Abstract
Objective
Bipolar disorder (BD) is emerging as an illness marred by neurocognitive deficits, many of which do not resolve on recovery. Deficits affecting working memory (WM) in particular appear to be significant. WM comprises temporally separated biological processes that involve the on-line retention and manipulation of information. Previous neuroimaging studies have not sought to dissect the individual contributions of WM and examined WM subprocesses in euthymic BD. In this study, we investigated the encode, delay and response components of WM to identify the neural substrates and respective contributions to the WM deficits found in BD.
Methods
We used event-related functional magnetic resonance imaging and a parametric WM task, incorporating 3 load conditions, to delineate individual WM subprocesses in 10 euthymic BD patients and 10 control subjects.
Results
Patients exhibited attenuated patterns of activity, predominantly in frontal brain regions, across all WM components.
Conclusions
Based on the attenuated activity observed in the patients, the clinical deficits in WM found in BD may reflect broad fronto-cortico-limbic dysfunction that is not confined to any single WM component. This is important in understanding the pathophysiology of BD and for future studies on executive functions in patients with this illness.
Medical subject headings: bipolar disorder, fMRI, gyrus, memory, mood disorders.
Abstract
Objectif
On commence à reconnaître que le trouble bipolaire (TB) est une maladie caractérisée par des déficits neurocognitifs dont beaucoup ne disparaissent pas au rétablissement. Les déficits qui touchent la mémoire de travail (MT) en particulier semblent importants. La MT comprend des processus biologiques séparés temporalement mettant en cause la rétention en ligne et la manipulation d'information. Les études antérieures de neuro-imagerie n'ont pas cherché à analyser les contributions individuelles de la MT et à examiner des sous-processus de la MT dans un cas de TB euthymique. Dans cette étude, nous nous sommes penchés sur les composantes encodage, retard et réponse de la MT afin d'identifier les substrats neuraux et les contributions respectives au déficit de la MT que l'on retrouve dans des cas de TB.
Méthodes
Nous avons utilisé l'imagerie par résonnance magnétique fonctionnelle reliée à un événement et une tâche MT paramétrisée, comportant trois conditions de charge, afin de délimiter des sous-processus individuels de la MT chez 10 patients atteints d'un TB euthymique et 10 sujets témoins.
Résultats
Les patients ont montré des tendances atténuées d'activité, principalement dans les régions frontales du cerveau, dans toutes les composantes de la MT.
Conclusions
Compte tenu de l'atténuation de l'activité observée chez les patients, les déficits cliniques de la MT que l'on constate dans les cas de TB peuvent refléter un dysfonctionnement frontocorticolimbique général qui n'est pas confiné à une seule composante de la MT. Cette constatation est importante si l'on veut comprendre la pathophysiologie du TB et pour des études futures des fonctions d'exécution chez les patients atteints de cette maladie.
Introduction
Bipolar disorder (BD) is a complex mental illness characterized by acute shifts in a person's mood, energy and ability to function. In BD, symptoms of depression and hypomania are more severe than the normal “ups and downs” experienced by healthy individuals, and episodes of apparent remission are deceptive, because impairments persist even while the patient is apparently well.1–3 Traditionally, BD has been regarded as a disorder of mood and, as a consequence, initial studies focused on structural abnormalities in limbic–thalamocortical networks, which are known to subserve mood. Collectively, the prefrontal cortex, medial temporal lobe and subcortical structures have been targeted and structural abnormalities identified in each of these regions.4–7 More recently, neuroimaging studies using positron emission tomography (PET) and single photon emission computed tomography (SPECT) have identified a wide range of metabolic dysfunctions involving the prefrontal cortex, cingulate, and temporal and parietal cortices.8 Moreover, functional MRI (fMRI) studies have highlighted functional aberrations at various locations along limbic–thalamocortical circuits.2,9–14 While the search for the precise structure–function relation underpinning the pathophysiology of BD has concentrated on mood-related circuits, it has been assumed that cognitive processes are unaffected and remain largely intact. Consequently, cognitive processes such as working memory (WM) have received far less attention than mood. This is surprising, given that many of the identified abnormalities occur in brain regions that clearly subserve functions other than emotional regulation. In BD, neurocognitive deficits have been identified3 that do not resolve during euthymia,15 and deficits in WM in particular appear extensive.16,17
Working memory
The term “working memory” refers to a complex set of biological processes involved in the on-line retention and manipulation of information,18 used by organisms to guide behaviour and successfully interact with the environment. WM plays a crucial role in many cognitive functions, such as spatial processing, reasoning, planning and language comprehension. Consequently, impairments in WM have a profound impact on cognition. Several studies have reported that dysfunctions in WM are the underlying cause of cognitive impairments observed in conditions such as schizophrenia,19 unipolar depression20 and Parkinson's disease.21 It has been postulated that WM is not a unitary process but, instead, is composed of 3 independent components that operate dynamically.18 According to this model, WM comprises a visuospatial sketchpad and phonological loop that stores and manipulates visuospatial and verbal information together with a central executive that regulates attention processes and controls information entry and the retention and release of memory from the visuospatial sketchpad and phonological loop. The precise neurobiological substrates of WM have yet to be elucidated. Results of recent neuroimaging studies lend tentative support for this 3-component model with the implication of such regions as the prefrontal cortex,22 parietal cortex23 and anterior cingulate.24
BD imaging studies
To date, only 2 WM fMRI studies have been conducted on patients with euthymic BD.25,26 The first study25 employed a 2-back WM task incorporated in a block design with 0-back as the baseline condition. The study reported that patients performed significantly worse than control subjects, with respect to the baseline condition 0-back. Despite performing poorly, patients showed qualitatively greater activation foci, compared with control subjects, across the entire brain; this pattern of activation persisted between group comparisons with healthy control subjects. The results from this study are counterintuitive, because poor performance would normally be associated with reduced activation, especially in regions associated with the WM. However, the paradigm employed in this study (2-back block design) did not draw on the load-capacity characteristics of the WM. The second study26 used 2 separate WM paradigms, the 2-back (block design) and the Sternberg Task (parametric design), in an attempt to dissociate the central executive from the phonological loop. The authors reported no significant between-group differences in reaction time performance in either of the tasks. In direct contrast to the previous25 study, the authors reported significantly decreased bilateral frontal, temporal and parietal activations for the BD group during the 2-back task, but no between-group differences were found during the Sternberg Task. The authors did not, however, adequately distinguish individual WM subprocesses; thus these findings may not be representative. Studies using the Sternberg Task reported reliable activations in frontal, parietal and subcortical areas.27,28 However, to accurately elucidate the brain regions involved by such WM tasks, it is necessary to disentangle the contributions of subprocesses such as encode, delay and response execution, because each of these may preferentially engage separate brain regions. Although studies25,26 have explored WM deficits in patients with BD using traditional n-back and Sternberg memory tasks, these studies that have used block-design paradigms are likely to be limited, because it is not possible to partition the subprocesses of WM when entire blocks are averaged.
