEM = 94.09% [SD 1.90%], data not shown).
D1 receptor binding
There was a significant effect of treatment on D1 binding in the dorsal striatum (2-way ANOVA: F1,34 = 5.20, p < 0.03; Fig. 3), with post-hoc testing indicating that D1 receptor binding was significantly increased after ECS treatment (p < 0.04). There was also a significant effect of treatment on D1 binding in the ventral striatum (F1,32 = 5.75, p < 0.03; Fig. 3) and, again, post-hoc testing indicated that D1 receptor binding was significantly increased after ECS treatment (p < 0.03). There were no significant main effects of hemisphere in these analyses, nor were there significant treatment × hemisphere interactions, indicating that ECS treatment increased D1 binding, regardless of the 6-OHDA lesion. There were no effects of either ECS treatment or 6-OHDA lesion on D1 binding in the fronto-parietal or cingulate cortices (data not shown).
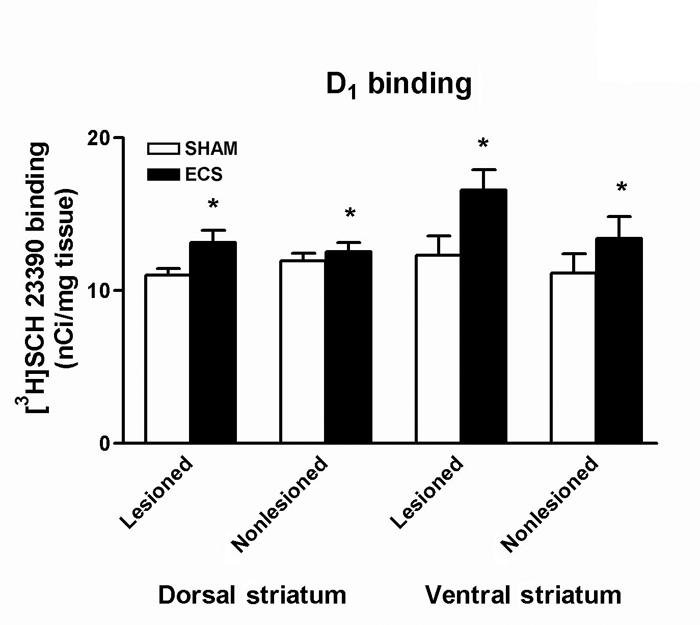
Fig. 3: Densitometric of autoradiographs representing [3H]SCH 23390 binding in the striatum. D1 binding was significantly increased after repeated electroconvulsive therapy (ECS) treatment in both the dorsal and ventral striatum (*p < 0.04). Values are mean ± SEM, n = 9–10 animals per group. SEM = standard error of the mean.
D2 receptor binding
There were no significant main effects of treatment, nor were there significant treatment × hemisphere interactions, indicating that ECS treatment had no effect on D2 receptor binding in either the dorsal or ventral striatum of either hemisphere. There was, however, a significant effect of hemisphere on D2 binding in the dorsal striatum (F1,34 = 47.45, p < 0.001; Fig. 4), with post hoc testing indicating that D2 receptor binding was significantly increased in the lesioned hemisphere (p < 0.001).
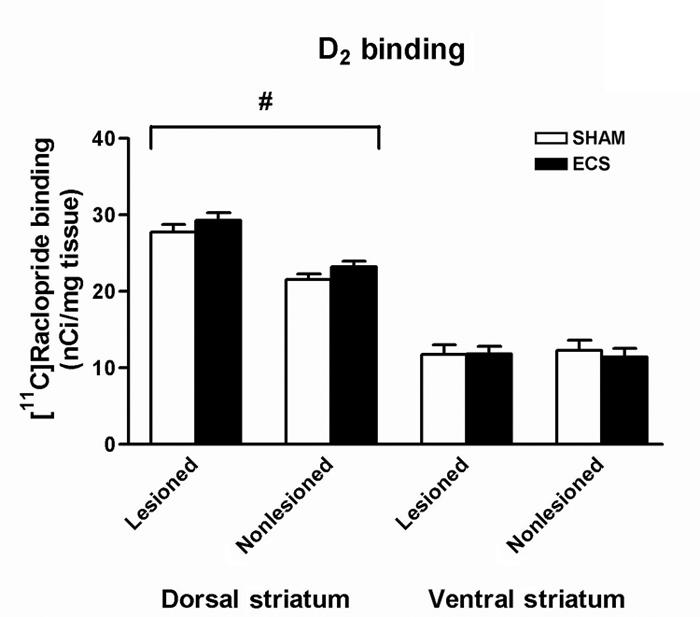
Fig. 4: Densitometric measurement of autoradiographs representing [11C]raclopride binding in the striatum. There was no effect of repeated ECS treatment on D2 binding, but it was significantly increased in the lesioned dorsal striatum (#p < 0.001). Values are mean ± SEM, n = 8–10 animals per group. SEM = standard error of the mean.
