Abstract
Objective
Low monoamine oxidase (MAO) activity and the neurotransmitter dopamine are 2 important factors in the development of alcohol dependence. MAO is an important enzyme associated with the metabolism of biogenic amines. Therefore, the present study investigates whether the association between the dopamine D2 receptor (DRD2) gene and alcoholism is affected by different polymorphisms of the MAO type A (MAOA) gene.
Methods
A total of 427 Han Chinese men in Taiwan (201 control subjects and 226 with alcoholism) were recruited for the study. Of the subjects with alcoholism, 108 had pure alcohol dependence (ALC) and 118 had both alcohol dependence and anxiety, depression or both (ANX/DEP ALC). All subjects were assessed with the Chinese Version of the Modified Schedule of Affective Disorders and Schizophrenia-Lifetime. Alcohol dependence, anxiety and major depressive disorders were diagnosed according to Diagnostic and Statistical Manual of Mental Disorders, fourth edition criteria.
Conclusion
The genetic variant of the DRD2 gene was only associated with the ANX/DEP ALC phenotype, and the genetic variant of the MAOA gene was associated with pure ALC. Subjects carrying the MAOA 3-repeat allele and genotype A1/A1 of the DRD2 were 3.48 times (95% confidence interval = 1.47–8.25) more likely to be ANX/DEP ALC than the subjects carrying the MAOA 3-repeat allele and DRD2 A2/A2 genotype. The MAOA gene may modify the association between the DRD2 gene and ANX/DEP ALC phenotype.
Medical subject headings: alcoholism, anxiety, depression, dopamine D2 receptor, monoamine oxidase
Abstract
Objectif
La faible activité de la monoamine oxydase (MAO) et la dopamine neurotransmettrice sont deux facteurs importants de l'apparition de l'accoutumance à l'alcool. La MAO est une enzyme importante associée au métabolisme des amines biogéniques. La présente étude vise donc à déterminer si des polymorphismes différents du gène de la MAO type A (MAOA) ont un effet sur le lien entre le gène du récepteur D2 de la dopamine (RDD2) et l'alcoolisme.
Méthodes
On a recruté au total, pour l'étude, 427 hommes chinois Han à Taiwan (201 sujets témoins et 226 atteints d'alcoolisme). Parmi les sujets alcooliques, 108 avaient une dépendance pure à l'alcool (ALC) et 118 avaient à la fois une dépendance à l'alcool et de l'anxiété, de la dépression, ou les deux (ANX/DEP ALC). On a évalué tous les sujets au moyen de la version chinoise du Guide modifié pour le diagnostic des troubles affectifs et de la schizophrénie. On a diagnostiqué la dépendance à l'alcool, l'anxiété et les troubles dépressifs majeurs selon les critères du Manuel diagnostique et statistique des troubles mentaux, quatrième édition.
Conclusion
On a établi un lien entre la variante génétique du gène du RDD2 et le phénotype ANX/DEP ALC seulement et entre la variante génétique du gène MAOA et la dépendance pure à l'alcool. Les sujets porteurs de l'allèle triple MAOA 3 et du génotype A1/A1 du gène du RDD2 étaient 3,48 fois (intervalle de confiance à 95 % = 1,47–8,25) plus susceptibles d'avoir le trouble ANX/DEP ALC que les sujets porteurs de l'allèle triple MAOA et du génotype A2/A2 du RDD2. Le gène MAOA a peu modifié le lien entre le gène du RDD2 et le phénotype ANX/DEP ALC.
Introduction
Family, twin and adoption studies suggest that heredity plays an important role in alcohol dependence and drinking behaviour and, therefore, that there are genetic risk factors for alcoholism.1–3 Alcohol dependence is a complex disorder that is probably regulated by several genes.4 Although several candidate genes have been studied, the results of these studies are controversial;5–12 the gene-to-gene interaction approach might be more revealing than the single-gene approach in the study of alcoholism.
Monoamine oxidase (MAO) is an important enzyme associated with the metabolism of biogenic amines and neurotransmitters, including dopamine as well as 5-hydroxytryptamine (5-HT) and norepinephrine.13,14 Cloninger15 proposed that the catecholamine neurotransmitters including dopamine, 5-HT and norepinephrine are related to some personality traits that might put an individual at increased risk for drinking behaviour and developing alcohol dependence. For example, low MAO activity might also be a risk factor for impulsive behaviour, personality disorder and alcoholism.16–19 Therefore, MAO activity may play a critical role in the regulation of catecholamines and in the pathogenesis of psychiatric disorders.13 A functional 30-base pair (bp) repeat polymorphism in the promoter region of the MAOA gene may alter transcriptional efficiency; the allele with 3 copies of the repeat sequence was transcribed about 2 times less efficiently than the allele with 4 copies of the repeat motif.20,21 The MAOA gene is considered to be a candidate gene of alcohol dependence susceptibility, because alcohol dependence is sensitive to allelic variation in the MAOA gene.5–7,22,23 Samochowiec and colleagues7 and Schmidt and colleagues23 reported that a low-activity 3-repeat allele of the MAOA promoter polymorphism is associated with antisocial alcoholism among German men, and Contini and colleagues5 confirmed that the 3-repeat allele increased susceptibility to alcohol dependence and antisocial behaviours in a Brazilian sample. However, the existence of an association between the MAOA gene and alcoholism with or without antisocial behaviour is not consistently reported. Further, several studies have found no association between alcoholism and the MAOA gene.8,24–26
In animal studies, alcohol can stimulate dopaminergic neurons in the ventral tegmental area,27,28 and the density of dopamine D2 receptors in the limbic system is lower in alcohol-preferring rats than in nonpreferring rats.29,30 Likewise, the number of striatal dopamine D2 receptors is less in alcohol-preferring humans than in healthy control subjects.31 Moreover, brain imaging studies of healthy volunteers have shown that individuals with an A1 allele of the DRD2 gene have a reduced number of dopamine D2 receptors.32,33 The A1 polymorphism of the DRD2 TaqI A loci has been considered as a risk factor for alcohol dependence,9,10,34,35 but the association between alcoholism and the DRD2 gene remains equivocal.11,12,34,36–38 The confounding effects of MAOA and DRD2 genes on alcohol dependence might be partly due to different definitions of control groups, ethnically or racially mixed study populations and phenotypic heterogeneity of alcoholism.39–41
To overcome these possible confounding effects and to reduce the probability of type I and type II errors, we recruited 2 different subtypes of patients dependent on alcohol: a group with pure alcohol dependence and no other comorbid diagnosis (pure ALC) and a group with alcohol dependence and comorbid anxiety, depression or both (ANX/DEP ALC). Our intent was to reduce the phenotypic heterogeneity of the overall sample. We also recruited unrelated healthy control subjects to evaluate the association between MAOA and DRD2 and alcohol dependence in the Han Chinese population of Taiwan.