We designed this study to investigate WM subprocesses (encode, delay and response execution) and to delineate the respective neural correlates. This study aimed to compare the patterns of brain activation in patients with euthymic BD and healthy control subjects during the performance of a delay-response memory task, based on a traditional Sternberg Parametric Memory Task.29 We hypothesized that patients with euthymic BD would exhibit an abnormal pattern of brain activation and that this would be reflected in increased reaction times. Further, we postulated that euthymic BD patients would exhibit an attenuated pattern of brain activations, especially in prefrontal cortical regions, during the delay WM condition.
Methods
Participants
Ten right-handed women with BD I and 10 age-and sex-matched control subjects participated in this study. A research psychiatrist made the diagnoses using the Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Version (SCID-P),30 which was supplemented by a case note review. All patients were in remission for at least 3 months. We excluded subjects who had a history of substance abuse, neurological disease or closed head injury. We also excluded subjects with an additional axis I or axis II psychiatric diagnosis based on the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV)31 or a medical disorder currently necessitating treatment.
Patients were aged 19–54 years (mean 32.4 [standard deviation {SD}] 10.8 yr). Symptoms were assessed with the 17-item Hamilton Depression Rating Scale (HAMD-17),32 Young Mania Rating Scale (YMRS), Montgomery–Asberg Depression Rating Scale33 and Global Assessment of Functioning Scale.31 Patients were defined as euthymic, with scores of 6 or less on the HAMD-17 and 6 or less on the YMRS.
The mean duration of illness from diagnosis was 8.8 (SD 5.8) years. The mean number of previous depressive episodes was 4.9 (SD 4.1) and of manic episodes was 3.2 (SD 2.6). None of the patients met DSM-IV criteria for rapid cycling. At the time of fMRI scanning, 7 patients were taking mood-stabilizing medications. Five patients were taking lithium (mean daily dosage 1340 [SD 230.2] mg) with a mean plasma level of 0.76 (SD 0.09) mmol/L. Two of these patients were taking lamotrigine 100 mg daily, and one of the 7 was taking carbamazepine alone (700 mg daily, plasma level of 40.0 μg/mL).
Patients were compared with 10 female volunteers matched for age (20–54 yr; mean 31.7 [SD 11.9] yr), handedness, level of education and premorbid IQ (see Table 1). Comparison subjects were screened for a history of neurological or psychiatric disorder (Structured Clinical Interview for DSM-IV-NonPatient [SCID-NP])34 or a family history of the same. The Prince of Wales Hospital and University of New South Wales ethics committees approved the study, and all participants provided written informed consent.
Table 1
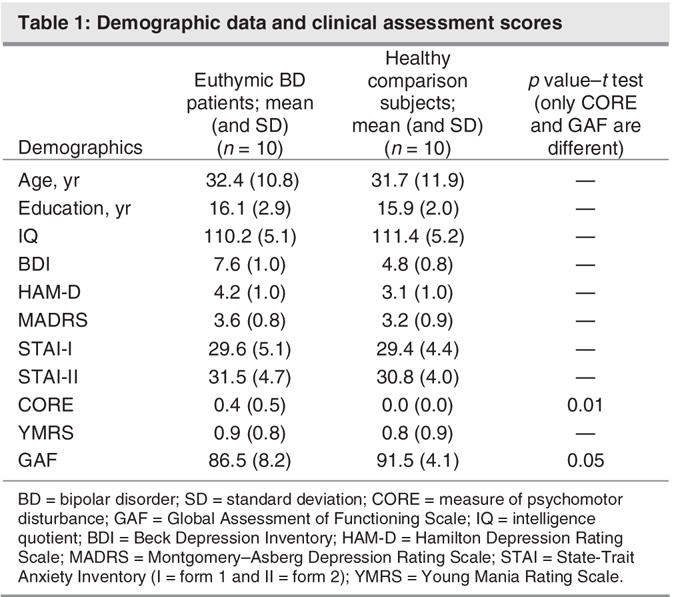
Behavioural paradigm
All subjects were scanned while performing a WM task based on the Sternberg Task (see Fig. 1). The task was an event-related parametric design that allowed investigation of the subprocesses that occur within a block and which are obscured by the traditional averaged measures. These subprocesses (encode, delay and response) could be quantified and spatially determined. Subjects observed a word list containing 3, 5 or 7 words and were instructed to memorize the list. The lists were presented for 8 seconds, after which they were erased from the screen and followed by a 4-second delay (blank screen) before a target word appeared. The target word was then presented on the screen for 2 seconds, at which time the subject had to decide whether the target word was part of the previously presented word list. The target was then erased, and a 1-second delay preceded the appearance of the next list. All subjects were asked to respond to the target words by pressing 1 of 2 reaction time buttons: Yes meant the target word was part of the word list and No meant it was not. Overall, 48 word list stimuli were presented (16 × 3, 16 × 5 and 16 × 7), and the total task duration was 12 minutes. All words were taken from the Lang Affective Norms for English Words database,35 and the word lists were balanced for affective valence, word length and word frequency. All word lists were different and were presented in a counterbalanced fashion. Word stimuli were computer-generated and back-projected onto a frosted screen, using an LCD video projector. Subjects viewed the screen through a mirror fixed to the head coil.
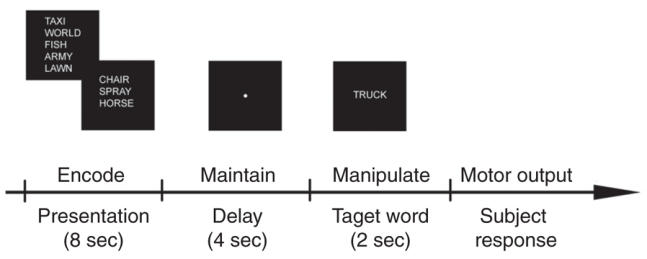
Fig. 1: A graphical representation of the experimental task. Depicted are the various stages of the Sternberg-like task in chronological order. The word lists were presented for 8 seconds, after which the screen was erased and a fixation point appeared. The fixation (delay period) screen lasted 4 seconds before the presentation of the target word. The target word was then presented for 2 seconds.
Reaction time responses were acquired with a fibre optic reaction time device (response window 50–2000 ms) specifically designed for use in the magnetic resonance (MR) scanner. We acquired the mean reaction time and each individual's reaction time to the 48 target stimuli.