D3 receptor binding
There was a significant effect of treatment on D3 binding in the dorsal striatum (2-way ANOVA: F1,34 = 4.55, p < 0.05; Fig. 5), with post-hoc testing indicating that D3 receptor binding was significantly increased after ECS treatment (p < 0.05). There was also a significant effect of treatment on D3 binding in the ventral striatum (F1,34 = 7.62, p < 0.01; Fig. 5), and again, post-hoc testing indicates that D3 receptor binding was significantly increased after ECS treatment (p < 0.01). There was also a significant effect of hemisphere on D3 binding in the ventral striatum (F1,34 = 4.55, p < 0.002; Fig. 5), with post-hoc testing indicating that D3 receptor binding was significantly decreased in the lesioned hemisphere (p < 0.002). No significant treatment × hemisphere interactions were found.
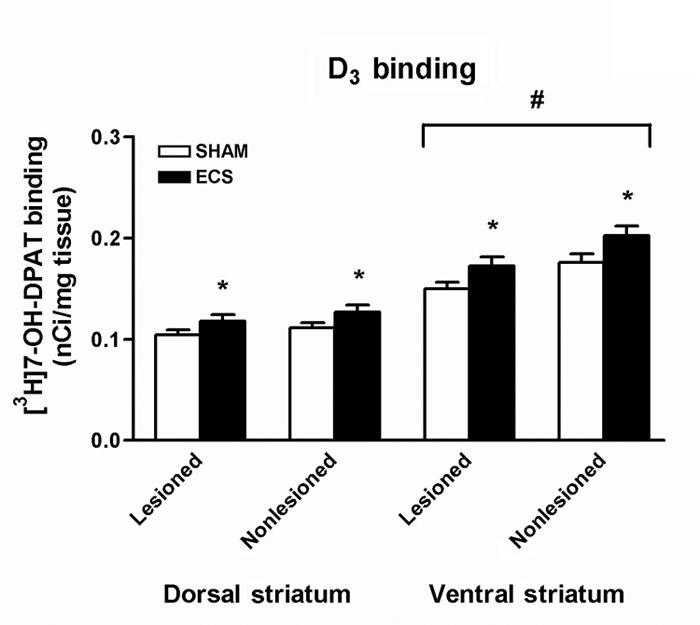
Fig. 5: Densitometric measurement of autoradiographs representing [3H]7-OH-DPAT binding in the striatum. D3 receptor binding was significantly increased in both the dorsal and ventral striatum after repeated electroconvulsive therapy (ECS) treatment (*p < 0.05), and significantly decreased in the lesioned ventral striatum (#p < 0.002). Values are mean ± SEM, n = 8–10 animals per group.
Discussion
The primary observation of the effects of ECT on PD patients is a fairly immediate and long-lasting improvement in their motor symptoms.7–9 Very few studies, however, have examined motor behaviour after ECS treatment in rodents. Those reports looked at drug-induced behaviours,13,38,39 with only one report on unilateral 6-OHDA-lesioned rats,40 and all of the studies showed significant increases in DA-mediated behaviours after repeated ECS treatment. We hypothesized that nonpharmacological motor behaviour of unilateral 6-OHDA-lesioned rats would also improve after a course of ECS treatment. Our results indicate no significant effect of repeated ECS treatment on forelimb use asymmetry in the cylinder test, but a significant improvement in hindlimb motor performance on the TB test.
