Abstract
Wnt-5a is a representative ligand that activates a β-catenin-independent pathway in Wnt signalling. In the present paper, the roles of the post-translational modifications in the actions of Wnt-5a were investigated. We found that Wnt-5a is modified with palmitate at Cys104 and glycans at Asn114, Asn120, Asn311 and Asn325. The palmitoylation was not essential for the secretion of Wnt-5a, but was necessary for its ability to suppress Wnt-3a-dependent T-cell factor transcriptional activity and to stimulate cell migration. Wnt-5a activated focal adhesion kinase and this activation also required palmitoylation. Wild-type Wnt-5a induced the internalization of Fz (Frizzled) 5, but a Wnt-5a mutant that lacks the palmitoylation site did not. Furthermore, the binding of Wnt-5a to the extracellular domain of Fz5 required palmitoylation of Wnt-5a. These results indicate that palmitoylation of Wnt-5a is important for the triggering of signalling at the cell surface level and, therefore, that the lipid-unmodified form of Wnt-5a cannot activate intracellular signal cascades. In contrast, glycosylation was necessary for the secretion of Wnt-5a, but not essential for the actions of Wnt-5a. Thus the post-translational palmitoylation and glycosylation of Wnt-5a are important for the actions and secretion of Wnt-5a.
Keywords: Frizzled 5 (Fz5), glycosylation, palmitoylation, post-translational modification, Wnt-5a
Abbreviations: CM, conditioned medium; CRD, cysteine-rich domain; ECM, extracellular matrix; FAK, focal adhesion kinase; Fz, Frizzled; GFP, green fluorescent protein; HEK-293, human embryonic kidney-293; HEK-293T cells, HEK-293 cells expressing the large T-antigen of SV40 (simian virus 40); JNK, c-Jun N-terminal kinase; Lef, lymphoid enhancer factor; LRP5/6, lipoprotein receptor-related protein 5/6; MALDI–TOF-MS, matrix-assisted laser-desorption ionization–time-of-flight MS; NLK, Nemo-like kinase; PKC, protein kinase C; PNGase F, peptide N-glycosidase F; siRNA, small interfering RNA; Tcf, T-cell factor; TFA, trifluoroacetic acid; WT, wild-type
INTRODUCTION
Wnt proteins are a large family of cysteine-rich secreted molecules that play essential roles in embryonic induction, cell polarity generation and cell fate specification in various species [1]. At least three major Wnt intracellular signalling pathways have been identified. The canonical β-catenin pathway has been studied most extensively. In this pathway, when Wnt acts on its cell-surface receptor consisting of Fz (Frizzled) and LRP5/6 (lipoprotein receptor-related protein 5/6), β-catenin escapes from degradation in the Axin complex [2–4]. The accumulated β-catenin is translocated to the nucleus, where it activates the transcription factor Tcf (T-cell factor)/Lef (lymphoid enhancer factor) [5]. The β-catenin pathway is involved in cell fate determination in development and in mammalian tumorigenesis [1,6]. A second Wnt pathway is the Ca2+ pathway, which activates calcium/calmodulin-dependent protein kinase II and PKC (protein kinase C) [7]. This pathway has been shown to control the dorso–ventral axis patterning in vertebrates. A third pathway is the planar cell polarity pathway in Drosophila, which activates small G-proteins, including Rac and Rho, JNK (c-Jun N-terminal kinase) and Rho-associated kinase [8]. This pathway controls hair polarity by regulating the subcellular location of the prehair, which is composed largely of actin filament bundles. In vertebrates, a counterpart pathway exists in the convergent extension movement of tissues during gastrulation and neurulation.
Wnt-5a is one of the Wnt proteins that activate a β-catenin-independent pathway. A knockout mouse study showed that Wnt-5a is essential for proper development [9]. Wnt-5a null mice exhibit prenatal lethality and fail to extend multiple structures that grow out from the primary body axis. The activity of Wnt-5a has been analysed by the use of overexpression of Wnt-5a, Wnt-5a CM (conditioned medium) and purified Wnt-5a protein. Wnt-5a inhibits the β-catenin pathway by inducing the downregulation of β-catenin through Siah2 [10] or by suppressing the transcriptional activity of Tcf/Lef [11,12]. However, Wnt-5a is able to activate the β-catenin pathway depending on the expression of combinations of distinct receptors [12]. In addition, it has been demonstrated that Wnt-5a inhibits the migration and invasiveness of thyroid tumour and colorectal cancer cell-lines [13,14], whereas, in contrast, it increases the cell motility and invasiveness of melanoma cells and breast cancer cells associated with macrophages [15,16]. Thus, Wnt-5a has been suggested to regulate cell migration, but how it regulates this process is not fully understood.
At least 19 Wnt members have been identified in mammals to date. Molecules that are secreted from cells are commonly post-translationally modified. Four asparagine-linked glycosylation sites of Wnt-1 have been identified and a Wnt-1 mutant that lacked the four glycosylation sites was still active in the C57MG transformation assay, although its activity was decreased [17]. It has been demonstrated that Wnt-3a is modified with palmitate and that this modification is necessary for the ability of Wnt-3a to induce the accumulation of β-catenin [18]. Thus, although the post-translational modifications of Wnt-1 and Wnt-3a have been shown to be important for their actions, those of other Wnt family members have not been systematically analysed. In the present paper, we analysed the roles of the different post-translational modifications of Wnt-5a on its actions. We have identified amino acids that are modified with palmitate and asparagine-linked glycans, and show that palmitoylation and glycosylation are important for the actions and secretion of Wnt-5a. In addition, we demonstrate that Wnt-5a induces the internalization of Fz5 and that it promotes cell migration by stimulating focal adhesion turnover.
EXPERIMENTAL
Materials and chemicals
TOP-fos-Luc and FOP-fos-Luc were provided by Dr H. Clevers (University Medical Centre, Utrecht, The Netherlands). pUC-EF-1α-β-cateninSA was provided by Dr A. Nagafuchi (Kumamoto University, Kumamoto, Japan). pEGFP-paxillin was provided by Dr H. Sabe (Osaka Bioscience Institute, Osaka, Japan), and pRK5-IgG was provided by Dr J. C. Hsieh (State University of New York, Stony Brook, NY, U.S.A.). Anti-(Wnt-5a) antibody was generated in rabbits by immunization with the synthetic peptide corresponding to residues 165–181 of mouse Wnt-5a. Wnt-3a protein was purified and anti-(Wnt-3a) antibody was prepared as described previously [19]. Standard recombinant DNA techniques were used to construct pPGK-neo-Wnt-5aC104A (Wnt-5a CA), pPGK-neo-Wnt-5aN325Q, pPGK-neo-Wnt-5aN114Q/N325Q, pPGK-neo-Wnt-5aN114Q/N311Q/N325Q, pPGK-neo-Wnt-5aN114Q/N120Q/N325Q, pPGK-neo-Wnt-5aN114Q/N120Q/N311Q/N325Q (Wnt-5a NQ) and pRK5-hFz5 (human Fz5)-CRD (cysteine-rich domain; amino acids 1–172)-IgG, which encodes the CRD of Fz5 fused to an immunoglobulin-γ Fc-tag. L cells (a variant of C3H mouse fibroblasts that do not adhere to one another and do not express cadherins) stably expressing WT (wild-type) Wnt-5a, Wnt-5a CA or Wnt-5a NQ were generated by selection with G418. HEK-293 (human embryonic kidney 293) or NIH3T3 cells stably expressing TOP-fos-Luc and HEK-293 cells stably expressing pUC-EF-1α-β-cateninSA were generated by selection with Blasticidin S. HeLaS3 cells stably expressing GFP (green fluorescent protein)–paxillin were generated by selection with puromycin [20]. The siRNA (small interfering RNA) for Wnt-5a was synthesized as described previously [21]. PNGase F (peptide N-glycosidase F), anti-FAK (focal adhesion kinase) and anti-(phospho-Tyr397-FAK) antibodies were purchased from Takara, BD Biosciences and BIO SOURCE respectively. Other materials were obtained from commercial sources.