We hypothesized that, if both the MAOA and DRD2 genes are associated with alcohol dependence, this would be revealed by an association study comparing subjects with pure ALC to well-matched control subjects. However, alcoholism is usually comorbid with anxiety or depression or both, and the mood disturbance might increase drinking behaviour. Thus, we hypothesized that the MAOA and DRD2 genes might increase susceptibility to ANX/DEP ALC. Dopamine is oxidatively deaminated by MAOA and, in a rat model, 90% of the metabolism is via deamination by MAOA in the corpus striatum to form 3,4-dihydroxyphenyl-acetaldehyde (DOPAL).38,42 These observations led us to hypothesize that the MAOA and DRD2 genes might interact to increase susceptibility to alcohol dependence and/or its subgroup. We therefore tested whether the relation between the DRD2 gene and alcoholism is affected by different polymorphisms of the MAOA gene.
Methods
Subjects and clinical assessments
The protocol of this study was approved by the Institutional Review Board for the Protection of Human Subjects at Tri-Service General Hospital (TSGH), a medical teaching hospital affiliated with the National Defence Medical Center in Taipei, Taiwan. Written informed consent was obtained from all participants after a full explanation of the study procedures.
To minimize the effects of ethnic differences on gene frequencies, all 427 subjects were recruited from the Han Chinese population in Taiwan; all participants were unrelated and were matched for ethnicity and geographic origin. Alcohol dependence was diagnosed and classified into 2 groups: pure ALC (108 participants) and ANX/DEP ALC (118 participants). A total of 201 participants were healthy control subjects. Subjects with pure ALC had a past or current history of alcohol dependence but no history of other mental disorders, including personality, anxiety, depressive, or affective disorders or illegal drug use disorders. Those with ANX/DEP ALC had a past or current history of major depression or anxiety disorder or both, as well as a diagnosis of alcohol dependence, but no history of other mental disorders or illegal drug use disorders. The healthy control subjects had no past or present major or minor mental illnesses (including affective disorder, schizophrenia, anxiety disorder, personality disorder or substance use disorders) and no family history of alcohol dependence or heavy alcohol consumption in first-degree relatives.
Subjects with alcohol dependence were recruited from the psychiatric clinical population, and control volunteers were recruited from the community. Each subject was interviewed by an attending psychiatrist, who made an initial evaluation, and then by a well-trained research psychologist, who identified the clinical subtype of alcohol dependence and selected the control subjects. All diagnoses of alcohol dependence, anxiety, major depressive disorders and other mental disorders were made according to Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) criteria.43 A well-trained research psychologist interviewed participants, using the Chinese Version of the Modified Schedule of Affective Disorders and Schizophrenia-LifeTime (SADS-L),44,45 in order to meet a DSM-IV diagnosis and to exclude other mental disorders (antisocial personality disorder and drug use disorder) in control subjects and in individuals with pure ALC. The interrater reliability kappa values of the Chinese Version of SADS-L were good-to-excellent for major depression (0.79), bipolar disorder (0.71), anxiety disorder (0.86), schizophrenia (0.95), alcohol abuse and dependence (1.00), and substance abuse and dependence (0.82).38
Blood samples and DNA extraction
With the informed consent of each participant, 20 mL of whole blood was drawn from the peripheral vein with vacutainer tubes containing 15% (K3) Ethylendiaminetetraacetic acid (EDTA) solution (Becton Dickinson vacutainer systems). Genomic DNA was extracted from the leukocytes using standard methods.
Genotyping of MAOA and DRD2 genes
The 30-bp repeat polymorphism of the MAOA-uVNTR gene (variable number of tandem repeats located upstream of the promoter region) was investigated with a modification of the polymerase chain reaction (PCR) method described by Zhu and colleagues.46 The EcoRV polymorphisms in exon 14 of the MAOA gene were detected with the modified PCR-RFLP (restriction fragment length polymorphism) method described by Hotamisligil and Breakefield.47 The MAOA EcoRV (-) polymorphism remained intact and was 703 bp long, whereas the MAOA EcoRV (+) polymorphism was cut into 2 DNA fragments of 340 bp and 363 bp by the EcoRV restriction enzymes.