Considering the severity of the unilateral lesion (> 90% depletion of striatal DA terminals), the absence of an effect of ECS on the use of the forelimb contralateral to the lesion in the cylinder test was not surprising. After unilateral 6-OHDA lesioning, the animal very rapidly (within days) learned to use its unimpaired forelimb almost exclusively for many tasks, including exploration, and this preferential use of the unimpaired forelimb has been shown to persist for a long time after severe unilateral 6-OHDA lesion.41,42 This preference for using the forelimb ipsilateral to the lesion makes it less likely for the animal to resume the use of the impaired forelimb, even when therapeutic interventions occur, especially in the case of mild or short-term therapeutic effects. Some recovery of impaired forelimb function in unilateral 6-OHDA-lesioned rats has been shown to occur after very specific interventions, such as deep brain stimulation (DBS),43 lentivector delivery of glial cell line-derived neurotrophic factor (GDNF) to the striatum and substantia nigra (SN),44 or through forced use of the impaired forelimb,45 likely as a result of increased striatal GDNF.46 Similarly, in our laboratory, we have observed recovery of function in the impaired forelimb on the cylinder test in unilateral 6-OHDA-lesioned rats after implantation of L-DOPA producing cells, retinal pigment epithelial (RPE) cells. Interestingly, this improvement only reached statistical significance 2–3 months after implantation, suggesting that a certain amount of time was necessary for the animals to relearn the use of the impaired forelimb.47 The literature therefore suggests that only interventions that bypass the striatal DA deficit, and act to inhibit the overactive basal ganglia output structures, provide strong DAergic trophic support to the SN and striatum, or provide long-term replacement of striatal DA appear to be able to improve forelimb use asymmetry on the cylinder test, and one course of ECS treatment may not be sufficient to accomplish this.
We did, however, see a significant improvement in TB test scores after repeated ECS treatment. The TB test measures different aspects of locomotion, and antiparkinsonian interventions should lead to a lack of performance errors, as opposed to an increase in use as in the cylinder test. Because the rat never has the choice to “ignore” its impaired hindlimb, it is likely that the degree of asymmetry between hindlimbs in unilateral 6-OHDA-lesioned rats is less than for specialized movements of the forelimbs. Thus, a smaller contralateral improvement may be more likely to improve scores on this test. We have shown the relation between performance on the task and the integrity of the striatal DA system26 and have found that unilaterally lesioned rats implanted with RPE cells show greater improvements on the TB test than on the cylinder test.47 In addition, in a genetic mouse model of PD, L-DOPA treatment significantly improved performance on an adaptation of the test for mice.48 The task is also widely used in models of stroke, where it has been shown to be sensitive to ischemic brain injury.30 In short, the TB test is a simple and valid test of gross and DA-dependent motor function, and our observation of significantly improved scores on this test after repeated ECS treatment in parkinsonian rats supports the hypothesis that enhanced DA function is part of the mechanism of action of ECT in PD.
In this study, we have also shown that repeated ECS treatment in unilateral 6-OHDA-lesioned rats increases binding to specific DA receptor subtypes in both the dorsal and ventral striatum. Binding to D1 and D3 receptors was increased in both the lesioned and nonlesioned hemispheres after repeated ECS treatment (although the increase in D1 binding was much smaller in the unlesioned versus lesioned striatum), whereas D2 receptor binding was unchanged by ECS treatment. There were also specific effects of the 6-OHDA lesion on D2 and D3 receptor binding, with D2 binding increased in the lesioned dorsal striatum and D3 binding decreased in the lesioned ventral striatum. These data on the effects of the lesion are in concordance with previous reports, and validate our lesion model: D2 receptor upregulation has been widely reported early after DA depletion in rodents,49,50 nonhuman primates51 and PD patients,52 whereas the 6-OHDA-induced decrease in D3 binding in the ventral striatum is also widely recognized.53–55
While the changes in D1 and D3 receptor binding occurred in both the lesioned and intact hemispheres, improved performance on the TB test was observed only in the hindlimb contralateral to the lesion. The bilateral changes in DA receptor binding are likely due to the fact that the ECS was applied bilaterally. On the TB test however, even normal animals make some mistakes with one or both hindlimbs on the narrowest section of the beam. This leads to a floor effect because bilateral scores on this task rarely approach zero and may explain why we saw no change in the performance of the ipsilateral hindlimb.