Image acquisition
Imaging was performed with a 1.5T Philips Intera MRI scanner. Sixteen axial slices (7 mm thickness, no gap) parallel to the anterior and posterior commissure covering the whole brain were imaged with a temporal resolution of 3 s, using a T2-weighted gradient echo planner imaging (EPI) sequence (echo time [TE] = 45 ms; repetition time [TR] = 3000 ms; matrix = 64 × 64; flip angle = 90°). The field of view (FOV) was 240 mm, and the effective inplane functional spatial resolution was 3.75 mm. For each functional run, 240 whole brain scans were collected. As an aid to localization of the activated voxels, we acquired T1-weighted high-resolution whole brain images with the following parameters: TR = 28 ms, TE = 5 ms, matrix 256 × 256, FOV 300 mm, acquired resolution = 0.45 × 0.45 × 1.0 mm.
Data analysis
We preprocessed all functional data, using SPM99. Images were interrogated and carefully scrutinized for movement and susceptibility artifacts. Data were corrected for movement and were smoothed using a 8 × 8 × 10 (full-width half-maximum [FWHM]) Gaussian kernel to compensate for residual spatial variability and to reduce the effect of MR signal variation between runs. We performed a within-group analysis on individual data, using MEDx 3.4.1 (Sensor Systems, Sterling Va.), with all functional data globally normalized to an empirically determined value of 1000. This involved proportional scaling of each voxel by the global mean at that time point and removal of low-frequency noise, using a high-pass filter (3 cycles/min) applied across the fMRI time series for each voxel.
The hemodynamic response related to intensity changes was predicted by a correlation model at each time point as being a linear function of the number of words contained in the lists in each volume acquisition. Thus, for each paradigm run, 48 volumes were submitted to a correlation analysis. The resultant Z-score maps were thresholded to a Bonferroni-corrected probability of p < 0.05, which corresponded to a statistically significant z score threshold of 4.00. We used thresholded Z-score maps to determine the presence of significant activation foci. We also analyzed individual preprocessed data, using the General Linear Model (GLM) and the theory of Gaussian random fields as implemented in the BrainVoyager software package (Brain Innovations, Netherlands). We performed a between-group analysis, using a random-effects model to provide an estimate of the error variance for each condition of interest across subjects, rather than across scans.36 We tested group differences between the control subjects and patients, with respect to increasing number of words in the word list. Individual contrast images reflecting activations from the WM load were created separately, and we used contrast analysis of the predictors to find regions in which the fMRI signal correlated with increasing number of words in the lists.
To identify between-group differences in performance at each level of difficulty, we analyzed reaction time data with a 2-way mixed analysis of variance (ANOVA) with repeated measures on WM load.
Results
Behavioural data
The patients with BD exhibited slower reaction times, compared with control subjects (see Fig. 2), for all levels of difficulty, but we observed no significant between-group effect (F1,18 = 1.25, p = 0.28). There was a significant main effect for the number of words contained in the presented word list (WM load) (F2,36 = 25.56, p < 0.001), such that the reaction time increased in line with increasing WM load in both control subjects and BD patients. There was no significant interaction effect between subject type (control subjects and BD patients) and the WM load on the reaction time (F2,36 = 0.71, p = 0.50). The mixed ANOVA for response accuracy indicated a significant main effect of group (F1,18 = 9.59, p = 0.006), reflecting poorer accuracy levels for patients compared with control subjects averaged across the 3 conditions. Subsequent t tests revealed a significant difference in response accuracy in the 7-item word list condition only (t18 = 2.23, p = 0.04); control subjects responded with 84% accuracy, whereas patients were 79% accurate. There was no significant group-by-WM load interaction (F2,36 = 0.52, p = 0.60). There was a significant main effect for WM load, with both patients and control subjects being less accurate as the task difficulty increased (F2,36 = 49.11, p = 0.0001).
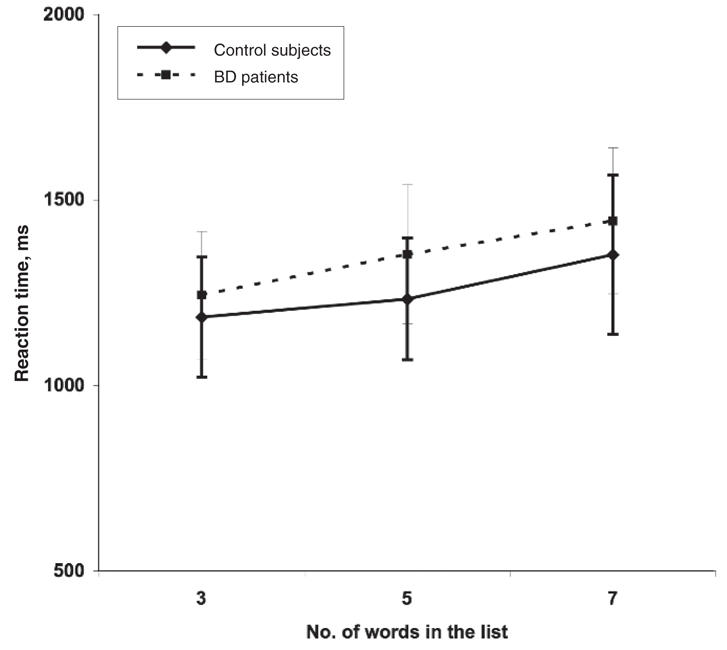
Fig. 2: A line chart depicting reaction time data for control and bipolar disorder (BD) groups as a function of increasing working memory load. The error bars indicate standard deviation.
Imaging data
We examined the functional data for increased blood oxygenated activity that correlated with increasing task difficulty within each group separately for the encode, delay and response conditions across the task.
Encode condition
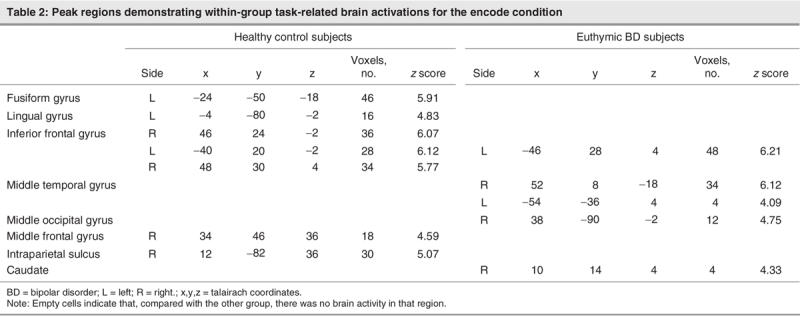
During the encode condition (see Fig. 3 and Table 2), the control subjects demonstrated activation foci in the fusiform gyrus, the ventrolateral prefrontal cortex in the region of the inferior frontal gyrus bilaterally, the lingual gyrus, the intraparietal sulcus on the right side, and the dorsolateral prefrontal cortex in the region of the middle frontal gyrus on the right. In contrast, BD patients displayed activity in the middle temporal gyrus and the inferior frontal gyrus, both lateralized to the left side; the middle occipital gyrus isolated to the right; and a region of activity within the right caudate nucleus.