TaqI “A” and TaqI “B” polymorphisms of the DRD2 gene were genotyped with the PCR-RFLP method. Cycling protocols, which were modified from those described by Castiglione and colleagues40 and Grandy and colleagues,48 were carried out on a Perkin Elmer 9700 thermal cycler (Boston, MA). The 310-bp A1 allele remained uncut, whereas the A2 allele was cut into 2 DNA fragments of 130 bp and 180 bp. The 459-bp TaqI B1 allele remained intact, and the TaqI B2 allele was cut into 2 DNA fragments of 267 bp and 192 bp.
Statistical analyses
The differences in the genotype and allele frequencies of the MAOA and DRD2 genes between the pure ALC and ANX/DEP ALC groups and the control groups were calculated with Pearson's chi-square (2-tailed), and Hardy–Weiberg equilibrium was assessed for each group. Fisher's exact test was substituted for the chi-square test when sample cell sizes were smaller than expected (< 5 subjects). One-way analysis of variance and Bonferroni post hoc test were employed to determine the difference of mean age among these subtypes. The Bonferroni post hoc test, Pearson's chi-square, Fisher's exact test and multiple logistic regression analyses were performed with SPSS (version 11.5, Taipei, Taiwan) for Windows. A p value of less than 0.05 was considered statistically significant. The frequency of the 2-repeat polymorphism of the MAOA gene was found in 0 subjects in the ANX/DEP ALC group, 1 subject in the pure ALC group and 4 subjects in the control group. Therefore, we did not include subjects with 2-repeat polymorphism of MAOA-uVNTR for data analysis.
Differences in haplotype frequencies, linkage disequilibrium coefficients (D), and standardized linkage disequilibrium coefficients (D′) between the TaqI A and TaqI B systems of the DRD2 gene were estimated with the Estimating haplotypes and Permutation and Model Free Analysis computer programs.49–51 Differences in haplotype frequency between study variables were estimated with Fisher's exact test when the cells were small. Moreover, the power analysis was performed with the use of G*Power computer software, and the effect size conventions were determined according to the method of Erdfelder and colleagues.52
Results
There were significant differences in mean age among these 3 study groups (F = 13.661; p < 0.001) and between the control and pure ALC groups (36.58 [standard deviation {SD} 9.57 yr v. 42.09 [SD 9.82] yr; p < 0.001) but not between the control and ANX/DEP ALC groups (36.58 [SD 9.57] yr v. 35.99 [SD10.51] yr; p = 0.611).
As shown in Table 1, the haplotype frequencies of A1B2 and A2B1 were less than 5%, and strong linkage disequilibrium between the TaqI A and TaqI B polymorphisms in the DRD2 gene (p < 0.001) was evident in each of the 3 study groups. Genotype distributions of TaqI A and TaqI B polymorphism of the DRD2 gene were in the Hardy–Weinberg equilibrium, both in the patients and in the control subjects (p > 0.1). There are significant differences in the haplotype frequencies of the DRD2 gene among the 3 study groups (p = 0.020). The frequency of the A1B1 haplotype was significantly higher in the ANX/DEP ALC group than in the control group (p = 0.006) but was not significantly different in the pure ALC group versus the normal control group (Table 1). There were no significant differences in the genotype frequency of MAOA-uVNTR (in the promoter region of the gene) and in EcoRV (in exon 14) polymorphisms among the 3 groups or the ANX/DEP ALC group versus the control group. However, the MAOA gene was significantly associated with pure ALC (p = 0.030 in MAOA-uVNTR and p = 0.039 in MAOA EcoRV, respectively; see Table 2).
Table 1
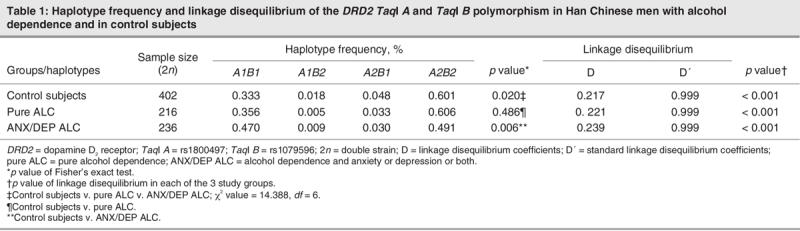
Table 2
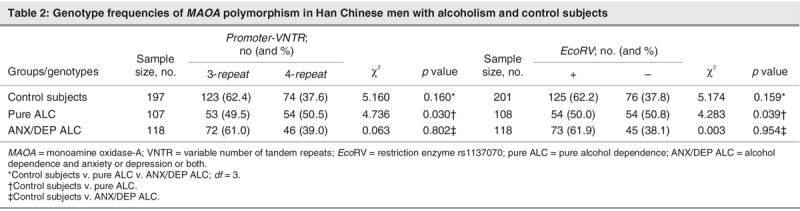
After stratifying the MAOA-uVNTR 3-repeat and MAOA-EchoRV(+) genotypes, the only significant difference in DRD2 haplotype was between the ANX/DEP ALC group and the control group. When the MAOA-uVNTR 4-repeat and MAOA-EchoRV(–) genotypes were stratified, respectively, there were no significant differences in the DRD2 haplotype between healthy control subjects and each of the other groups, respectively (Table 3).