Our results on the effects of repeated ECS treatment on DA receptor binding in 6-OHDA-lesioned rats are consistent with the previous literature in normal rodents. Using both homogenate and autoradiographic receptor binding techniques, upregulation of D1 receptors is a common finding in the normal striatum after a course of ECS,56–58 whereas D2 receptors are typically unchanged in the dorsal striatum,59–61 and D2 and D3 receptors have been reported to be upregulated in the ventral striatum.58,62 Also consistent with the literature,58,63 we found no change in D1 receptor binding in the fronto-parietal or cingulate cortices after either ECS or 6-OHDA treatment. Our observations, then, of increased striatal D1 and D3 binding, without concomitant changes in D2 binding after repeated ECS treatment in 6-OHDA-lesioned rats, are in agreement with the literature on the effects of repeated ECS on these receptors in normal animals.
The D3 receptor is most abundant in the Islands of Calleja and ventral striatum (nucleus accumbens shell) and is expressed at very low levels in the dorsal striatum and the rest of the rat brain under normal circumstances.37,64 The striatal expression of the D3 receptor can, however, be upregulated by specific interventions, including long-term antidepressant treatment62 and long-term treatment with L-DOPA.55,65 In unilateral 6-OHDA-lesioned rats, chronic pulsatile L-DOPA leads to behavioural sensitization (the rodent homologue of L-DOPA-induced dyskinesia) and a dramatic increase in the expression of the D3 receptor in the lesioned dorsal striatum.55,65
It appears that 3 conditions must be met to increase the dorsal striatal expression of the D3 receptor in the rat brain after chronic pulsatile L-DOPA treatment: 1) severe depletion of striatal DA, 2) activation of the D1 receptor and 3) elevated BDNF levels.55,65,66 These 3 conditions may also be met in our model. Our animals were severely unilaterally lesioned, and repeated ECS treatment increased binding to striatal D1 receptors, which could lead to greater D1 activation. In addition, there is strong evidence that BDNF activity is increased after repeated ECS treatment, not only in the hippocampus,14,67,68 but also in the striatum of normal15 rats, and we have preliminary evidence (unpublished) of increased striatal BDNF expression after repeated ECS treatment in 6-OHDA-lesioned rats. These 3 events (D1 activation, enhanced BDNF activity, and D3 upregulation) may represent compensatory and regulatory changes that underlie both L-DOPA induced behavioural sensitization and improved motor performance after repeated ECS treatment.
The increased D3 receptor binding that we observed in the dorsal striatum after repeated ECS treatment was less pronounced than in L-DOPA-induced behavioural sensitization (12%–15% in this study vs. 130%–680% in studies by van Kampen and Stoessl55 and Bordet and colleagues65) an observation that may be a result of differences in the duration of treatment in these 2 models (twice daily treatment with L-DOPA for several weeks in behavioural sensitization v. daily treatment with ECS for 10 days in our study). Indeed, the fact that the D3 receptor was only moderately upregulated by repeated ECS treatment compared with behavioural sensitization to L-DOPA treatment may, in fact, be advantageous. In the rat, the induction of the D3 receptor in the dorsal striatum after long-term L-DOPA treatment occurs mainly in dynorphin/substance P (and D1) expressing neurons of the direct striatonigral pathway.69 Co-expression of D1 and D3 receptors has been shown to have both opposite and synergistic effects on cAMP and on gene expression.70 When the 2 receptor subtypes are in synergy, the relative abundance of the receptors may dictate the functional outcome. For example, synergy between D1 and D3 occurs in L-DOPA-induced behavioural sensitization, but in this case, the D3 receptor is expressed at high levels, leading to overactivity of the direct pathway of the basal ganglia and the development of sensitization.65,69 If D3 receptors are expressed in a low-to-moderate ratio compared with the D1 receptor, however, the synergy between them may enhance the activity in the direct pathway without causing excessive stimulation. The nature of the synergistic relation between D1 and D3 therefore may depend on the relative expression of the 2 receptor subtypes, with moderate levels of D3 being advantageous and high levels being detrimental. If ECT treatment enhances D3 expression only moderately, then activity in the direct pathway will be enhanced but not excessive.
In conclusion, this is the first study to show improvements in nonpharmacological motor performance and increased binding to specific DA receptor subtypes after repeated ECS-treatment in 6-OHDA-lesioned rats. In this preliminary report, we did not measure the timecourse or persistence of the behavioural and receptor binding changes, but these issues will be addressed in future studies. ECT is a noninvasive and safe treatment and is widely used to treat psychiatric disorders. The clinical evidence suggests that, in some patients, ECT can provide almost immediate and fairly long-lasting relief of the motor symptoms of PD. ECT should be considered in PD patients with poor response to medication; before surgical intervention in patients with severe motor symptoms; and, given its potential neurotrophic effects, perhaps in patients early in the course of the disease. While the mechanism of action is not completely known, and further research is necessary, this study increases our understanding of the effects of ECT on the brain, and provides support for the continued use and study of ECT as a potential adjunct treatment for PD.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research and a Senior Graduate Scholarship from the Michael Smith Foundation for Health Research to Dr. Strome. Thanks to Dr. I. Cepeda for his help in the development of the behavioural tests.