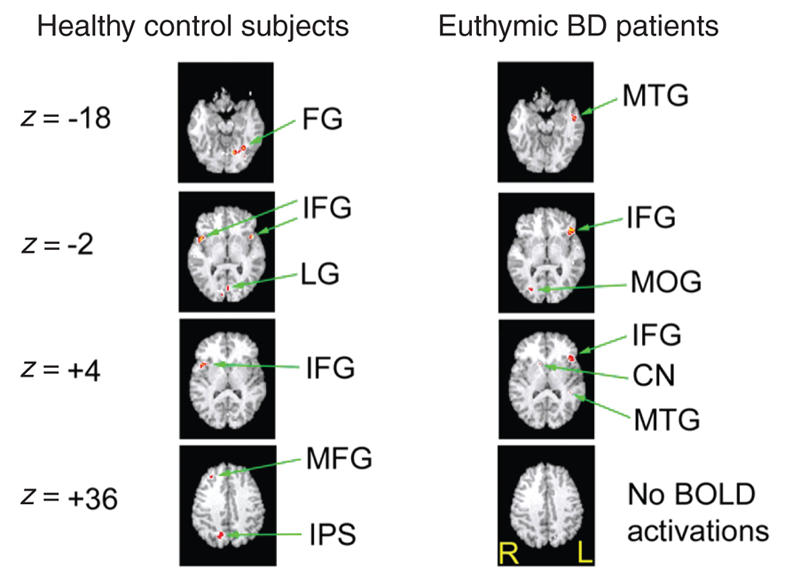
Fig. 3: Activation foci depicting within-group parametric BOLD changes for the control and bipolar disorder (BD) groups for the encode condition. All activations depicted represent Bonferroni corrected z scores greater than 4.0, which corresponds to a corrected whole brain p value less than 0.05. All images are radiologically oriented. FG = fusiform gyrus; MTG = middle temporal gyrus; IFG = inferior frontal gyrus; LG = lingual gyrus; MOG = middle occipital gyrus; CN = caudate nucleus; MFG = middle frontal gyrus; IPS = intraparietal sulcus; BOLD = blood oxygen–level dependent.
Table 2
Delay condition
For the delay condition (see Fig. 4 and Table 3), control subjects demonstrated significant activation foci in the subcallosal and parahippocampal gyri approximately midline and the inferior occipital gyrus bilaterally. Ventral prefrontal activation foci were detected in the inferior frontal gyrus lateralized to the right. Activations were present in the right-middle temporal gyrus, the cuneus bilaterally, the right pulvinar, the superior temporal sulcus and the middle occipital gyrus. Activation foci were present in the middle frontal and supramarginal gyri on the right side, the precuneus on the left side and the intraparietal sulcus on the right side. In contrast, patients with BD exhibited a pattern of activation in the left inferior occipital gyrus and superior temporal gyri, the medial frontal gyrus centrally and the left cuneus. A small area of right-sided activity was identified in the inferior frontal gyrus and the caudate.
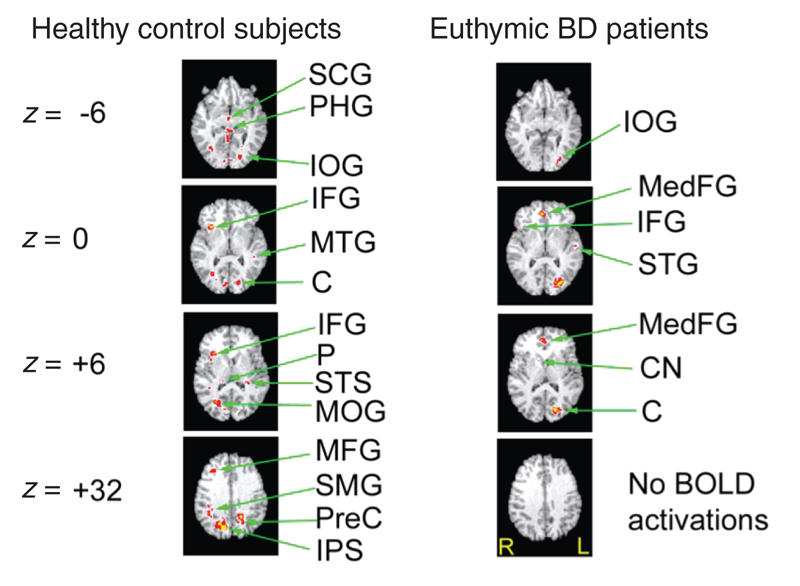
Fig. 4: Activation foci depicting within-group parametric BOLD changes for the control and bipolar disorder (BD) groups in the delay condition. All activations depicted represent Bonferroni corrected z scores greater than 4.0, which corresponds to a corrected whole brain p value less than 0.05. All images are radiologically oriented. SCG = subcallosal gyrus; PHG = parahippocampal gyrus; IOG = inferior occipital gyrus; IFG = inferior frontal gyrus; MedFG = medial frontal gyrus; MTG = middle temporal gyrus; C = cuneus; STG = superior temporal sulcus; P = precuneus; STS = superior temporal sulcus; MOG = middle occipital gyrus; CN = caudate nucleus; MFG = middle frontal gyrus; SMG = supramarginal gyrus; PreC = precuneus; IPS = intraparietal sulcus; BOLD = blood oxygen–level dependent.