Purification of Wnt-5a protein
Wnt-5a CM (1 litre) was adjusted to 1% (v/v) Triton X-100 and applied to a Blue Sepharose HP column (2.6×20 cm; Amersham Biosciences) equilibrated with binding buffer [20 mM Tris/HCl, pH 7.5 and 1% (w/v) CHAPS] containing 150 mM KCl. After the column was washed with 250 ml of binding buffer, elution was performed in a stepwise manner by adding 250 ml of binding buffer containing 1.5 M KCl at a flow rate of 5 ml/min. Fractions of 12.5 ml were collected. An aliquot (20 μl) of each fraction was subjected to Western blotting and probed with anti-(Wnt-5a) antibody, and the results (not shown) revealed that a single peak of reactive protein is eluted in fractions 5–8. These fractions contained an active substance that induced the phosphorylation of Dvl in NIH3T3 cells (as determined by Western blotting; results not shown). The active fractions of the Blue Sepharose column chromatography (50 ml; 3.45 mg of protein) were pooled and concentrated to 5 ml using a 30 kDa molecular mass cut-off membrane (Pall Corporation). The concentrate (5 ml; 3.16 mg of protein) was applied to a HiLoad™ Superdex™ 200 column (2.6×60 cm; Amersham Biosciences) equilibrated with PBS and 1% (w/v) CHAPS. Elution was performed by the same buffer at a flow rate of 2.5 ml/min and 2.5 ml fractions were collected. When an aliquot (20 μl) of each fraction was subjected to Western blotting and probed with anti-(Wnt-5a) antibody, a single broad peak was observed in fractions 72–80 (results not shown). These fractions contained an active substance that phosphorylated Dvl in NIH3T3 cells (as determined by Western blotting; results not shown). The active fractions of the HiLoad™ Superdex™ 200 column chromatography (22.5 ml; 0.096 mg of protein) were applied to a HiTrap Heparin column (0.75×2.5 cm; Amersham Biosciences) equilibrated with PBS and 1% (w/v) CHAPS. After the column was washed with 10 ml of the same buffer, elution was performed by a 10 ml linear gradient of NaCl (0–1 M) in PBS and 1% (w/v) CHAPS at a flow rate of 0.5 ml/min. Western blotting of an aliquot (20 μl) of each fraction with anti-(Wnt-5a) antibody revealed that a single broad peak is eluted in fractions 9–16 (results not shown). Wnt-5a in fractions 10–14 was nearly homogeneous, as judged by SDS/PAGE (results not shown). These fractions contained an active substance that phosphorylated Dvl in NIH3T3 cells (as determined by Western blotting; results not shown). The active fractions (2.5 ml; 37.5 μg of protein) were collected and used for experiments. Wnt-5a CA was also purified from Wnt-5a CA CM using the same procedures.
MALDI–TOF-MS (matrix-assisted laser-desorption ionization–time-of-flight MS) analyses
Purified Wnt-5a was reduced and alkylated, and then digested with trypsin. A portion (14 μg) of the digest was lyophilized and dissolved in 50 μl of 0.1% TFA (trifluoroacetic acid) solution, desalted using ZipTip μC18, and subjected to MALDI–TOF-MS analysis. All MALDI–TOF mass spectra were obtained in the reflector positive mode using α-cyano-4-hydroxycinnamic acid [saturated solution in 50% (v/v) acetonitrile with 0.1% TFA] as the matrix. Analytes were prepared by mixing 0.5 μl of peptide sample with 0.5 μl of matrix solution on a MALDI plate and allowed to air dry at room temperature in a safety cabinet before being inserted into the spectrometer. External calibration was conducted using adrenocorticoid hormone (1–7) and bradykinin. Peptides were identified by using the Mascot search program (Matrix Science) to perform theoretical trypsin digests and searching for potential unmodified tryptic peptides (with up to one missed cleavage) or suspected modified species. Methionine residues were considered to be either normal methionine or the oxidized form (Met-ox), and cysteine residues were considered to be normal cysteine or palmitoylated cysteine to produce Cys-S-palmitate.
Statistical analyses
All of the luciferase assays and migration assays were performed repeatedly in at least three separate experiments. Results are presented as means±S.D. Statistical analyses were performed using Microsoft Excel. An unpaired t test with P<0.05 was used to determine statistical significance.
Deglycosylation of Wnt-5a in vitro
To generate deglycosylated Wnt-5a, 75 or 300 ng of purified Wnt-5a protein was incubated with 0.125 to 0.5 milli-units of PNGase F for 2 h at 37 °C in 25 μl of reaction mixture [0.1 M Tris/HCl, pH 8.6, and 1% (w/v) CHAPS]. The samples were then subjected to Silver staining or used for examining the ability of Wnt-5a to inhibit the Wnt-3a-dependent Tcf-4 activity and to stimulate cell migration.
Preparation of lysates, ECM (extracellular matrix) and CM from Wnt-5a-producing cells
HEK-293T cells [HEK-293 cells expressing the large T-antigen of SV40 (simian virus 40)] expressing Wnt-5a WT or Wnt-5a NQ (60-mm-diameter dishes) were lysed in 250 μl of Nonidet P40 buffer [50 mM Tris/HCl, pH 8.0, 130 mM NaCl and 1% (v/v) Nonidet P40] containing the following protease inhibitors: 1 μg/ml leupeptin, 1 μg/ml aprotinin and 1 mM PMSF. The samples were used as the lysate fraction. ECM remaining on the dishes was extracted at 100 °C for 5 min in 375 μl of Laemmli sample buffer after washing with PBS and Nonidet P40 buffer three times. These samples were used as the ECM fraction. To concentrate Wnt-5a in CM, one third of the CM (1 ml) was incubated with Blue Sepharose 4 Fast Flow beads (Amersham Biosciences) for 2 h at 4 °C and the beads were precipitated by centrifugation at 20000 g for 15 s at 4 °C. The precipitates were used as the CM fraction.
Receptor binding assay
Fz5-CRD–IgG and control IgG were produced in HEK-293T cells via transient transfection [22]. The secreted proteins were harvested after 48 h. For binding assays, Fz5-CRD–IgG and control IgG were purified using Protein A–Sepharose. The indicated amounts of Wnt-5a WT and Wnt-5a CA were incubated with Fz5-CRD–IgG or control IgG (10 nM) for 1 h on ice. Protein A–Sepharose was collected by centrifugation at 20000 g for 15 s at 4 °C and the precipitates were resolved by SDS/PAGE and probed with anti-(Wnt-5a) antibody.