Footnotes
Contributors: All authors designed the study. Dr. Strome acquired the data, and all authors analyzed it. Dr. Strome wrote the article, and all authors revised it. All authors gave final approval for the article to be published.
Competing interests: None declared.
Correspondence to: Dr. Elissa Strome, University of British Columbia, Pacific Parkinson's Research Centre, Purdy Pavilion M36, Vancouver BC. V6T 2B5; fax 604 822-7866; strome@interchange.ubc.ca
References
- 1.Bernheimer H, Birkmayer W, Hornykiewicz O, et al. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci 1973;20:415-55. [DOI] [PubMed]
- 2.Jankovic J. Motor fluctuations and dyskinesias in Parkinson's disease: clinical manifestations. Mov Disord 2005;20(Suppl 11):S11-6. [DOI] [PubMed]
- 3.Leentjens AF. Depression in Parkinson's disease: conceptual issues and clinical challenges. J Geriatr Psychiatry Neurol 2004;17:120-6. [DOI] [PubMed]
- 4.Kho KH, van Vreeswijk MF, Simpson S, et al. A meta-analysis of electroconvulsive therapy efficacy in depression. J ECT 2003;19: 139-47. [DOI] [PubMed]
- 5.Pagnin D, de Quriroz V, Pini S, et al. Efficacy of ECT in depression: a meta-analytic review. J ECT 2004;20:13-20. [DOI] [PubMed]
- 6.Kennedy R, Mittal D, O'Jile J. Electroconvulsive therapy in movement disorders: an update. J Neuropsychiatry Clin Neurosci 2003;15:407-21. [DOI] [PubMed]
- 7.Fall PA, Ekman R, Granerus AK, et al. ECT in Parkinson's disease. Changes in motor symptoms, monoamine metabolites and neuropeptides. J Neural Transm Park Dis Dement Sect 1995;10:129-40. [DOI] [PubMed]
- 8.Zervas IM, Fink M. ECT for refractory Parkinson's disease. Convuls Ther 1991;7:222-3. [PubMed]
- 9.Andersen K, Balldin J, Gottfries CG, et al. A double-blind evaluation of electroconvulsive therapy in Parkinson's disease with “on-off” phenomena. Acta Neurol Scand 1987;76:191-9. [DOI] [PubMed]
- 10.Goodwin GM, Green AR, Johnson P. 5-HT2 receptor characteristics in frontal cortex and 5-HT2 receptor-mediated head-twitch behaviour following antidepressant treatment to mice. Br J Pharmacol 1984;83:235-42. [DOI] [PMC free article] [PubMed]
- 11.Green AR, Heal DJ, Johnson P, et al. Antidepressant treatments: effects in rodents on dose-response curves of 5-hydroxytryptamine-and dopamine-mediated behaviours and 5-HT2 receptor number in frontal cortex. Br J Pharmacol 1983;80:377-85. [DOI] [PMC free article] [PubMed]
- 12.Yoshida K, Higuchi H, Kamata M, et al. Single and repeated electroconvulsive shocks activate dopaminergic and 5-hydroxytryptaminergic neurotransmission in the frontal cortex of rats. Prog Neuropsychopharmacol Biol Psychiatry 1998;22:435-44. [DOI] [PubMed]
- 13.Green AR, Johnson P, Nimgaonkar V. Increased 5-HT2 receptor number in brain as a probable explanation for the enhanced 5-hydroxytryptamine-mediated behaviour following repeated electroconvulsive shock administration to rats. Br J Pharmacol 1983;80:173-7. [DOI] [PMC free article] [PubMed]
- 14.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci 1995;15:7539-47. [DOI] [PMC free article] [PubMed]
- 15.Angelucci F, Aloe L, Jimenez-Vasquez P, et al. Electroconvulsive stimuli alter the regional concentrations of nerve growth factor, brain-derived neurotrophic factor, and glial cell line-derived neurotrophic factor in adult rat brain. J ECT 2002;18:138-43. [DOI] [PubMed]
- 16.Malberg JE, Eisch AJ, Nestler EJ, et al. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 2000;20:9104-10. [DOI] [PMC free article] [PubMed]