Table 3
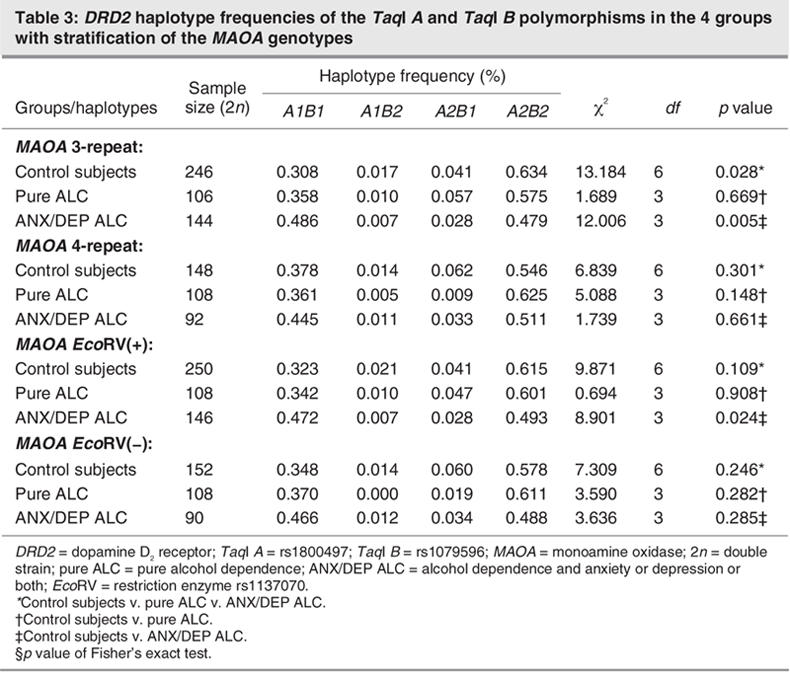
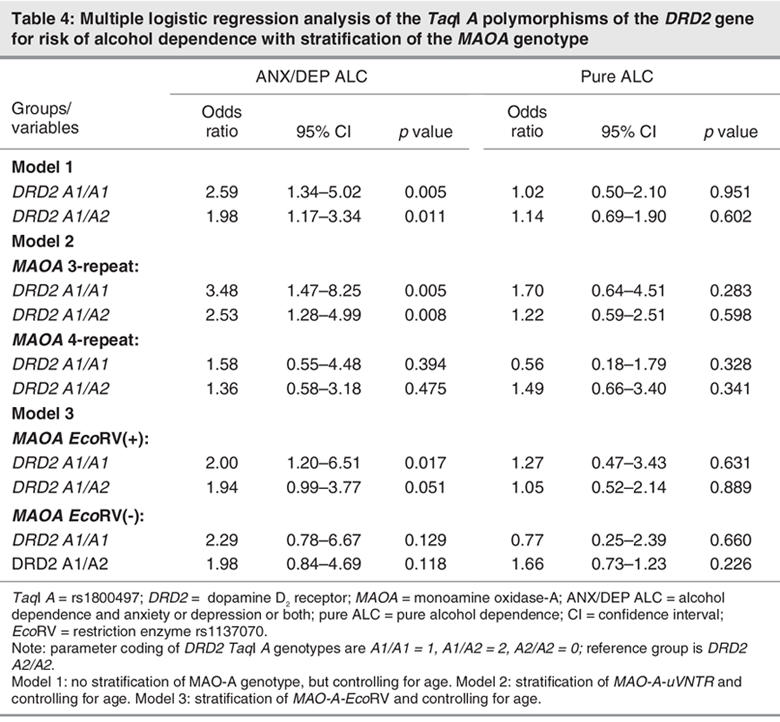
Logistic regression analysis of the DRD2 TaqI A polymorphism as a risk factor for alcohol dependence and correction for age showed that the DRD2 A1/A1 and A1/A2 genotypes were associated with higher risk for ANX/DEP ALC than the DRD2 A2/A2 genotype (approximately 1.98–2.59-fold), but the DRD2 A1/A1 genotype was not significantly associated with pure ALC (model 1, Table 4). After stratifying MAOA genotypes and correcting for age, the association of the DRD2 A1/A1 and A1/A2 genotypes with ANX/DEP ALC persisted only after stratification by MAOA-uVNTR 3-repeat (odds ratio [OR] = 3.48, p = 0.005 for A1/A1 genotype; OR = 2.53, p = 0.008 for A1/A2 genotype) and MAOA-EchoRV(+) genotypes (OR = 2.80, p = 0.017 for EchoRV(+) genotype), but the association had not been found under stratification of the MAOA-uVNTR 4-repeat and MAOA-EchoRV(-) genotypes, respectively (Table 4). These results led us to suggest that the DRD2 gene plays an important role in the ANX/DEP ALC group and that the MAOA gene might modify the association between the DRD2 gene and alcoholism.
Table 4
The study power was around 0.41–0.56 to detect a small effect and 0.99 to detect a medium and large effect in the haplotype and/or genotype frequencies. With a power of 0.93, we detected an effect size of 0.15 for detecting a significance difference in haplotype distributions. After stratification and logistic regression analysis, this study had a power of 0.22–0.35 to detect a small effect, 0.98–0.99 to detect a medium effect and 0.99 to detect a large effect. In the present power analysis, effect size conventions were determined according to the method of Erdfelder and colleagues,52 as follows: small effect size = 0.10, medium effect size = 0.30, large effect size = 0.50 (α = 0.05).
Discussion
We found that the DRD2 gene is associated with ANX/DEP ALC and that the frequency of the A1/B1 haplotype is higher in subjects with alcohol dependence. These results are consistent with our previous studies in mixed-sex subjects,38 but results differ from those of the small study of 20 subjects with alcoholism and mood disorder.53 We hypothesized that, if the DRD2 gene is associated with alcohol dependence, this would be revealed by an association study of subjects with pure ALC and well-matched control subjects, but these results do not support this association. The foregoing observations led us to suggest that the DRD2 gene is associated with alcoholism only in patients with anxiety and depression among the Han Chinese population of Taiwan. Thus, it may be easier to detect an association between the DRD2 gene and alcohol dependence in specific population subgroups.