Receptor internalization assay
The receptor internalization assay was performed as previously described [23]. HEK-293 cells expressing Fz5–GFP were stimulated with Wnt-5a WT or Wnt-5a CA CM for 1 h at 4 °C. After unbound Wnt-5a was removed by washing with ice-cold PBS three times, internalization was initiated by adding warm Dulbecco's modified Eagle's medium and the dishes were transferred to a heated chamber (37 °C) in a 5% CO2/95% air atmosphere. The cells were viewed directly with a confocal microscope (LSM510, Zeiss) to detect Fz5–GFP. To quantify the internalization of Fz5–GFP, the appearance of the intracellular localization of Fz5 was classified into three types with regard to the distribution of this protein and the number of puncta in the cytosol. The first type showed clear localization to the cell surface, with a few puncta in the cytosol. The second type showed localization to both the cell surface and puncta in the cytosol. The third type showed the little or no cell surface distribution with more than 20 puncta in the cytosol. More than 100 cells were evaluated in each experiment.
Others
The immunocytochemical analyses of the cultured cells were performed as described previously [20,24]. The Triton X-114 phase separation assay was done as described previously [18]. Tcf-4 transcriptional activity and cellular proliferation were measured as described [24,25]. Assays for cell adhesion, migration, Matrigel invasion and live imaging of focal adhesions were performed as described previously [20].
RESULTS
Palmitoylation of Wnt-5a protein
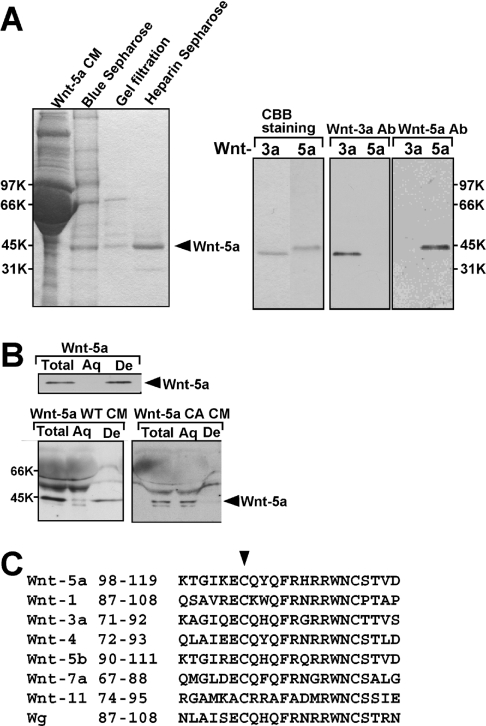
To examine the post-translational modifications of Wnt-5a, we purified Wnt-5a to homogeneity by three successive types of column chromatography (Figure 1A). Approx. 37.5 μg of Wnt-5a protein was purified from 1 litre of Wnt-5a CM (Table 1). Purified Wnt-5a was recognized with its specific antibody (Figure 1A). In the Triton X-114 phase-separation method, all of the purified Wnt-5a was recovered in the detergent-enriched phase (Figure 1B), which is characteristic of hydrophobic proteins. To analyse the hydrophobic properties of Wnt-5a, we subjected trypsin-induced proteolytic peptide fragments of Wnt-5a to LC tandem MS, which identifies the molecular masses of the ionized peptides. We found a peak corresponding to the palmitoylated peptide fragment at m/z 1211.97 (Cal. 1211.642 Da), which was consistent with the peptide (ECQYOFR: 972.421 Da) containing cysteine (Cys104) modified with palmitate (239.221 Da). To examine whether this cysteine residue was in fact modified, we mutated Cys104 to alanine in Wnt-5a (Wnt-5a CA) and expressed the Wnt-5a mutant in L cells. Wnt-5a CA was secreted as efficiently as Wnt-5a WT, suggesting that this cysteine residue is not essential for secretion. While most of the Wnt-5a WT in the CM partitioned in the detergent phase in the Triton X-114 phase-separation assay, Wnt-5a CA in the CM was recovered in the aqueous phase (Figure 1B). These results indicate that palmitoylation of Wnt-5a at Cys104 is essential for the hydrophobicity of the protein.
Figure 1. Palmitoylation of Wnt-5a protein.
(A) Left-hand panel: Coomassie Brilliant Blue staining of an SDS/PAGE 10% (v/v) gel containing fractions from all steps of the purification. Right-hand panel: the purified Wnt-3a and Wnt-5a proteins were stained with Coomassie Brilliant Blue (CBB) or subjected to Western blotting and probed with anti-(Wnt-3a) or anti-(Wnt-5a) antibodies. (B) Purified Wnt-5a protein and CM containing Wnt-5a WT or Wnt-5a CA were subjected to the Triton X-114 phase-separation assay. An aliquot of each fraction was subjected to Western blotting and probed with anti-(Wnt-5a) antibody. Aq, aqueous phase; De, detergent phase. (C) Sequence alignment of Wnt proteins. The region of residues 98–119 of mouse Wnt-5a is aligned to mouse Wnt-1, Wnt-3a, Wnt-4, Wnt-5b, Wnt-7b, Wnt-11 and Drosophila Wg. The arrowhead shows the conserved cysteine.
Table 1. Purification of Wnt-5a.
The concentration of Wnt-5a protein in the CM was determined by comparing its signal intensity on a Wnt-5a immunoblot to that of serial dilutions of a known amount of purified Wnt-5a protein.
| Volume (ml) | Protein concentration (mg/ml) | Total protein (mg) | Wnt-5a concentration (μg/ml) | Wnt-5a (μg) | |
|---|---|---|---|---|---|
| Wnt-5a CM | 1000 | 3.89 | 3890 | 0.11 | 110 |
| Blue Sepharose | 50 | 0.069 | 3.45 | 1.64 | 82 |
| Gel filtration | 22.5 | 4.26* | 0.096 | 2.0 | 45 |
| Heparin Sepharose | 2.5 | 15.0* | 0.0375 | 15.0 | 37.5 |
*Concentration in μg/ml.
Cys77 in mouse Wnt-3a and Cys51 in Drosophila Wnt-8 have been shown to be modified with palmitate [18]. Cys104 in mouse Wnt-5a corresponded to these cysteine residues, in the sense that palmitoylated cysteine residues are the most N-terminally conserved cysteine residues of the Wnt proteins (Figure 1C). Other Wnt family proteins also contain this conserved cysteine residue, suggesting that other Wnt proteins may be palmitoylated at the same sites.
Wnt-5a has been reported to have at least two cellular functions. One is the ability to regulate the β-catenin pathway and the other is the ability to regulate cell migration. Next we tested the role of palmitoylation of Wnt-5a in these functions.
Requirement for palmitoylation of Wnt-5a for its ability to inhibit Tcf-4 activity
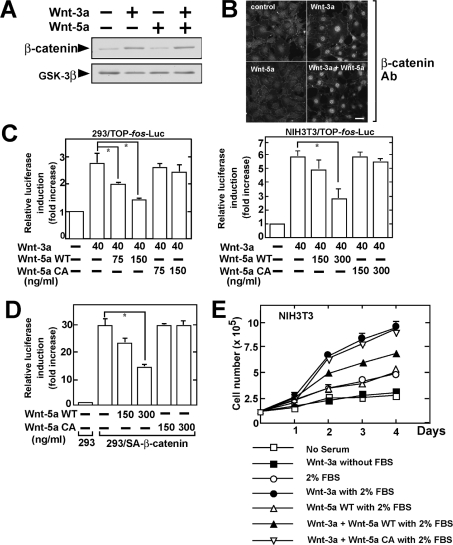
Purified Wnt-5a affected neither Wnt-3a-dependent stabilization of β-catenin nor Wnt-3a-induced nuclear accumulation of β-catenin in HEK-293 and NIH3T3 cells (Figures 2A and 2B). However, purified Wnt-5a inhibited the Wnt-3a-dependent transcriptional activity of Tcf-4 in these cells in a dose-dependent manner (Figure 2C). Furthermore, the β-catenin-induced Tcf-4 activity was also inhibited by purified Wnt-5a (Figure 2D). These findings are basically consistent with recently reported observations made using purified Wnt-5a [12]. To analyse the roles of palmitoylation of Wnt-5a we purified Wnt-5a CA, which lacks palmitoylation, and examined whether this purified Wnt-5a mutant inhibits Tcf-4 activity. Purified Wnt-5a CA did not inhibit the Wnt-3a- or β-catenin-dependent activation of Tcf-4 (Figures 2C and 2D).