- 17.Vaidya VA, Siuciak JA, Du F, et al. Hippocampal mossy fiber sprouting induced by chronic electroconvulsive seizures. Neuroscience 1999;89:157-66. [DOI] [PubMed]
- 18.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry 1997;54:597-606. [DOI] [PubMed]
- 19.Jackson DM, Westlind-Danielsson A. Dopamine receptors: molecular biology, biochemistry and behavioural aspects. Pharmacol Ther 1994;64:291-369. [DOI] [PubMed]
- 20.Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature 1979;277:93-6. [DOI] [PubMed]
- 21.Gerfen CR, Engber TM, Mahan LC, et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 1990;250:1429-32. [DOI] [PubMed]
- 22.Hernandez TD, Schallert T. Seizures and recovery from experimental brain damage. Exp Neurol 1988;102:318-24. [DOI] [PubMed]
- 23.Feeney DM, Bailey BY, Boyeson MG, et al. The effect of seizures on recovery of function following cortical contusion in the rat. Brain Inj 1987;1:27-32. [DOI] [PubMed]
- 24.Schmanke T, Barth TM. Amphetamine and task-specific practice augment recovery of vibrissae-evoked forelimb placing after unilateral sensorimotor cortical injury in the rat. J Neurotrauma 1997; 14:459-68. [DOI] [PubMed]
- 25.Schmanke TD, Avery RA, Barth TM. The effects of amphetamine on recovery of function after cortical damage in the rat depend on the behavioral requirements of the task. J Neurotrauma 1996;13:293-307. [DOI] [PubMed]
- 26.Strome EM, Cepeda IL, Sossi V, et al. Evaluation of the integrity of the dopamine system in a rodent model of Parkinson's disease: small animal PET compared to behavioral assessment and autoradiography. Mol Imaging Biol 2006;8:292-9. [DOI] [PubMed]
- 27.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd ed. San Diego: Academic Press; 1997.
- 28.Schallert T, Fleming SM, Leasure JL, et al. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 2000;39:777-87. [DOI] [PubMed]
- 29.Schallert T, Woodlee MT. Orienting and placing. In: Whishaw IQ, Kolb B, editors. The behavior of the laboratory rat: a handbook with tests. Oxford: Oxford University Press; 2005. p. 129-140.
- 30.Zhao CS, Puurunen K, Schallert T, et al. Behavioral and histological effects of chronic antipsychotic and antidepressant drug treatment in aged rats with focal ischemic brain injury. Behav Brain Res 2005;158:211-20. [DOI] [PubMed]
- 31.Zar JH. Biostatistical analysis. 4th ed. Upper Saddle River (NJ): Prentice-Hall; 1999.
- 32.Rasmussen KG, Jarvis MR, Zorumski CF. Ketamine anesthesia in electroconvulsive therapy. Convuls Ther 1996;12:217-23. [PubMed]
- 33.Zetterstrom TSC, Pei Q, Grahame-Smith DG. Repeated electroconvulsive shock extends the duration of enhanced gene expression for BDNF in rat brain compared with a single administration. Brain Res Mol Brain Res 1998;57:106-10. [DOI] [PubMed]
- 34.DaSilva JN, Carey JE, Sherman PS, et al. Characterization of [11C]tetrabenazine as an in vivo radioligand for the vesicular monoamine transporter. Nucl Med Biol 1994;21:151-6. [DOI] [PubMed]
- 35.Liberatore GT, Wong JYF, Krenus D, et al. Tissue fixation prevents contamination of tritium-sensitive storage phosphor imaging plates. Biotechniques 1999;26:432-4. [DOI] [PubMed]
- 36.Strome EM, Jivan S, Doudet DJ. Quantitative in vitro phosphor imaging using [3H] and [18F] radioligands: the effects of chronic desipramine treatment on serotonin 5-HT2 receptors. J Neurosci Methods 2005;141:143-54. [DOI] [PubMed]
- 37.Levesque D, Diaz J, Pilon C, et al. Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-n-propyl-2-aminotetralin. Proc Natl Acad Sci U S A 1992;89:8155-9. [DOI] [PMC free article] [PubMed]