Table 3
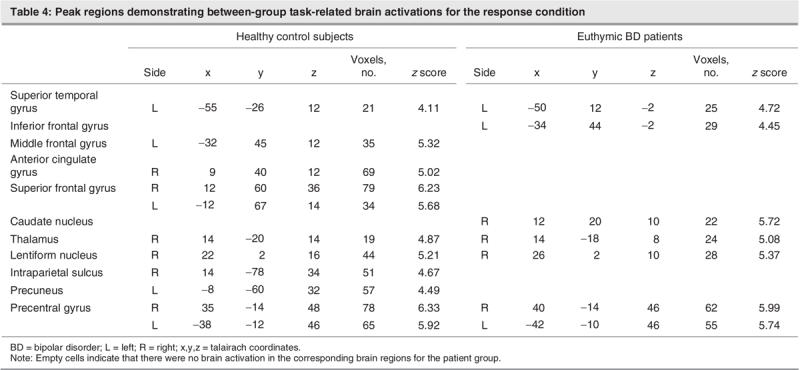
Response condition
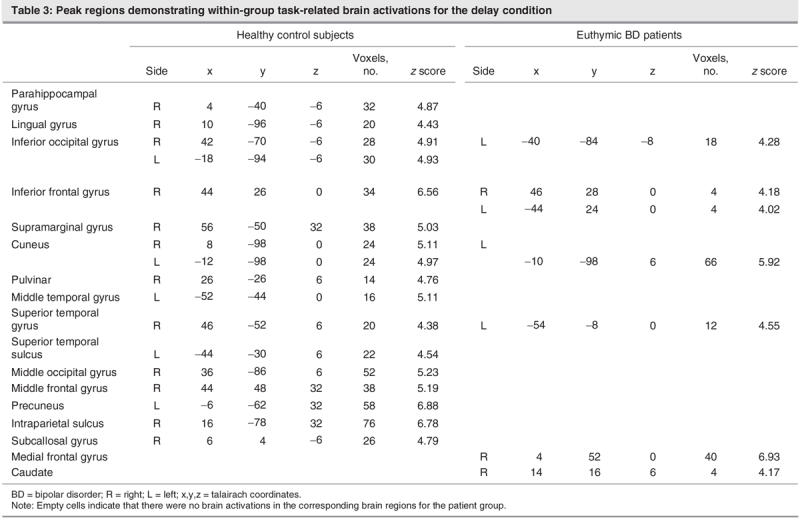
During the response condition, subjects had to respond by pressing a button identifying whether the word was originally part of the presented list (see Fig. 5 and Table 4). The healthy control subjects demonstrated activations bilaterally in the superior frontal and precentral gyrus, along with activations in the middle frontal and superior temporal gyri and the left precuneus. Activations were also present on the right side for the anterior cingulate, thalamus, lentiform nucleus and intraparietal sulcus. The pattern of activations in the control subjects encompassed frontal, temporal and subcortical regions. Conversely, patients attenuated frontal activation on the left side for the superior and inferior frontal gyri and exhibited subcortical activation (all right-sided) in the caudate, thalamus and lentiform nuclei. Patients also had bilateral precentral gyrus activations.
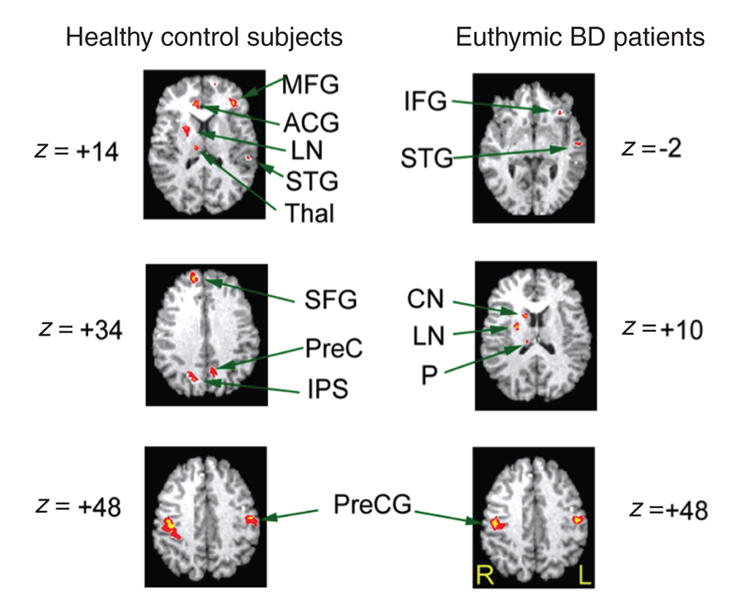
Fig. 5: Activation foci depicting within-group parametric blood oxygen–level dependent. changes for the control and bipolar disorder (BD) groups in the response condition. All activations depicted represent Bonferroni corrected z scores greater than 4.0, which correspond to a corrected whole brain p value less than 0.05. All images are radiologically oriented. MFG = middle frontal gyrus; ACG = anterior cingulate; LN = lentiform nuclei; STG = superior temporal gyrus; PreC = precuneus; IPS = intraparietal sulcus; CN = caudate nucleus; P = thalamus (pulvinar); PreCG = precentral gyrus; R = right; L= left.
Table 4
Group differences
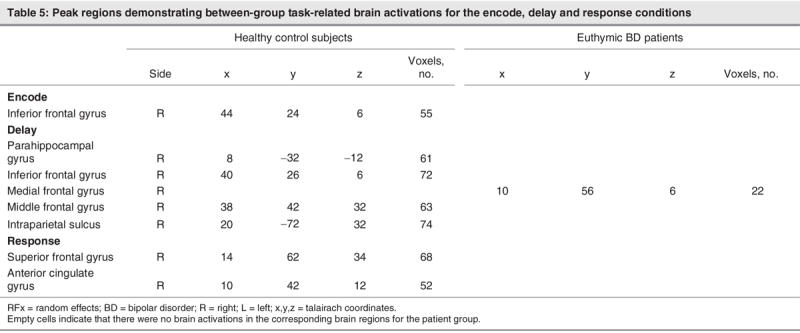
We conducted a GLM group analysis of contrast differences separately across the encode, delay and response conditions between control subjects and patients; the analysis revealed that, during encoding, control subjects had greater activity in the right inferior frontal gyrus compared with patients. During delay, control subjects had greater activity in the right parahippocampal, inferior and middle frontal gyri and within the intraparietal sulcus. Patients demonstrated significantly greater activity in the medial frontal gyrus. Finally, during the response condition, control subjects had significantly greater activity in the superior frontal and anterior cingulate gyri, whereas patients did not demonstrate any significantly greater activity during this period, compared with control subjects (Table 5).
Table 5
Discussion
Although a useful model for BD has not yet been formulated, emerging evidence suggests it is associated with discernible cognitive impairment that is not limited to periods of manifest illness.3,37 Surprisingly, these cognitive deficits resemble those observed in patients with frontal lobe damage38 and likely contribute to changes in social behaviour associated with BD.39 Early neuroimaging studies measuring regional cerebral blood flow (rCBF) and glucose metabolism (regional cerebral glucose metabolic rate [rCMRglu]) in patients with BD found frontal-lobe abnormalities such as marked hypofrontality; this has since been replicated in treatment-resistant BD patients with depression.40 BD patients with depression, therefore, appear compromised with respect to frontal-lobe affective processing. fMRI investigations in BD have also examined affective processing in the context of its interplay with cognitive and executive functions, and abnormal prefrontal patterns have been noted in BD patients.2,11,13,41 Along with these neuroimaging findings, there is growing neuropsychological interest in cognitive impairment, and a complex picture of subtle but clinically significant deficits is emerging.16,17,42,43 There are, however, conflicting views regarding whether these deficits resolve when patients recover.3,44
The primary objective of this study was to explore whether patients with euthymic BD have impaired WM and whether this is reflected by fMRI changes in prefrontal cortical activation. Specifically, we chose a design to identify changes in association with particular components of WM. Hence this study sought to extrapolate from standard block-design paradigms25,26 where WM components have been traditionally averaged across an fMRI trial block. This more sophisticated approach partitioned WM subprocesses (encode, delay and response conditions), which are normally subsumed within a single parameter with block averaging techniques.