Figure 2. Palmitoylation of Wnt-5a is required for its ability to suppress the β-catenin pathway.
(A) After HEK-293 cells were stimulated with 40 ng/ml Wnt-3a or/and 150 ng/ml Wnt-5a for 1 h, the lysates were subjected to Western blotting and probed with the indicated antibodies. The blotting of GSK-3β was used as a loading control. (B) After NIH3T3 cells were stimulated with 40 ng/ml Wnt-3a or/and 150 ng/ml Wnt-5a for 1 h, the cells were stained with anti-(β-catenin) antibody. Scale bar, 10 μm. (C) HEK-293 (left-hand panel) and NIH3T3 (right-hand panel) cells stably expressing TOP-fos-Luc were incubated with 40 ng/ml Wnt-3a with or without the indicated amounts of Wnt-5a WT or Wnt-5a CA for 8 h. The luciferase activity was measured and expressed as the fold increase compared with the level observed in the cells without treatment. *, P<0.05. (D) After HEK-293 cells (293) or HEK-293 cells stably expressing β-cateninSA (293/SA-β-catenin) were transfected with pEF-BOS-hTcf-4E (0.1 μg) and TOP-fos-Luc (0.5 μg), they were incubated with the indicated amounts of Wnt-5a WT or Wnt-5a CA for 8 h. The luciferase activity was measured and expressed as the fold increase compared with the level observed in control HEK-293 cells. *, P<0.05. (E) NIH3T3 cells were incubated with serum-free (without FBS) or 2% (v/v) serum-containing (with 2% FBS) medium in the absence or presence of 100 ng/ml Wnt-3a and/or 300 ng/ml Wnt-5a, and then the cells were trypsinized and counted at each time point.
Purified Wnt-3a stimulated the cellular proliferation of NIH3T3 cells in the presence of 2% (v/v) serum, although Wnt-3a alone was not able to affect cell growth (Figure 2E). Purified Wnt-5a WT did not affect the 2% (v/v) serum-dependent cell growth, but it suppressed Wnt-3a-induced cellular proliferation. In addition, purified Wnt-5a CA did not suppress Wnt-3a-dependent cell proliferation. These results indicate that Wnt-5a suppresses the transcriptional activity of Tcf-4 and cell growth by acting downstream of β-catenin and that palmitoylation of Wnt-5a is necessary for the inhibitory activity against the β-catenin pathway.
Requirement for palmitoylation of Wnt-5a for its ability to stimulate cell migration
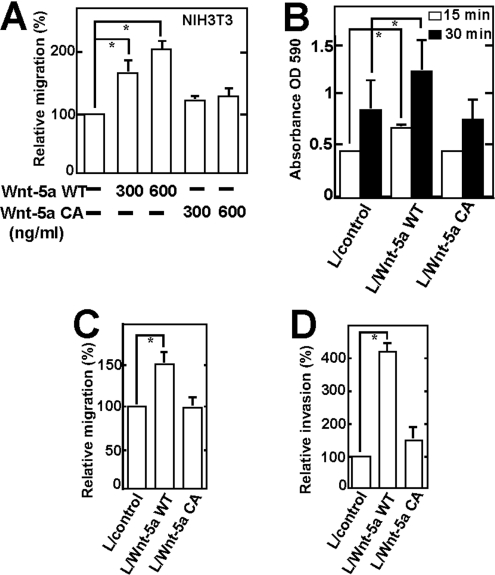
To examine the cell migration activity of Wnt-5a, transwell assays were performed using a modified Boyden chamber. When purified Wnt-5a WT was added to the lower chamber, migration of NIH3T3 cells to the lower side of the upper chamber was stimulated in a dose-dependent manner, whereas purified Wnt-5a CA did not stimulate cell migration (Figure 3A). We also transfected the plasmid of Wnt-5a WT or Wnt-5a CA in L cells and tested the adhesion, migration and invasion abilities of these cells. Overexpression of Wnt-5a WT in L cells stimulated adhesion, migration and invasion, but overexpression of Wnt-5a CA did not affect them (Figures 3B–3D). Taken together, these findings indicate that palmitoylation of Wnt-5a is necessary for its ability to stimulate cell migration.
Figure 3. Palmitoylation of Wnt-5a is required for its ability to stimulate cell migration.
(A) NIH3T3 cells were subjected to the transwell migration assay in the presence of purified Wnt-5a WT or Wnt-5a CA. *, P<0.05. (B) L cells containing empty vector or stably expressing Wnt-5a WT or Wnt-5a CA were subjected to the adhesion assay for the indicated time (*P<0.05). (C) and (D) L cells containing empty vector or stably expressing Wnt-5a WT or Wnt-5a CA were placed in non-coated (C) or Matrigel-coated (D) transwell chambers respectively. *, P<0.05.
Molecular mechanism of stimulation of cell migration by Wnt-5a
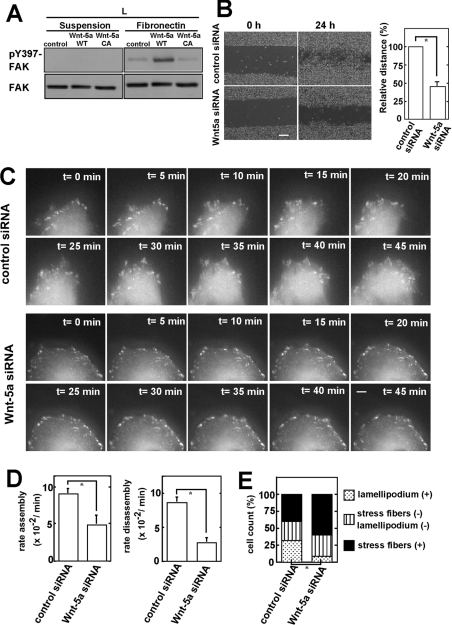
Several possible mechanisms by which Wnt-5a may inhibit the β-catenin pathway have been proposed [10,11]. However, how Wnt-5a regulates cell migration is not well understood. Focal adhesion complexes provide the main sites of cell adhesion to the ECM to control cell migration and activation of FAK is necessary for cell migration [26]. FAK is activated via autophosphorylation at Tyr397, which is initiated by the engagement of integrin with its ligand [27]. Adhesion-dependent FAK activation was enhanced by overexpression of Wnt-5a WT, but not by that of Wnt-5a CA (Figure 4A).
Figure 4. Molecular mechanism by which Wnt-5a stimulates cell migration.