The association of MAOA EcoRV polymorphism with alcoholism has been reported in the Han Chinese population6 but remains controversial.8,24 We found that the polymorphism of promoter and EcoRV in the MAOA gene are associated with pure ALC but not with other subgroups of alcohol dependence. The significant association between the MAOA gene and pure ALC is consistent with previous studies on alcoholism6,22 but contradicts certain other reports.8,24,25 Moreover, our finding of no MAOA gene association with ANX/DEP ALC is consistent with the some reports8,24 but not others.6,23
There are several possible reasons for these contradictory results. First, definition of the “normal control” group varies between studies. Some studies use a “super-control” (that is, the healthy control subjects had no past or present major or minor mental illnesses, including affective disorder, schizophrenia, anxiety disorder, personality disorder or substance use disorders), while others do not.10 In genetic association studies, use of suitable control subjects is very important.10,54,55 Several studies have shown that the MAOA and DRD2 genes are associated with several substance abuse or mood disorders.56–59 Previous studies suggested that the prevalence of the A1 allele is significantly higher in unscreened control subjects (not excluding people with alcoholism or nicotine addiction) than in assessed control subjects (with exclusion).10 Using unscreened individuals as control subjects may unwittingly include an excess of patients with A1 alleles and further attenuate the association between the DRD2 A1 allele and alcohol dependence.55,60 Thus, the control group should probably exclude subjects with substance use disorders, other major or minor mental disorders and/or a family history of mental disorders. In this study, all potential control subjects were screened by an attending psychiatrist and interviewed by a well-trained psychologist to reduce the confounding factors. If comorbid disorders were found, these participants were excluded. In the study by Lu and colleagues,24 patient subtype of alcohol dependence was not determined, even though alcohol dependence is a complex phenotype with a heterogeneous etiology.6,24 To establish a precise phenotype, Cloninger15 proposed a neurobiological learning model that subdivided alcoholism into 2 subtypes. People with type I alcoholism (late-onset) often have a high incidence of comorbidity with mood disorders; they show high harm-avoidance and low novelty-seeking behaviours. People with type II alcoholism (early-onset) often have antisocial personality traits and show low harm-avoidance and high novelty-seeking. Cloninger's classification was not confirmed by subsequent studies.61–64 The use of Cloninger's Tri-dimensional Personality Questionnaire (TPQ) lacks a cut-off point to distinguish the various subtypes of alcoholism, and the definition of personality traits may vary according to sociocultural differences.65 In recent studies, the DSM-IV criteria have been considered more reliable for clinical use and have also been used in clinical research.7,23,38 It is important to use a well-defined or quantitative phenotype in the association studies of candidate genes of complex disorders because it can lead to a dramatic increase in statistical power.66 Thus, we suggest that using the SADS-L for initial assessment and the DSM-IV diagnosis to further subgroup patients might reveal novel associations between candidate genes and specific subtypes of alcohol dependence. Another potential confounding factor in prior studies is that there are racial and ethnic differences in gene frequency. The frequencies of DRD2 and MAOA genes are known to vary among different racial or ethnic groups.10,12,39–41 The DRD2 TaqI A polymorphism of the A1 allele frequency in our control samples (35.1%) is similar to other Asian populations (35%–37%)53,67 but is much higher than in Caucasian samples (11%–20%).10 Finally, the MAOA promoter polymorphism, high-activity allele (4-repeat) has a prevalence of 40% in Asian populations24,68 but in Caucasian populations is as high as 60%–70%.7,23,25 These differences may be partially responsible for the divergent association results.
Several findings from our study suggest that MAOA genes modify the association between the DRD2 gene and alcoholism. The DRD2 gene was associated with ANX/DEP ALC before stratifying by MAOA genotype; after stratification, an association with the MAOA 3-repeat and MAOA EcoRV(+) genotypes was revealed, even though the DRD2 gene was not associated with ANX/DEP ALC in those with MAOA 4-repeats and EcoRV(-) genotypes (Table 3). After stratifying MAOA genotypes and correcting for age, multiple logistic regression analysis showed that the risk of alcohol dependence differed in people with different DRD2 TaqI A genotypes. The risk for ANX/DEP ACL was much higher in subjects with A1/A1 and A1/A2 genotypes (2.53–3.48 times) than in those with the A2/A2 genotype. That difference in risk was significant only after stratification into the MAOA-uVNTR 3-repeat genotype and into those with the EcoRV(+) genotype (Table 4). Thus, the MAOA genes appear to modulate the effect of the DRD2 gene in the ANX/DEP ALC group but not in the pure ALC group. A possible reason for this result is that dopaminergic tone might be lower in people with alcoholism who have mood disorders than in control subjects and others with different subtypes of alcohol dependence. The lower reinforcement of the dopamine-related reward system might be compensated for by the low-enzyme activity genotype of the MAOA gene. Our results seem to favour a hypothesis that MAOA genes modulate the relation between the DRD2 gene and ANX/DEP ALC, but additional studies are needed. Such studies should use large samples in different populations to determine whether DRD2 and MAOA genes are jointly involved in the development of alcohol dependence.
A potential weakness of our study is that only men were recruited. This is because men with alcoholism are about 10 times more frequent than women with alcoholism in the Han Chinese population in Taiwan,69 and the MAOA gene locus is located on the short arm of the X chromosome (Xp11.23).70 Therefore, the relation is easier to study in men than in women. Nevertheless, alcoholism is more frequent in women with depression and anxiety than in women without mood disorders.38 Thus a study is needed to investigate the relation between the MAOA gene and the DRD2 gene in women with alcoholism.