Imaging results
The results from this study corroborate the findings from WM neuroimaging studies45–49 and identify significant activation foci in well-described networks.27,50 Distinct patterns of activity are associated with various components of WM with some modest overlap. Several studies47,49 provide evidence for a ventral-dorsal partitioning of WM networks based on its subprocesses. These studies have suggested that the ventrolateral prefrontal cortex (VLPFC) and, in particular, the inferior frontal gyrus49 is a region of the brain where information is initially collected and organized. The dorsolateral prefrontal cortex (DLPFC), a region encompassing the middle frontal gyrus,49 is then recruited and corresponds to the delay and response components. However, the role of the DLPFC in WM is a topic of ongoing debate, with studies equally divided on its putative role. While several studies have reported DLPFC activations during the delay condition of WM tasks,28,51 others have reported no such activity.52,53 Task attributes such as WM load, type of information being processed and whether response preparation is required are all possible explanations for the heterogeneity of findings in this region.
Results in the control subjects in our study showed bilateral VLPFC activation during the encode condition, unilateral activation during the delay condition and no activity during the response condition. The observed overlap in neural activity during encode and delay is consistent with single-unit recording of delay-response tasks in monkeys.54,55 Moreover, the VLPFC activations in our study encompass Broca's area, and activity in this region might reflect verbal rehearsal strategies engaging the phonological loop. In contrast, BD patients demonstrated activity in the inferior frontal gyrus across all 3 components of WM. This would indicate that patients found the task more challenging and had greater engagement of rehearsal strategies. Although task performance was not significantly different between the BD patients and control subjects, the patients exhibited a trend for slower reaction times across all levels of difficulty, which would be consistent with such an explanation.
The DLPFC was activated across all WM conditions for the control subjects in our study. Recently it has been reported that neurons in the DLPFC have the capacity to be engaged during the delay and response conditions.50,56 However, in contrast to these studies, our control subjects also activated the DLPFC during the encode condition. Activation in the DLPFC reportedly reflects encoding of items and possibly activity in the central executive.50,57 A possible explanation for the additional recruitment of this region during the encode condition may be a corollary of the adaptation of the Sternberg Task, in which entire words formed components in each list, rather than the conventional use of numbers or single letters. This methodological difference made the task more challenging and, as such, might have resulted in participants having to recruit greater executive processing to adequately perform the task. On direct comparison, the euthymic BD patients demonstrated a dramatically altered pattern of DLPFC activation. The most prominent feature was the failure to engage the DLPFC during any of the WM conditions. This is interesting because, behaviourally, the patients did not perform significantly worse when compared with the control subjects. Based on the assumption that the DLPFC is actively engaged during information encoding, this result might reflect impairment of efficient encoding and retrieval strategies involving the central executive. Thus (during the delay condition), euthymic BD patients may have to recruit the medial frontal gyrus as a compensatory mechanism, a strategy that could diminish DLPFC availability.
Additional fMRI prefrontal activity that occurred in control subjects but not patients involved the superior frontal and anterior cingulate gyri. The anterior cingulate is vital to cognitive functions, such as reward anticipation, decision-making, empathy and emotion. Moreover, a recent study suggests the anterior cingulate may also be involved in rendering new memories permanent.58
Notably, intraparietal sulcus (IPS) fMRI activity was absent in BD patients. This is significant because the IPS plays a pivotal role in a frontoparietal network and is preferentially engaged during the shifting of spatial attention.59–61 It has been suggested that its function subserves the suppression of irrelevant distractors,62 facilitating attention through integration of stimulus features.63 Several studies have reported that euthymic BD patients exhibit deficits on sustained attention tasks, both with and without a WM component.44,64
Parahippocampal gyrus activation was also absent in the BD group, whereas control subjects exhibited robust blood-oxygen–level dependent (BOLD) activity in this region during the delay condition. The parahippocampal gyrus, in addition to its putative role in the formation of memory, also acts as a temporary mnemonic bridge to more permanent neocortical storage. It is reported that circuits within the parahippocampal gyrus and hippocampus adapt their outputs to maintain attention and motivational salience during task-related delays that may vary.65 Moreover, it is thought that these regions modulate cortical recognition by means of a neuronal model without encoding the representational information that the cortex normally encodes.66 The absence of parahippocampal and IPS activation in BD patients may therefore reflect a deficit in the internal control of attention and may have wider implications in terms of long-term memory.
Additionally, within-group differences in regional brain activation were also present, indicating a difference in the networks that BD patients recruit when completing the WM task. Control subjects appear to recruit a distributed network that incorporates the precuneus and supramarginal gyrus — neither of which are activated in patients with BD. These 2 regions contribute significantly to the brain's ability to facilitate the extraction of pertinent information for further evaluation and have wider implications for higher-order cognitive processes, such as the formation of memory. The precuneus is a multimodal association area that is involved in episodic memory retrieval,67 and it has been mooted that the prefrontal regions drive memory retrieval and that successful retrieval prompts the reactivation of engrams stored in the precuneus.67 The failure of BD patients to activate the precuneus may be a direct consequence of prefrontal hypoactivity resulting in a lack of retrieval drive. This is in keeping with the lack of supramarginal gyrus activation in BD patients that perhaps reflects precuneus output compromise because it is reportedly involved in the postprocessing of salient information for evaluation and subsequent categorization.68
Behavioural results
Although there was no significant between-group differences in reaction time, the overall accuracy of the control subjects and patients significantly decreased with increasing WM loads. The patients, however, were found to have significantly poorer performance on the 7-item WM load condition, which would be consistent with the observed decreased prefrontal activity in this group. Given the relatively small sample size and low statistical power of this study, these results require replication.
Several limitations of this study require clarification. Medication is an important potential confound: 7 patients were taking mood stabilizers at the time of scanning, with lithium being the most common stabilizer. It has been reported that lithium can alter vascular smooth muscle contractility in the brain that might affect neurovascular coupling, which the BOLD response is predicated on.69 This is more likely to produce global effects than specific regional changes, although it is important to acknowledge that global blood flow changes can affect local BOLD responses and the coupling between local neuronal activity and hemodynamics. The relatively small sample size used in this study might have diminished the power of the study, particularly at the between-group level, with respect to behavioural responses. The wider application of our findings is somewhat limited; in limiting patients to those suitable for scanning, we extrapolated those with rapid cycling or BD II. This may not be appropriate, especially because comorbid illnesses such as substance misuse and anxiety disorders were excluded and only females were scanned.