(A) L cells stably expressing Wnt-5a WT or Wnt-5a CA were suspended in serum-free medium and were kept in suspension or replated onto fibronectin-coated dishes. After 1 h, the cells were lysed, subjected to Western blotting and probed with anti-(phospho-Tyr397-FAK) or anti-FAK antibodies. (B) Left-hand panel: HeLaS3 cells transfected with the control siRNA or Wnt-5a siRNA for 24 h were wounded. Scale bar, 200 μm. Right-hand panel: distance of migration was calculated by subtracting the width of the wound at 24 h from that at 0 h. The distance of migration with control siRNA was expressed as 100%. The relative migration distance with Wnt-5a siRNA was expressed as a percentage of control siRNA-treated cells. *, P<0.05. (C) Dynamics of GFP–paxillin in HeLaS3 cells treated with Wnt-5a siRNA were visualized by time-lapse fluorescence microscopy. Scale bar, 5 μm. (D) Rate constants of assembly and disassembly of GFP–paxillin shown in (C) were calculated. *, P<0.05. (E) HeLaS3 cells transfected with control siRNA or Wnt-5a siRNA were stained with FITC–phalloidin for visualizing F-actin 12 h after wounding. Then the cells (n=100) at the leading edge were counted and classified into three groups according to the appearance of F-actins: mainly stress fibres were observed; lamellipodium were visible clearly; neither stress fibres nor lamellipodium were clearly observed. Percentages of the respective groups in control siRNA- and Wnt-5a siRNA-treated cells are shown in the bar graph. *, P<0.05.
The migration of HeLaS3 cells in scratch-wound cultures was suppressed by knockdown of Wnt-5a (Figure 4B). Paxillin is an important focal adhesion-associated adaptor molecule and a substrate for the Src–FAK complex [26]. Phosphorylation of paxillin is critical for focal adhesion dynamics. To examine the effects of Wnt-5a on the dynamics of focal adhesions, we expressed GFP–paxillin in HeLaS3 cells and analysed the turnover of dynamics by live fluorescence imaging. At the cell front in control cells, paxillin-containing adhesions disassembled as new adhesions were formed near the leading edge (Figure 4C) [28]. The rate constants of the assembly and disassembly of GFP–paxillin from adhesion sites were decreased in Wnt-5a knockdown cells (Figure 4D). The initial stages of cell adhesion and focal adhesion formation involve the lamellipodium. The percentage of lamellipodium-containing cells among those at the leading edge of the migrating cells was reduced significantly in Wnt-5a-knockdown cells, whereas the percentage showing stress fibres was increased slightly (Figure 4E). These results suggest that Wnt-5a regulates the turnover of adhesion complexes, thereby stimulating cell migration.
Requirement for palmitoylation of Wnt-5a for its binding to receptor
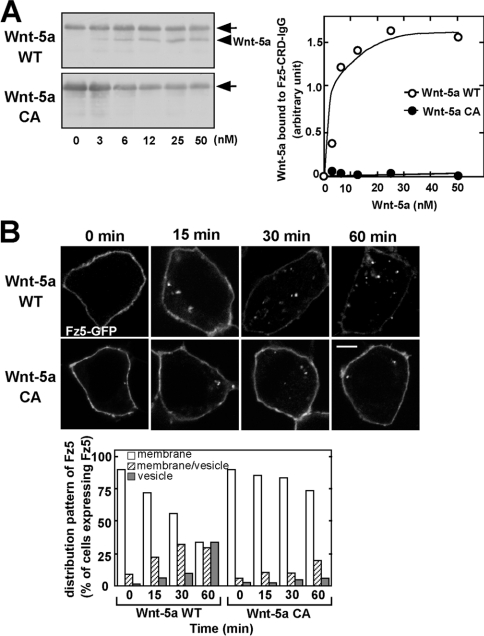
Among the ten Fz receptors, Fz5 has been suggested to be a candidate receptor for Wnt-5a [15]. To test whether Wnt-5a directly binds to Fz5, we performed a binding assay using the CRD of Fz5 tagged with an immunoglobulin-γ Fc epitope (Fz5-CRD–IgG). Purified Wnt-5a WT bound to Fz5-CRD–IgG in a dose-dependent manner with a Kd of approx. 5 nM, whereas purified Wnt-5a CA did not (Figure 5A). Purified Wnt-5a WT did not interact with the IgG-Fc epitope alone (results not shown).
Figure 5. Palmitoylation is required for the binding to Fz5 and its internalization.
(A) Left-hand panel: the indicated amounts of Wnt-5a WT and Wnt-5a CA were incubated with Fz5-CRD–IgG. Fz5-CRD–IgG was precipitated with Protein A–Sepharose, and the precipitates were subjected to Western blotting and probed with anti-(Wnt-5a) antibody. The arrows indicate non-specific bands of protein A crossreacted with anti-Wnt-5a antibody. The arrowhead indicates Wnt-5a. Right-hand panel: the amounts of precipitated Wnt-5a WT (○) and Wnt-5a CA (●) were quantified by densitometric tracing. The results are means of two independent experiments. (B) HEK-293 cells expressing Fz5–GFP were stimulated with CM containing Wnt-5a WT or Wnt-5a CA for the indicated periods of times and the fixed cells were directly processed for microscopy. Upper panel, confocal images; lower panel, quantification of internalized Fz5–GFP. Scale bar, 5 μm.
To examine whether Wnt-5a induces the internalization of Fz5, we expressed Fz5–GFP in HEK-293 cells. In the absence of Wnt-5a, Fz5–GFP was present predominantly at the cell surface (Figure 5B). When HEK-293 cells were stimulated with Wnt-5a, Fz5–GFP was internalized and the receptor was observed in the intracellular vesicles at 15–60 min (Figure 5B). Before stimulation with Wnt-5a, Fz5–GFP was present at the cell surface in 80% of the cells. When the cells were stimulated with Wnt-5a, the percentage of cells exhibiting cell surface localization of Fz5–GFP decreased and the percentage in which Fz5–GFP was observed as cytoplasmic vesicles increased in a time-dependent manner. However, Wnt-5a CA did not induce the internalization of Fz5–GFP (Figure 5B). Thus, palmitoylation of Cys104is essential for the binding of Wnt-5a to its receptor, and therefore the lipid-unmodified form does not induce the internalization of Fz5 and subsequent signalling.
Glycosylation of Wnt-5a
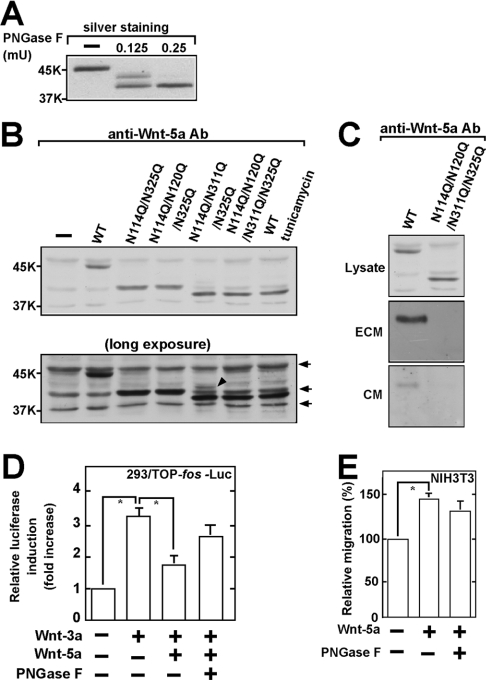
A series of experiments using tunicamycin have shown that Wnt-5a is modified with asparagine-linked glycans [29]. However, which amino acids are modified has not been determined. When purified Wnt-5a was completely deglycosylated with PNGase F, which cleaves off asparagine-linked glycans, a single fast-migrating band of Wnt-5a was observed by SDS/PAGE (Figure 6A). Partial deglycosylation of Wnt-5a using less PNGase F produced at least two fast-migrating bands (Figure 6A), indicating that Wnt-5a has multiple glycosylation sites.