- 38.Wielosz M. Increased sensitivity to dopaminergic agonists after repeated electroconvulsive shock (ECS) in rats. Neuropharmacology 1981;20:941-5. [DOI] [PubMed]
- 39.Smith SE, Sharp T. Evidence that the enhancement of dopamine function by repeated electroconvulsive shock requires concomitant activation of D1-like and D2-like dopamine receptors. Psychopharmacology (Berl) 1997;133:77-84. [DOI] [PubMed]
- 40.Green AR, Heal DJ, Grahame-Smith DG. Further observations on the effect of repeated electroconvulsive shock on the behavioural responses of rats produced by increases in the functional ability of brain 5-hydroxytryptamine and dopamine. Psychopharmacology (Berl) 1977;52:195-200. [DOI] [PubMed]
- 41.Dunnett SB, Whishaw IQ, Rogers DC, et al. Dopamine-rich grafts ameliorate whole body motor asymmetry and sensory neglect but not independent limb use in rats with 6-hydroxydopamine lesions. Brain Res 1987;415:63-78. [DOI] [PubMed]
- 42.Evenden JL, Robbins TW. Effects of unilateral 6-hydroxydopamine lesions of the caudate-putamen on skilled forepaw use in the rat. Behav Brain Res 1984;14:61-8. [DOI] [PubMed]
- 43.Shi L-H, Woodward DJ, Luo F, et al. High-frequency stimulation of the subthalamic nucleus reverses limb-use asymmetry in rats with unilateral 6-hydroxydopamine lesions. Brain Res 2004;1013:98-106. [DOI] [PubMed]
- 44.Dowd E, Monville C, Torres EM, et al. Lentivector-mediated delivery of GDNF protects complex motor functions relevant to human Parkinsonism in a rat lesion model. Eur J Neurosci 2005;22:2587-95. [DOI] [PubMed]
- 45.Tillerson JL, Cohen AD, Philhower J, et al. Forced limb-use effects on the behavioral and neurochemical effects of 6-hydroxydopamine. J Neurosci 2001;21:4427-35. [DOI] [PMC free article] [PubMed]
- 46.Cohen AD, Tillerson JL, Smith AD, et al. Neuroprotective effects of prior limb use in 6-hydroxydopamine-treated rats: possible role of GDNF. J Neurochem 2003;85:299-305. [DOI] [PubMed]
- 47.Cepeda IL, Flores J, Cornfeldt ML, et al. Intrastriatal implantation of microcarrier attached human retinal pigment epithelial cells ameliorates motor deficits in a rat model of Parkinson's disease (PD). Soc Neurosci Abstr 2004;790-18.
- 48.Hwang DY, Fleming SM, Ardayfio P, et al. 3,4-dihydroxyphenylalanine reverses the motor deficits in Pitx3-deficient aphakia mice: behavioral characterization of a novel genetic model of Parkinson's disease. J Neurosci 2005;25:2132-7. [DOI] [PMC free article] [PubMed]
- 49.Narang N, Wamsley JK. Time dependent changes in DA uptake sites, D1 and D2 receptor binding and mRNA after 6-OHDA lesions of the medial forebrain bundle in the rat brain. J Chem Neuroanat 1995;9:41-53. [DOI] [PubMed]
- 50.Graham WC, Crossman AR, Woodruff GN. Autoradiographic studies in animal models of hemi-parkinsonism reveal dopamine D2 but not D1 receptor supersensitivity. I. 6-OHDA lesions of ascending mesencephalic dopaminergic pathways in the rat. Brain Res 1990;514:93-102. [DOI] [PubMed]
- 51.Doudet DJ, Holden JE, Jivan S, et al. In vivo PET studies of the dopamine D2 receptors in rhesus monkeys with long-term MPTP-induced parkinsonism. Synapse 2000;38:105-13. [DOI] [PubMed]
- 52.Kaasinen V, Ruottinen HM, Nagren K, et al. Upregulation of putaminal dopamine D2 receptors in early Parkinson's disease: a comparative PET study with [11C] raclopride and [11C]N-methylspiperone. J Nucl Med 2000;41:65-70. [PubMed]
- 53.Stanwood GD, Artymyshyn RP, Kung MP, et al. Quantitative autoradiographic mapping of rat brain dopamine D3 binding with [125I]7-OH-PIPAT: evidence for the presence of D3 receptors on dopaminergic and nondopaminergic cell bodies and terminals. J Pharmacol Exp Ther 2000;295:1223-31. [PubMed]