Acknowledgments
Thanks to Dr. Adrian M. Owen for his comments on this paper. Thanks to the Australian Rotary Health Research Fund, the National Health and Medical Research Council for financial support, and the National Institute of Schizophrenia and Allied Disorders for infrastructure support.
Footnotes
Contributors: Drs Lagopoulos and Malhi designed the study. Dr. Lagopoulos and Ms. Ivanovski aquired the data, and all the authors analyzed it. All authors contributed to the writing and revision of the article. All authors gave final approval for the article to be published.
Competing interests: None declared.
Correspondence to: Dr. Jim Lagopoulos, Discipline of Psychological Medicine, Northern Clinical School, Level 5 Building 34, Royal North Shore Hospital, St Leonards, Sydney, Australia 2065; fax 61 2 9926 7730; jlagopoulos@med.usyd.edu.au
References
- 1.Bearden CE, Hoffman KM, Cannon TD. The neuropsychology and neuroanatomy of bipolar affective disorder: a critical review. [Discussion 151-3]. Bipolar Disord 2001;3:106-50. [DOI] [PubMed]
- 2.Malhi GS, Lagopoulos J, Sachdev P, et al. Cognitive generation of affect in hypomania: an fMRI study. Bipolar Disord 2004;6:271-85. [DOI] [PubMed]
- 3.Olley A, Malhi GS, Mitchell PB, et al. When euthymia is just not good enough: the neuropsychology of bipolar disorder. J Nerv Ment Dis 2005;193:323-30. [DOI] [PubMed]
- 4.Dewan MJ, Haldipur CV, Lane EE, et al. Bipolar affective disorder. I. Comprehensive quantitative computed tomography. Acta Psychiatr Scand 1988;77:670-6. [DOI] [PubMed]
- 5.Drevets WC, Price JL, Simpson JR Jr, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature 1997;386:824-7. [DOI] [PubMed]
- 6.Sax KW, Strakowski SM, Zimmerman ME, et al. Frontosubcortical neuroanatomy and the continuous performance test in mania. Am J Psychiatry 1999;156:139-41. [DOI] [PubMed]
- 7.Weinberger DR, DeLisi LE, Perman GP, et al. Computed tomography in schizophreniform disorder and other acute psychiatric disorders. Arch Gen Psychiatry 1982;39:778-83. [DOI] [PubMed]
- 8.Rubinsztein JS, Fletcher PC, Rogers RD, et al. Decision-making in mania: a PET study. Brain 2001;124:2550-63. [DOI] [PubMed]
- 9.Blumberg HP, Leung HC, Skudlarski P, et al. A functional magnetic resonance imaging study of bipolar disorder: state-and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry 2003;60:601-9. [DOI] [PubMed]
- 10.Caligiuri MP, Brown GG, Meloy MJ, et al. A functional magnetic resonance imaging study of cortical asymmetry in bipolar disorder. Bipolar Disord 2004;6:183-96. [DOI] [PubMed]
- 11.Chang K, Adleman NE, Dienes K, et al. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Arch Gen Psychiatry 2004;61:781-92. [DOI] [PubMed]
- 12.Elliott R, Ogilvie A, Rubinsztein JS, et al. Abnormal ventral frontal response during performance of an affective go/no go task in patients with mania. Biol Psychiatry 2004;55:1163-70. [DOI] [PubMed]
- 13.Malhi GS, Lagopoulos J, Ward PB, et al. Cognitive generation of affect in bipolar depression: an fMRI study. Eur J Neurosci 2004;19:741-54. [DOI] [PubMed]
- 14.Yurgelun-Todd DA, Gruber SA, Kanayama G, et al. fMRI during affect discrimination in bipolar affective disorder. Bipolar Disord 2000;2:237-48. [DOI] [PubMed]
- 15.Asarnow RF, MacCrimmon DJ. Span of apprehension deficits during the postpsychotic stages of schizophrenia. A replication and extension. Arch Gen Psychiatry 1981;38:1006-11. [DOI] [PubMed]
- 16.Ferrier IN, Stanton BR, Kelly TP, et al. Neuropsychological function in euthymic patients with bipolar disorder. Br J Psychiatry 1999;175:246-51. [DOI] [PubMed]
- 17.van Gorp WG, Altshuler L, Theberge DC, et al. Declarative and procedural memory in bipolar disorder. Biol Psychiatry 1999;46:525-31. [DOI] [PubMed]
- 18.Baddeley A. Working memory. New York: Oxford University Press; 1986.
- 19.Kim J, Glahn DC, Nuechterlein KH, et al. Maintenance and manipulation of information in schizophrenia: further evidence for impairment in the central executive component of working memory. Schizophr Res 2004;68:173-87. [DOI] [PubMed]
- 20.Merriam EP, Thase ME, Haas GL, et al. Prefrontal cortical dysfunction in depression determined by Wisconsin Card Sorting Test performance. Am J Psychiatry 1999;156:780-2. [DOI] [PubMed]
- 21.Lewis SJ, Cools R, Robbins TW, et al. Using executive heterogeneity to explore the nature of working memory deficits in Parkinson's disease. Neuropsychologia 2003;41:645-54. [DOI] [PubMed]
- 22.D'Esposito M, Postle BR, Ballard D, et al. Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cogn 1999;41:66-86. [DOI] [PubMed]
- 23.Jonides J, Schumacher EH, Smith EE, et al. The role of parietal cortex in verbal working memory. J Neurosci 1998;18:5026-34. [DOI] [PMC free article] [PubMed]
- 24.Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci 1999;10:49-57. [DOI] [PubMed]
- 25.Adler CM, Holland SK, Schmithorst V, et al. Changes in neuronal activation in patients with bipolar disorder during performance of a working memory task. Bipolar Disord 2004;6:540-9. [DOI] [PubMed]
- 26.Monks PJ, Thompson JM, Bullmore ET, et al. A functional MRI study of working memory task in euthymic bipolar disorder: evidence for task-specific dysfunction. Bipolar Disord 2004;6:550-64. [DOI] [PubMed]
- 27.Manoach DS, Gollub RL, Benson ES, et al. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry 2000;48:99-109. [DOI] [PubMed]
- 28.Rypma B, Prabhakaran V, Desmond JE, et al. Load-dependent roles of frontal brain regions in the maintenance of working memory. Neuroimage 1999;9:216-26. [DOI] [PubMed]
- 29.Sternberg S. Memory-scanning: mental processes revealed by reaction-time experiments. Am Sci 1969;57:421-57. [PubMed]
- 30.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition, Version 2.0. New York (NY): New York State Psychiatric Institute; 1995.
- 31.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56-62. [DOI] [PMC free article] [PubMed]
- 32.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382-9. [DOI] [PubMed]
- 33.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: The Association; 1994.