Figure 6. Glycosylation of Wnt-5a protein.
(A) Purified Wnt-5a (75 ng) was treated with the indicated amounts of PNGase F and the samples were subjected to SDS/PAGE [10% (v/v) gels] and silver staining. (B) Upper panel: the indicated Wnt-5a mutants were expressed in HEK-293T cells and the lysates were subjected to Western blotting and probed with anti-(Wnt-5a) antibody. As a control, the cells expressing Wnt-5a WT were treated with tunicamycin. Lower panel: longer exposure of the same immunoblotting. The arrows indicate non-specific bands that were detected with anti-(Wnt-5a) antibody and the arrowhead a slowly migrating band of Wnt-5aN114Q/N311Q/N325Q. (C) Secretion of Wnt-5a. Wnt-5a WT or Wnt-5a NQ was expressed in HEK-293T cells and the lysates and ECM were probed by Western blotting with anti-(Wnt-5a) antibody. Wnt-5a in the CM was concentrated by precipitation with Blue Sepharose and the precipitates were probed with anti-(Wnt-5a) antibody. The expression levels of Wnt-5a in the lysate and ECM fractions can be compared directly and reflect the amount of Wnt-5a produced by confluent HEK-293T cells expressing Wnt-5a in a 60-mm-diameter dish. The expression level of Wnt-5a in the CM reflects the amount of Wnt-5a produced by one third of confluent HEK-293T cells expressing Wnt-5a. (D) HEK-293 cells stably expressing TOP-fos-Luc were incubated with 40 ng/ml Wnt-3a and 150 ng/ml Wnt-5a pre-treated or not pre-treated with PNGase F. The luciferase activity was measured and expressed as the fold increase as compared with the level observed in control cells. *, P<0.05. (E) NIH3T3 cells were subjected to the transwell migration assay in the presence of 300 ng/ml Wnt-5a pre-treated or not pre-treated with PNGase F. *, P<0.05.
There are indeed four potential consensus sequences for glycosylation of Wnt-5a: Asn114-Cys-Ser; Asn120-Thr-Ser; Asn311-Glu-Ser; and Asn325-Cys-Thr. The asparagine residues in these sequences are possible candidates as the glycosylation sites. To identify the glycosylation sites of Wnt-5a, we generated several Wnt-5a mutants in which these asparagine residues were replaced with glutamine and expressed these mutants in HEK-293T cells. We have recently found that Wnt-3a is glycosylated at Asn87 and Asn298 (H. Komekado, H. Yamamoto, T. Chiba and A. Kikuchi, unpublished work), which correspond to Asn114 and Asn325 of Wnt-5a WT respectively. Therefore, we first generated the Wnt-5aN114Q/N325Q mutant. Wnt-5aN114Q/N325Q migrated more slowly than Wnt-5a treated with tunicamycin by SDS/PAGE. Although Asn120 was additionally mutated, Wnt-5aN114Q/N120Q/N325Q showed similar electrophoretic mobility to Wnt-5aN114Q/N325Q (Figure 6B). When Wnt-5aN114Q/N325Q was additionally mutated at Asn311, most of Wnt-5aN114Q/N311Q/N325Q exhibited electrophoretic mobility similar to Wnt-5aN114Q/N120Q/N311Q/N325Q (Wnt-5a NQ) (Figure 6B), but in addition it still showed a slowly migrating band (Figure 6B, lower panel). Wnt-5a NQ exhibited electrophoretic mobility similar to that of Wnt-5a WT in the cells treated with tunicamycin (Figure 6B). These results indicate that Wnt-5a is glycosylated at Asn114, Asn120, Asn311 and Asn325.
Consistent with previous observations [30], much of the Wnt-5a WT that was secreted was bound to components of the ECM, and the remainder was recovered in the culture medium (Figure 6C). However, Wnt-5a NQ was not detected in the ECM or CM even though it was present at high levels in the cell lysates (Figure 6C), indicating that glycosylation is necessary for the secretion of Wnt-5a. To examine the effect of glycosylation on the activity of Wnt-5a, purified Wnt-5a was incubated with PNGase F to produce the deglycosylated form of Wnt-5a. Deglycosylation only partially affected the ability of Wnt-5a to inhibit the Wnt-3a-dependent Tcf-4 activity or to stimulate cell migration and the reduction was not considered significant (Figures 6D and 6E). Therefore, glycosylation is not essential for Wnt-5a activity, unlike palmitoylation.
DISCUSSION
In the present paper, we have identified the palmitoylation and glycosylation sites of Wnt-5a and demonstrated that post-translational palmitoylation and glycosylation are important for the actions and secretion of Wnt-5a.
Activities of Wnt-5a
Purified Wnt-5a inhibits Wnt-3a-dependent Tcf-4 transcriptional activity and cellular proliferation in various cell lines. Although it has been reported that Wnt-5a activates the β-catenin-dependent pathway when Fz5 is expressed or Fz4 and LRP5 are co-expressed [12,31], and that Wnt-5a induces the downregulation of β-catenin [10], purified Wnt-5a did not affect the stability of β-catenin at least in WT cells without ectopic expression of receptors. Rather, Wnt-5a suppressed Wnt-3a-dependent gene expression downstream of β-catenin, consistent with previous observations [11,12]. Although NLK (Nemo-like kinase) has been shown to mediate Wnt-5a-induced inhibition of Tcf-4 activity [11], purified Wnt-5a could not activate NLK and a kinase-negative form of NLK did not inhibit the action of Wnt-5a in our experiments (results not shown). The molecular mechanism by which Wnt-5a inhibits the β-catenin pathway still remains to be clarified.
Purified Wnt-3a alone did not affect cell proliferation of NIH3T3 cells but enhanced serum-dependent cell growth. Since Wnt-3a did not stimulate the incorporation of [3H]thymidine in L, HEK-293 or PC12 cells (results not shown), Wnt-3a signalling alone is not sufficient for the growth of these cell lines. Overexpression of β-catenin, due to genetic alterations of the β-catenin and APC (adenomatous polyposis coli) genes in cancer cells is known to play an important role in tumorigenesis [6]. However, it has also been reported that ectopically overexpressed β-catenin stimulates cell growth in the presence, but not in the absence, of serum [32,33]. Therefore, the physiological level of β-catenin increased by Wnt-3a may co-operate with other factors in the serum to stimulate cell growth. Wnt-5a suppressed Wnt-3a-dependent, but not serum-dependent, cellular proliferation. Since Wnt-5a acts downstream of β-catenin, Wnt-5a would suppress β-catenin-mediated cell growth specifically. These observations are consistent with previous reports showing that Wnt-5a acts as a tumour suppressor [13,14].