- 54.Levesque D, Martres MP, Diaz J, et al. A paradoxical regulation of the dopamine D3 receptor expression suggests the involvement of an anterograde factor from dopamine neurons. Proc Natl Acad Sci U S A 1995;92:1719-23. [DOI] [PMC free article] [PubMed]
- 55.van Kampen JM, Stoessl AJ. Effects of oligonucleotide antisense to dopamine D3 receptor mRNA in a rodent model of behavioural sensitization to levodopa. Neuroscience 2003;116:307-14. [DOI] [PubMed]
- 56.Fochtmann LJ, Cruciani R, Aiso M, et al. Chronic electroconvulsive shock increases D-1 receptor binding in rat substantia nigra. Eur J Pharmacol 1989;167:305-6. [DOI] [PubMed]
- 57.Nowak G, Zak J. Repeated electroconvulsive shock (ECS) enhances striatal D-1 dopamine receptor turnover in rats. Eur J Pharmacol 1989;167:307-8.2687010
- 58.Barkai AI, Durkin M, Nelson HD. Localized alterations of dopamine receptor binding in rat brain by repeated electroconvulsive shock: an autoradiographic study. Brain Res 1990;529:208-13. [DOI] [PubMed]
- 59.Bergstrom DA, Kellar KJ. Effect of electroconvulsive shock on monoaminergic receptor binding sites in rat brain. Nature 1979;278: 464-6. [DOI] [PubMed]
- 60.Reches A, Wagner HR, Barkai AI, et al. Electroconvulsive treatment and haloperidol: effects on pre-and postsynaptic dopamine receptors in rat brain. Psychopharmacology (Berl) 1984;83:155-8. [DOI] [PubMed]
- 61.Martin KF, Phillips I, Cheetham SC, et al. Dopamine D2 receptors: a potential pharmacological target for nomifensine and tranylcypromine but not other antidepressant treatments. Pharmacol Biochem Behav 1995;51:565-9. [DOI] [PubMed]
- 62.Lammers CH, Diaz J, Schwartz JC, et al. Selective increase of dopamine D3 receptor gene expression as a common effect of chronic antidepressant treatments. Mol Psychiatry 2000;5:378-88. [DOI] [PubMed]
- 63.Araki T, Tanji H, Kato H, et al. Sequential changes of dopaminergic receptors in the rat brain after 6-hydroxydopamine lesions of the medial forebrain bundle. J Neurol Sci 1998;160:121-7. [DOI] [PubMed]
- 64.Diaz J, Levesque D, Lammers CH, et al. Phenotypical characterization of neurons expressing the dopamine D3 receptor in the rat brain. Neuroscience 1995;65:731-45. [DOI] [PubMed]
- 65.Bordet R, Ridray S, Carboni S, et al. Induction of dopamine D3 receptor expression as a mechanism of behavioral sensitization to levodopa. Proc Natl Acad Sci U S A 1997;94:3363-7. [DOI] [PMC free article] [PubMed]
- 66.Guillin O, Diaz J, Carrol P, et al. BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature 2001;411:86-9. [DOI] [PubMed]
- 67.Altar CA, Whitehead RE, Chen R, et al. Effects of electroconvulsive seizures and antidepressant drugs on brain-derived neurotrophic factor protein in rat brain. Biol Psychiatry 2003;54:703-9. [DOI] [PubMed]
- 68.Jacobsen JP, Mork A. The effect of escitalopram, desipramine, electroconvulsive seizures and lithium on brain-derived neurotrophic factor mRNA and protein expression in the rat brain and the correlation to 5-HT and 5-HIAA levels. Brain Res 2004;1024:183-92. [DOI] [PubMed]
- 69.Bordet R, Ridray S, Schwartz JC, et al. Involvement of the direct striatonigral pathway in levodopa-induced sensitization in 6-hydroxydopamine-lesioned rats. Eur J Neurosci 2000;12:2117-23. [DOI] [PubMed]
- 70.Ridray S, Griffon N, Mignon V, et al. Coexpression of dopamine D1 and D3 receptors in islands of Calleja and shell of nucleus accumbens of the rat: opposite and synergistic functional interactions. Eur J Neurosci 1998;10:1676-86. [DOI] [PubMed]