- 34.Spitzer R.L, Williams JBW, Gibbon M, et al. Structured clinical interview for DSM-III-R–NonPatient ed. (version 1.0) Washington: American Psychiatric Press; 1990.
- 35.Lang PJ, Bradley MM, Cuthbert BN. Emotion and motivation: measuring affective perception. J Clin Neurophysiol 1998;15:397-408. [DOI] [PubMed]
- 36.Holmes AP, Friston KJ. Generalisability, random effects and population inference. Neuroimage 1998;7:S754.
- 37.Rubinsztein JS, Michael A, Paykel ES, et al. Cognitive impairment in remission in bipolar affective disorder. Psychol Med 2000;30:1025-36. [DOI] [PubMed]
- 38.Owen AM, Sahakian BJ, Semple J, et al. Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia 1995;33:1-24. [DOI] [PubMed]
- 39.Dickerson FB, Sommerville J, Origoni AE, et al. Outpatients with schizophrenia and bipolar I disorder: Do they differ in their cognitive and social functioning? Psychiatry Res 2001;102:21-7. [DOI] [PubMed]
- 40.Ketter TA, Kimbrell TA, George MS, et al. Effects of mood and subtype on cerebral glucose metabolism in treatment-resistant bipolar disorder. Biol Psychiatry 2001;49:97-109. [DOI] [PubMed]
- 41.Blumberg HP, Martin A, Kaufman J, et al. Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. Am J Psychiatry 2003;160:1345-7. [DOI] [PubMed]
- 42.Clark L, Iversen SD, Goodwin GM. A neuropsychological investigation of prefrontal cortex involvement in acute mania. Am J Psychiatry 2001;158:1605-11. [DOI] [PubMed]
- 43.Zubieta JK, Huguelet P, O'Neil RL, et al. Cognitive function in euthymic bipolar I disorder. Psychiatry Res 2001;102:9-20. [DOI] [PubMed]
- 44.Harmer CJ, Clark L, Grayson L, et al. Sustained attention deficit in bipolar disorder is not a working memory impairment in disguise. Neuropsychologia 2002;40:1586-90. [DOI] [PubMed]
- 45.D'Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Exp Brain Res 2000;133:3-11. [DOI] [PubMed]
- 46.Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci 2000;23:475-83. [DOI] [PubMed]
- 47.Owen AM, Herrod NJ, Menon DK, et al. Redefining the functional organization of working memory processes within human lateral prefrontal cortex. Eur J Neurosci 1999;11:567-74. [DOI] [PubMed]
- 48.Owen AM, Stern CE, Look RB, et al. Functional organization of spatial and nonspatial working memory processing within the human lateral frontal cortex. Proc Natl Acad Sci U S A 1998;95:7721-6. [DOI] [PMC free article] [PubMed]
- 49.Petrides M. Frontal lobes and working memory: evidence from investigations of the effects of cortical excisions in nonhuman primates. In: Boller F, editor. Handbook of neuropsychology. Amsterdam: Elsevier Science; 1994.
- 50.Rypma B, D'Esposito M. The roles of prefrontal brain regions in components of working memory: effects of memory load and individual differences. Proc Natl Acad Sci U S A 1999;96:6558-63. [DOI] [PMC free article] [PubMed]
- 51.Leung HC, Gore JC, Goldman-Rakic PS. Sustained mnemonic response in the human middle frontal gyrus during on-line storage of spatial memoranda. J Cogn Neurosci 2002;14:659-71. [DOI] [PubMed]
- 52.Rowe JB, Passingham RE. Working memory for location and time: activity in prefrontal area 46 relates to selection rather than maintenance in memory. Neuroimage 2001;14:77-86. [DOI] [PubMed]
- 53.Rowe JB, Toni I, Josephs O, et al. The prefrontal cortex: response selection or maintenance within working memory? Science 2000;288:1656-60. [DOI] [PubMed]
- 54.Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol 1989;61:331-49. [DOI] [PubMed]
- 55.Fuster JM. Unit activity in prefrontal cortex during delayed-response performance: neuronal correlates of transient memory. J Neurophysiol 1973;36:61-78. [DOI] [PubMed]
- 56.Constantinidis C, Franowicz MN, Goldman-Rakic PS. The sensory nature of mnemonic representation in the primate prefrontal cortex. Nat Neurosci 2001;4:311-6. [DOI] [PubMed]
- 57.Braver TS, Cohen JD, Nystrom LE, et al. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 1997;5:49-62. [DOI] [PubMed]
- 58.Wiltgen BJ, Brown RA, Talton LE, et al. New circuits for old memories: the role of the neocortex in consolidation. Neuron 2004;44:101-8. [DOI] [PubMed]
- 59.Corbetta M, Akbudak E, Conturo TE, et al. A common network of functional areas for attention and eye movements. Neuron 1998;21:761-73. [DOI] [PubMed]
- 60.Gitelman DR, Nobre AC, Parrish TB, et al. A large-scale distributed network for covert spatial attention: further anatomical delineation based on stringent behavioural and cognitive controls. Brain 1999;122:1093-106. [DOI] [PubMed]
- 61.Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci 2000;3:284-91. [DOI] [PubMed]
- 62.Wojciulik E, Kanwisher N. The generality of parietal involvement in visual attention. Neuron 1999;23:747-64. [DOI] [PubMed]
- 63.Treisman M. The multiregional and single origin hypotheses of the evolution of modern man: a reconciliation. J Theor Biol 1995;173:23-9. [DOI] [PubMed]
- 64.Clark L, Iversen SD, Goodwin GM. Sustained attention deficit in bipolar disorder. Br J Psychiatry 2002;180:313-9. [DOI] [PubMed]
- 65.Buzsaki G, Penttonen M, Nadasdy Z, et al. Pattern and inhibition-dependent invasion of pyramidal cell dendrites by fast spikes in the hippocampus in vivo. Proc Natl Acad Sci U S A 1996;93:9921-5. [DOI] [PMC free article] [PubMed]
- 66.Francis G, Grossberg S. Cortical dynamics of boundary segmentation and reset: persistence, afterimages, and residual traces. Perception 1996;25:543-67. [DOI] [PubMed]
- 67.Krause BJ, Schmidt D, Mottaghy FM, et al. Episodic retrieval activates the precuneus irrespective of the imagery content of word pair associates. A PET study. Brain 1999;122:255-63. [DOI] [PubMed]
- 68.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain 1995;118:279-306. [DOI] [PubMed]
- 69.Dehpour AR, Ghafourifar P, Samenian J, et al. The effect of lithium on endothelial-dependent relaxation in rat isolated aorta. Gen Pharmacol 1995;26:1003-7. [DOI] [PubMed]