The addition of purified Wnt-5a and knockdown of Wnt-5a stimulated and inhibited cell migration and invasion respectively. Although Wnt-5a has been reported to be capable of activating JNK [34], which regulates convergent extension movement in Xenopus embryos, the molecular mechanism by which Wnt-5a regulates cell migration is unclear. Cell migration is a complex cellular behaviour that involves protrusion and adhesion at the cell front, and contraction and detachment at the rear [35]. FAK activation is best understood in the context of the engagement of integrins at the cell surface [36]. In the present paper, we have demonstrated that Wnt-5a is involved in the activation of FAK, the turnover of paxillin and membrane ruffling. It has also been shown that Wnt-5a activates PKC [7] and that JNK-dependent phosphorylation of paxillin and PKC-dependent activation of FAK are important for cell migration [37,38]. These findings suggest that Wnt-5a activates PKC and JNK, thereby leading to the activation of FAK. The pathways would mediate Wnt-5a signalling in order to regulate cell motility through focal adhesion turnover. Taken together, these results make it conceivable that Wnt-5a and ECM component(s) bind to Fz and integrin and co-operatively activate a signalling cascade to stimulate cell migration.
Roles of palmitoylation in Wnt-5a functions
Using MALDI–TOF-MS analyses, we demonstrated that Wnt-5a purified from the CM of L cells is palmitoylated at Cys104. Since this amino acid is conserved among Wnt family members, this result strongly suggests that the conserved cysteine residue is palmitoylated in Wnt proteins. Therefore, it will be important to examine whether other Wnt proteins are also modified with palmitate. Palmitoylation of Cys104 was not necessary for the secretion of Wnt-5a, but was essential for the ability of Wnt-5a to inhibit Tcf-4 transcriptional activation and to stimulate cell migration. This is similar to the finding that palmitoylation of Wnt-3a is required for the Wnt-3a-dependent accumulation of β-catenin [18].
The interaction of Wnt proteins with their receptors on the cell surface is the first step in transducing an extracellular signal into intracellular responses [22]. In humans and mice, ten Fz proteins, which are members of the family of seven-pass transmembrane receptors, have been identified as Wnt receptors [1]. It has been reported that Wnt-5a induces the internalization of Fz4 through the binding of Dvl to β-arrestin-2 [39], suggesting that Fz4 is one of the Wnt-5a receptors. Ror2, a member of the Ror family of receptor tyrosine kinases, has also been shown to bind to Wnt-5a and mediate Wnt-5a-dependent inhibition of Tcf-4 [12,40]. In the present paper, we have shown that Wnt-5a binds to Fz5 and induces the internalization of Fz5. Activation of PKC was required for the Wnt-5a-induced internalization of Fz4 [39], but not for the Wnt-5a-dependent internalization of Fz5 (results not shown). These results indicate that there are multiple receptors for Wnt-5a, which makes the diverse and complex signalling of Wnt-5a possible. Palmitoylation was necessary for the binding of Wnt-5a to Fz5 and, therefore, the lipid-unmodified form could not induce the internalization of Fz5. Palmitoylation may be important for the anchoring of Wnt-5a to the cell surface membrane, because it has been reported that lipidation of Wingless is required for its targeting to the lipid raft microdomains in Drosophila S2 cells [41]. In addition, binding of Wnt to lipoprotein particles has been shown to be important for long-range gradient formation [42]. Palmitoylation may be necessary for the binding of Wnt to lipoprotein particles.
Roles of glycosylation of Wnt-5a functions
In the present paper, we have shown that Wnt-5a is glycosylated at Asn114, Asn120, Asn311 and Asn325. Asparagine-linked glycans play pivotal roles in protein folding, oligomerization, quality control and transport [43]. The secretion of unglycosylated Wnt-5a was impaired, although the mechanism by which glycosylated Wnt-5a is transported to the cell surface from the endoplasmic reticulum is not known. It was recently reported that Wntless/Evi is required for the secretion of Wnt proteins in Drosophila and mammalian cells [44,45]. Therefore, the unglycosylated form of Wnt-5a may not be able to bind to Wntless. However, when purified Wnt-5a was deglycosylated by PNGase F, unglycosylated Wnt-5a retained the ability to inhibit the β-catenin pathway and to stimulate cell migration. It is noteworthy that glycosylation is not usually essential for maintaining the overall folded structure once a glycoprotein has folded [46]. This was also the case for Wnt-5a. Although the presence of glycans influences the properties of the polypeptide moiety, for example by increasing stability, the effect is usually rather small. Consequently, glycans can, as a rule, be removed from a folded glycoprotein without major effects on protein conformation. Thus, both palmitoylation and glycosylation are necessary for Wnt-5a to be secreted in its active form.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research and for Scientific Research on Priority Areas from the Ministry of Education, Science and Culture, Japan (2003, 2004 and 2005). We thank Dr H. Clevers, Dr A. Nagafuchi, Dr H. Sabe and Dr J. C. Hsieh for donating plasmids.
References
- 1.Wodarz A., Nusse R. Mechanisms of Wnt signalling in development. Annu. Rev. Cell Dev. Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- 2.Ikeda S., Kishida S., Yamamoto H., Murai H., Koyama S., Kikuchi A. Axin, a negative regulator of the Wnt signalling pathway, forms a complex with GSK-3β and β-catenin and promotes GSK-3β-dependent phosphorylation of β-catenin. EMBO J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kikuchi A. Roles of Axin in the Wnt signalling pathway. Cell. Signalling. 1999;11:777–788. doi: 10.1016/s0898-6568(99)00054-6. [DOI] [PubMed] [Google Scholar]
- 4.He X., Semënov M., Tamai K., Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/β-catenin signalling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- 5.Hurlstone A., Clevers H. T-cell factors: turn-ons and turn-offs. EMBO J. 2002;21:2303–2311. doi: 10.1093/emboj/21.10.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kikuchi A. Tumor formation by genetic mutations in the components of the Wnt signaling pathway. Cancer Sci. 2003;94:225–229. doi: 10.1111/j.1349-7006.2003.tb01424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kühl M., Sheldahl L. C., Park M., Miller J. R., Moon R. T. The Wnt/Ca2+ pathway: a new vertebrate Wnt signalling pathway takes shape. Trends Genet. 2000;16:279–283. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- 8.Adler P. N. Planar signalling and morphogenesis in Drosophila. Dev. Cell. 2002;2:525–535. doi: 10.1016/s1534-5807(02)00176-4. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi T. P., Bradley A., McMahon A. P., Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- 10.Topol L., Jiang X., Choi H., Garrett-Beal L., Carolan P. J., Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent β-catenin degradation. J. Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishitani T., Kishida S., Hyodo-Miura J., Ueno N., Yasuda J., Waterman M., Shibuya H., Moon R. T., Ninomiya-Tsuji J., Matsumoto K. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca2+ pathway to antagonize Wnt/β-catenin signalling. Mol. Cell. Biol. 2003;23:131–139. doi: 10.1128/MCB.23.1.131-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikels A. J., Nusse R. Purified Wnt5a protein activates or inhibits β-catenin-TCF signalling depending on receptor context. PLoS Biol. 2006;4:570–582. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kremenevskaja N., von Wasielewski R., Rao A. S., Schofl C., Andersson T., Brabant G. Wnt-5a has tumor suppressor activity in thyroid carcinoma. Oncogene. 2005;24:2144–2154. doi: 10.1038/sj.onc.1208370. [DOI] [PubMed] [Google Scholar]