The ANX/DEP ALC subtype of alcohol dependence might play an important role in a candidate gene study of alcoholism. The present study suggests that the MAOA VNTR allelic variants may modify the effect of the DRD2 gene in subjects with ANX/DEP ALC. However, dopamine is not only degraded by MAO but is also subjected to O-methylation by catechol-O-methyltransferase (COMT) and aldehyde dehydrogenase (ALDH). Our results suggest that the effect of other genes on alcoholism, including gene variants of metabolic enzymes involved in the metabolism of dopamine (e.g., ALDH and COMT genes), should be further investigated.
Acknowledgments
This study was supported in part by National Science Council Grants NSC 92-2314-B-006-151, NSC93-2314-B-006-108, NSC94-2314-B-006-116, NSC94-2314-B-016-016 and NSC95-2314-B-016-019; the Department of Health Grants DOH 88-TD-1107 and DOH94-TD-D-113-040; Tri-Service General Hospital Grant TSGH-C94-76, and by National Cheng Kung University Project of Promoting Academic Excellence and Developing World Class Research Centers, Taiwan, Republic of China. Thanks to Mr. Cheng-Chang Huang, A-Lan Tang and Fu-Kuei Chang for their assistance in preparing this manuscript.
Footnotes
Contributors: Drs. Huang, Lin, Ko, and Lu, Ms. Chang and Ms. Wu designed the study. Drs. Huang, Wan, Wang and Lu, Ms. Chang and Ms. Wu aquired the data, which Drs. Huang, Lin, and Lu, and Ms. Wu analyzed. Drs. Huang and Lu wrote the article, and all authors revised it. All authors gave final approval for the article to be published.
Competing interests: None declared.
Correspondence to: Dr. Ru-Band Lu, Department of Psychiatry, College of Medicine and Hospital, National Cheng Kung University, No. 138, Sheng-Li Road, 70428, Tainan, Taiwan, ROC; fax 886-6-302-8012; rblu@mail.ncku.edu.tw
References
- 1.Bierut LJ, Dinwiddie SH, Begleiter H, et al. Familial transmission of substance dependence: alcohol, marijuana, cocaine, and habitual smoking: a report from the Collaborative Study on the Genetics of Alcoholism. Arch Gen Psychiatry 1998;55:982-8. [DOI] [PubMed]
- 2.Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Arch Gen Psychiatry 1981;38:861-8. [DOI] [PubMed]
- 3.Pickens RW, Svikis DS, McGue M, et al. Heterogeneity in the inheritance of alcoholism: a study of male and female twins. Arch Gen Psychiatry 1991;48:19-28. [DOI] [PubMed]
- 4.Peltonen L, McKusick VA. Genomics and medicine. Dissecting human disease in the postgenomic era. Science 2001;291:1224-9. [DOI] [PubMed]
- 5.Contini V, Marques FZ, Garcia CE, et al. MAOA-uVNTR polymorphism in a Brazilian sample: further support for the association with impulsive behaviors and alcohol dependence. Am J Med Genet B Neuropsychiatr Genet 2006;141:305-8. [DOI] [PubMed]
- 6.Hsu YP, Loh EW, Chen WJ, et al. Association of monoamine oxidase A alleles with alcoholism among male Chinese in Taiwan. Am J Psychiatry 1996;153:1209-11. [DOI] [PubMed]
- 7.Samochowiec J, Lesch KP, Rottmann M, et al. Association of a regulatory polymorphism in the promoter region of the monoamine oxidase A gene with antisocial alcoholism. Psychiatry Res 1999;86:67-72. [DOI] [PubMed]
- 8.Lu RB, Lin WW, Lee JF, et al. Neither antisocial personality disorder nor antisocial alcoholism association with MAOA gene among Han Chinese males in Taiwan. Alcohol Clin Exp Res 2003;27:889-93. [DOI] [PubMed]
- 9.Goldman D. Candidate genes in alcoholism. Clin Neurosci 1995;3:174-81. [PubMed]
- 10.Noble EP. D2dopamine receptor gene in psychiatric and neurologic disorders and its phenotypes. Am J Med Genet B Neuropsychiatr Genet 2003;116:103-25. [DOI] [PubMed]
- 11.Lu RB, Ko HC, Chang FM. No association between alcoholism and multiple polymorphisms at the dopamine D2receptor gene (DRD2) in three distinct Taiwanese populations. Biol Psychiatry 1996;39:419-29. [DOI] [PubMed]
- 12.Noble EP. The DRD2 gene in psychiatric and neurological disorders and its phenotypes. Pharmacogenomics 2000;1:309-33. [DOI] [PubMed]
- 13.Shih JC, Thompson RF. Monoamine oxidase in neuropsychiatry and behavior. Am J Hum Genet 1999;65:593-8. [DOI] [PMC free article] [PubMed]
- 14.Weyler W, Hsu YP, Breakefield XO. Biochemistry and genetics of monoamine oxidase. Pharmacol Ther 1990;47:391-417. [DOI] [PubMed]
- 15.Cloninger CR. Neurogenic adaptive mechanisms in alcoholism. Science 1987;236:410-6. [DOI] [PubMed]
- 16.Devor EJ, Cloninger CR, Hoffman PL, et al. Association of monoamine oxidase (MAO) activity with alcoholism and alcoholic subtypes. Am J Med Genet 1993;48:209-13. [DOI] [PubMed]