- 14.Dejmek J., Dejmek A., Safholm A., Sjolander A., Andersson T. Wnt-5a protein expression in primary Dukes B colon cancers identifies a subgroup of patients with good prognosis. Cancer Res. 2005;65:9142–9146. doi: 10.1158/0008-5472.CAN-05-1710. [DOI] [PubMed] [Google Scholar]
- 15.Weeraratna A. T., Jiang Y., Hostetter G., Rosenblatt K., Duray P., Bittner M., Trent J. M. Wnt5a signalling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1:279–288. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 16.Pukrop T., Klemm F., Hagemann T., Gradl D., Schulz M., Siemes S., Trumper L., Binder C. Wnt 5a signalling is critical for macrophage-induced invasion of breast cancer cell lines. Proc. Natl. Acad. Sci. U.S.A. 2006;103:5454–5459. doi: 10.1073/pnas.0509703103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mason J. O., Kitajewski J., Varmus H. E. Mutational analysis of mouse Wnt-1 identifies two temperature-sensitive alleles and attributes of Wnt-1 protein essential for transformation of a mammary cell line. Mol. Biol. Cell. 1992;3:521–533. doi: 10.1091/mbc.3.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willert K., Brown J. D., Danenberg E., Duncan A. W., Weissman I. L., Reya T., Yates J. R. I., Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 19.Kishida S., Yamamoto H., Kikuchi A. Wnt-3a and Dvl induce neurite retraction by activating Rho-associated kinase. Mol. Cell. Biol. 2004;24:4487–4501. doi: 10.1128/MCB.24.10.4487-4501.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi T., Hino S.-I., Oue N., Asahara T., Zollo M., Yasui W., Kikuchi A. Glycogen synthase kinase 3 and h-prune regulate cell migration by modulating focal adhesions. Mol. Cell. Biol. 2006;26:898–911. doi: 10.1128/MCB.26.3.898-911.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurayoshi M., Oue N., Yamamoto H., Kishida M., Inoue A., Asahara T., Yasui W., Kikuchi A. Expression of Wnt-5a is correlated with aggresiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res. 2006;66:10439–10448. doi: 10.1158/0008-5472.CAN-06-2359. [DOI] [PubMed] [Google Scholar]
- 22.Cong F., Schweizer L., Varmus H. Wnt signals across the plasma membrane to activate the β-catenin pathway by forming oligomers containing its receptors, Frizzled and LRP. Development. 2004;131:5103–5115. doi: 10.1242/dev.01318. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto H., Komekado H., Kikuchi A. Caveolin is necessary for Wnt-3a-dependent internalization of LRP6 and accumulation of β-catenin. Dev. Cell. 2006;11:213–223. doi: 10.1016/j.devcel.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto H., Ihara M., Matsuura Y., Kikuchi A. Sumoylation is involved in β-catenin-dependent activation of Tcf-4. EMBO J. 2003;22:2047–2059. doi: 10.1093/emboj/cdg204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okazaki M., Kishida S., Murai H., Hinoi T., Kikuchi A. Ras-interacting domain of Ral GDP dissociation stimulator like (RGL) reverses v-Ras-induced transformation and Raf-1 activation in NIH3T3 cells. Cancer Res. 1996;56:2387–2392. [PubMed] [Google Scholar]
- 26.Carragher N. O., Frame M. C. Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion. Trends Cell Biol. 2004;14:241–249. doi: 10.1016/j.tcb.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 27.Schaller M. D., Hildebrand J. D., Shannon J. D., Fox J. W., Vines R. R., Parsons J. T. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol. Cell. Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Webb D. J., Parsons J. T., Horwitz A. F. Adhesion assembly, disassembly and turnover in migrating cells – over and over and over again. Nat. Cell Biol. 2002;4:E97–E100. doi: 10.1038/ncb0402-e97. [DOI] [PubMed] [Google Scholar]
- 29.Smolich B. D., McMahon J. A., McMahon A. P., Papkoff J. Wnt family proteins are secreted and associated with the cell surface. Mol. Biol. Cell. 1993;4:1267–1275. doi: 10.1091/mbc.4.12.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burrus L. W., McMahon A. P. Biochemical analysis of murine Wnt proteins reveals both shared and distinct properties. Exp. Cell Res. 1995;220:363–373. doi: 10.1006/excr.1995.1327. [DOI] [PubMed] [Google Scholar]
- 31.He X., Saint-Jeannet J. P., Wang Y., Nathans J., Dawid I., Varmus H. A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science. 1997;275:1652–1654. doi: 10.1126/science.275.5306.1652. [DOI] [PubMed] [Google Scholar]
- 32.Young C. S., Kitamura M., Hardy S., Kitajewski J. Wnt-1 induces growth, cytosolic β-catenin, and Tcf/Lef transcriptional activation in Rat-1 fibroblasts. Mol. Cell. Biol. 1998;18:2474–2485. doi: 10.1128/mcb.18.5.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orford K., Orford C. C., Byers S. W. Exogenous expression of β-catenin regulates contact inhibition, anchorage-independent growth, anoikis, and radiation-induced cell cycle arrest. J. Cell Biol. 1999;146:855–868. doi: 10.1083/jcb.146.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamanaka H., Moriguchi T., Masuyama N., Kusakabe M., Hanafusa H., Takada R., Takada S., Nishida E. JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 2002;3:69–75. doi: 10.1093/embo-reports/kvf008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 36.Parsons J. T. Focal adhesion kinase: the first ten years. J. Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 37.Bruce-Staskal P. J., Bouton A. H. PKC-dependent activation of FAK and src induces tyrosine phosphorylation of Cas and formation of Cas–Crk complexes. Exp. Cell Res. 2001;264:296–306. doi: 10.1006/excr.2000.5137. [DOI] [PubMed] [Google Scholar]
- 38.Huang C., Rajfur Z., Borchers C., Schaller M. D., Jacobson K. JNK phosphorylates paxillin and regulates cell migration. Nature. 2003;424:219–223. doi: 10.1038/nature01745. [DOI] [PubMed] [Google Scholar]
- 39.Chen W., ten Berge D., Brown J., Ahn S., Hu L. A., Miller W. E., Caron M. G., Barak L. S., Nusse R., Lefkowitz R. J. Dishevelled 2 recruits β-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science. 2003;301:1391–1394. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- 40.Oishi I., Suzuki H., Onishi N., Takada R., Kani S., Ohkawara B., Koshida I., Suzuki K., Yamada G., Schwabe G. C., et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 41.Zhai L., Chaturvedi D., Cumberledge S. Drosophila wnt-1 undergoes a hydrophobic modification and is targeted to lipid rafts, a process that requires porcupine. J. Biol. Chem. 2004;279:33220–33227. doi: 10.1074/jbc.M403407200. [DOI] [PubMed] [Google Scholar]
- 42.Panakova D., Sprong H., Marois E., Thiele C., Eaton S. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature. 2005;435:58–65. doi: 10.1038/nature03504. [DOI] [PubMed] [Google Scholar]
- 43.Helenius A., Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291:2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- 44.Bänziger C., Soldini D., Schütt C., Zipperlen P., Hausmann G., Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signalling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 45.Bartscherer K., Pelte N., Ingelfinger D., Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Imperiali B., O'Connor S. E. Effect of N-linked glycosylation on glycopeptide and glycoprotein structure. Curr. Opin. Chem. Biol. 1999;3:643–649. doi: 10.1016/s1367-5931(99)00021-6. [DOI] [PubMed] [Google Scholar]