- 17.Eensoo D, Paaver M, Harro M, et al. Predicting drunk driving: contribution of alcohol use and related problems, traffic behaviour, personality and platelet monoamine oxidase (MAO) activity. Alcohol Alcohol 2005;40:140-6. [DOI] [PubMed]
- 18.Faraj BA, Davis DC, Camp VM, et al. Platelet monoamine oxidase activity in alcoholics, alcoholics with drug dependence, and cocaine addicts. Alcohol Clin Exp Res 1994;18:1114-20. [DOI] [PubMed]
- 19.Verkes RJ, Van der Mast RC, Kerkhof AJ, et al. Platelet serotonin, monoamine oxidase activity, and [3H]paroxetine binding related to impulsive suicide attempts and borderline personality disorder. Biol Psychiatry 1998;43:740-6. [DOI] [PubMed]
- 20.Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet 1998;103:273-9. [DOI] [PubMed]
- 21.Deckert J, Catalano M, Syagailo YV, et al. Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Hum Mol Genet 1999;8:621-4. [DOI] [PubMed]
- 22.Vanyukov MM, Moss HB, Yu LM, et al. Preliminary evidence for an association of a dinucleotide repeat polymorphism at the MAOA gene with early onset alcoholism/substance abuse. Am J Med Genet 1995;60:122-6. [DOI] [PubMed]
- 23.Schmidt LG, Sander T, Kuhn S, et al. Different allele distribution of a regulatory MAO-A gene promotor polymorphism in antisocial and anxious-depressive alcoholics. J Neural Transm 2000;107:681-9. [DOI] [PubMed]
- 24.Lu RB, Lee JF, Ko HC, et al. No association of the MAO-A gene with alcoholism among Han Chinese males in Taiwan. Prog Neuropsychopharmacol Biol Psychiatry 2002;26:457-61. [DOI] [PubMed]
- 25.Koller G, Bondy B, Preuss UW, et al. No association between a polymorphism in the promoter region of the MAOA gene with anti-social personality traits in alcoholics. Alcohol Alcohol 2003;38:31-4. [DOI] [PubMed]
- 26.Saito T, Lachman HM, Diaz L, et al. Analysis of monoamine oxidase A (MAOA) promoter polymorphism in Finnish male alcoholics. Psychiatry Res 2002;109:113-9. [DOI] [PubMed]
- 27.Brodie MS, Shefner SA, Dunmiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat. Brain Res 1990;508:65-9. [DOI] [PubMed]
- 28.Brodie MS. Increased ethanol excitation of dopaminergic neurons of the ventral tegmental area after chronic ethanol treatment. Alcohol Clin Exp Res 2002;26:1024-30. [DOI] [PubMed]
- 29.McBride WJ, Chernet E, Dyr W, et al. Densities of dopamine D2 receptors are reduced in CNS regions of alcohol-preferring P rats. Alcohol 1993;10:387-90. [DOI] [PubMed]
- 30.Stefanini E, Frau M, Garau MG, et al. Alcohol-preferring rats have fewer dopamine D2 receptors in the limbic system. Alcohol Alcohol 1992;27:127-30. [PubMed]
- 31.Hietala J, West C, Syvalahti E, et al. Striatal D2 dopamine receptor binding characteristics in vivo in patients with alcohol dependence. Psychopharmacology (Berl) 1994;116:285-90. [DOI] [PubMed]
- 32.Pohjalainen T, Rinne JO, Nagren K, et al. The A1 allele of the human D2dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol Psychiatry 1998;3:256-60. [DOI] [PubMed]
- 33.Jonsson EG, Nothen MM, Grunhage F, et al. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry 1999;4:290-6. [DOI] [PubMed]
- 34.Noble EP, Blum K, Ritchie T. Allele association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch Gen Psychiatry 1991;48:648-54. [DOI] [PubMed]
- 35.Reich T, Hinrichs A, Culverhouse R, et al. Psychiatric genetics '99 genetic studies of alcoholism and substance dependence. Am J Hum Genet 1999;65:599-605. [DOI] [PMC free article] [PubMed]
- 36.Blum K, Noble EP, Sheridam PJ. Allelic association of human dopamine D2 receptor gene in alcoholism. JAMA 1990;263:2055-60. [PubMed]
- 37.Bolos AM, Dean M, Lucas-Derse S, et al. Population and pedigree studies reveal a lack of association between the dopamine D2receptor gene and alcoholism. JAMA 1990;264:3156-60. [PubMed]
- 38.Huang SY, Lin WW, Ko HC, et al. Possible interaction of alcohol dehydrogenase and aldehyde dehydrogenase genes with the dopamine D2receptor gene in anxiety-depressive alcohol dependence. Alcohol Clin Exp Res 2004;28:374-84. [DOI] [PubMed]
- 39.Barr CL, Kidd KK. Population frequencies of the A1 allele at the dopamine D2 receptor locus. Biol Psychiatry 1993;34:204-9. [DOI] [PubMed]
- 40.Castiglione CM, Deinard AS, Speed WC, et al. Evolution of haplotypes at the DRD2 locus. Am J Hum Genet 1995;57:1445-56. [PMC free article] [PubMed]
- 41.Kidd KK, Morar B, Castiglione CM, et al. A global survey of haplotype frequencies and linkage disequilibrium at the DRD2 locus. Hum Genet 1998;103:211-27. [DOI] [PubMed]
- 42.Westerink BH, de Vries JB. On the origin of dopamine and its metabolite in predominantly noradrenergic innervated brain areas. Brain Res 1985;330:164-6. [DOI] [PubMed]
- 43.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: The Association; 1994.
- 44.Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry 1978;35: 837-44. [DOI] [PubMed]
- 45.Merikangas KR, Stevens DE, Fenton B, et al. Co-morbidity and familial aggregation of alcoholism and anxiety disorders. Psychol Med 1998;28:773-88. [DOI] [PubMed]
- 46.Zhu QS, Grimsby J, Chen K, et al. Promoter organization and activity of human monoamine oxidase (MAO) A and B genes. J Neurosci 1992;12:4437-46. [DOI] [PMC free article] [PubMed]
- 47.Hotamisligil GS, Breakefield XO. Human monoamine oxidase A gene determines levels of enzyme activity. Am J Hum Genet 1991; 49:383-92. [PMC free article] [PubMed]
- 48.Grandy DK, Zhang Y, Civelli O. PCR detection of the TaqA RFLP at the DRD2 locus. Hum Mol Genet 1993;2:2197. [DOI] [PubMed]
- 49.Xie X, Ott J. Testing linkage disequilibrium between a disease gene and marker loci. Am J Hum Genet 1993;53(Suppl):1107.8105690
- 50.Zhao JH, Curtis D, Sham PC. Model-free analysis and permutation tests for allelic associations. Hum Hered 2000;50:133-9. [DOI] [PubMed]
- 51.Zhao JH, Sham PC. Faster allelic association analysis using unrelated subjects. Hum Hered 2002;53:36-41. [DOI] [PubMed]
- 52.Erdfelder E, Faul F, Buchner A. G*POWER: a general power analysis program. Behav Res Methods Instrum Comput 1996;28:1-11.
- 53.Kono Y, Yoneda H, Sakai T, et al. Association between early-onset alcoholism and the dopamine D2 receptor gene. Am J Med Genet 1997;74:179-82. [DOI] [PubMed]
- 54.Buckland PR. Genetic association studies of alcoholism problems with the candidate gene approach. Alcohol Alcohol 2001;36:99-103. [DOI] [PubMed]
- 55.Lawford BR, Young RM, Rowell JA, et al. Association of the D2 dopamine receptor A1 allele with alcoholism: medical severity of alcoholism and type of controls. Biol Psychiatry 1997;41:386-93. [DOI] [PubMed]
- 56.Comings DE, Muhleman D, Ahn C, et al. The dopamine D2receptor gene: a genetic risk factor in substance abuse. Drug Alcohol Depend 1994;34:175-80. [DOI] [PubMed]
- 57.Comings DE, Ferry L, Bradshaw-Robinson S, et al. The dopamine D2 receptor (DRD2) gene: a genetic risk factor in smoking. Pharmacogenetics 1996;6:73-9. [DOI] [PubMed]
- 58.Massat I, Souery D, Del-Favero J, et al. Positive association of dopamine D2 receptor polymorphism with bipolar affective disorder in a European multicenter association study of affective disorders. Am J Med Genet 2002;114:177-85. [PubMed]
- 59.Jin Y, Chen D, Hu Y, et al. Association between monoamine oxidase gene polymorphisms and smoking behaviour in Chinese males. Int J Neuropsychopharmacol 2006;9:557-64. [DOI] [PubMed]
- 60.Noble EP. The D2dopamine receptor gene: a review of association studies in alcoholism and phenotypes. Alcohol 1998;16:33-45. [DOI] [PubMed]
- 61.Nixon SJ, Parsons OA. Application of the Tridimensional Personality Questionnaire to a population of alcoholics and other substance abusers. Alcohol Clin Exp Res 1990;14:513-7. [DOI] [PubMed]
- 62.Earleywine M, Finn PR, Peterson JB, et al. Factor structure and correlates of The Tridimensional Personality Questionnaire. J Stud Alcohol 1992;53:233-8. [DOI] [PubMed]
- 63.Howard MO, Kivlahan D, Walker RD. Cloninger's tridimensional theory of personality and psychopathology: applications to substance use disorders. J Stud Alcohol 1997;58:48-66. [DOI] [PubMed]
- 64.Mulder RT. Alcoholism and personality. Aust N Z J Psychiatry 2002;36:44-52. [DOI] [PubMed]
- 65.Svrakic DM, Przybeck TR, Cloninger CR. Further contribution to the conceptual validity of the unified biosocial model of personality: US and Yugoslav data. Compr Psychiatry 1991;32:195-209. [DOI] [PubMed]
- 66.Rice JP, Saccone NL, Rasmussen E. Definition of the phenotype. In: Rao DC, Province MA, editors. Genetic dissection of complex traits. San Diego: Academic Press; 2001. p. 70-4.
- 67.Chen WJ, Lu ML, Hsu YP, et al. Dopamine D2receptor gene and alcoholism among four aboriginal groups and Han in Taiwan. Am J Med Genet 1997;74:129-36. [PubMed]
- 68.Yu YW, Tsai SJ, Hong CJ, et al. Association study of a functional MAOA-uVNTR gene polymorphism and cognitive function in healthy females. Neuropsychobiology 2005;52:77-82. [DOI] [PubMed]
- 69.Hwu HG, Yeh YL, Wang JD, et al. Alcoholism among Taiwan aborigines defined by the Chinese Diagnostic Interview Schedule: a comparison with alcoholism among Chinese. Acta Psychiatr Scand 1990;82:374-80. [DOI] [PubMed]
- 70.Kochersperger LM, Parker EL, Siciliano M, et al. Assignment of genes for human monoamine oxidases A and B to the X chromosome. J Neurosci Res 1986;16:601-16. [DOI] [PubMed]


