Abstract
Mammalian metallothioneins are redox-active metalloproteins. In the case of zinc metallothioneins, the redox activity resides in the cysteine sulfur ligands of zinc. Oxidation releases zinc, whereas reduction re-generates zinc-binding capacity. Attempts to demonstrate the presence of the apoprotein (thionein) and the oxidized protein (thionin) in tissues posed tremendous analytical challenges. One emerging strategy is differential chemical modification of cysteine residues in the protein. Chemical modification distinguishes three states of the cysteine ligands (reduced, oxidized and metal-bound) based on (i) quenched reactivity of the thiolates when bound to metal ions and restoration of thiol reactivity in the presence of metal-ion-chelating agents, and (ii) modification of free thiols with alkylating agents and subsequent reduction of disulfides to yield reactive thiols. Under normal physiological conditions, metallothionein exists in three states in rat liver and in cell lines. Ras-mediated oncogenic transformation of normal HOSE (human ovarian surface epithelial) cells induces oxidative stress and increases the amount of thionin and the availability of cellular zinc. These experiments support the notion that metallothionein is a dynamic protein in terms of its redox state and metal content and functions at a juncture of redox and zinc metabolism. Thus redox control of zinc availability from this protein establishes multiple methods of zinc-dependent cellular regulation, while the presence of both oxidized and reduced states of the apoprotein suggest that they serve as a redox couple, the generation of which is controlled by metal ion release from metallothionein.
Keywords: 7-fluorobenz-2-oxa-1,3-diazole-4-sulfonamide (ABD-F); metallothionein (MT); redox state; thionein; thionin; zinc signalling
Abbreviations: ABD-F, 7-fluorobenz-2-oxa-1,3-diazole-4-sulfonamide; AM, acetoxymethyl ester; DMF, dimethylformamide; DPBS, Dulbecco's PBS; DTNB, 5,5′-dithiobis-(2-nitrobenzoic acid); HOSE, human ovarian surface epithelial; 6-IAF, 6-iodoacetamidofluorescein; MT, metallothionein; MTF-1, metal-response-element-binding transcription factor-1; NEM, N-ethylmaleimide; T, apo-MT; TR, reduced apo-MT (thionein); TO, oxidized apo-MT (thionin); ABD-T, ABD-F-modified TR; TCEP, tris-(2-carboxyethyl)phosphine; TNB, 5-thio-2-nitrobenzoic acid; TPEN, N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine
INTRODUCTION
MT (metallothionein) was discovered as a protein binding cadmium and zinc and received its name on the basis of its high metal and sulfur content [1]. The analysis of the protein from horse kidneys gave 5.9% cadmium, 2.2% zinc, 0.2% iron and 0.1% copper, which corresponds to 4.0 g-atom of cadmium, 2.6 g-atom of zinc, 0.3 g-atom of iron and 0.1 g-atom of copper based on a metal complement of seven metal ions [2]. In mammalian livers, the protein contains mainly zinc [3], with the exception of neonatal livers and conditions of copper overload, where it contains significant amounts of copper [4,5]. The isolation of MT is largely on the basis of its metal content and therefore precludes the detection of other forms that do not contain metals. Because the isolated protein is often heterogeneous in its metal load and redox state, a defined species is obtained by preparing the apoprotein thionein (T) through acidification and reduction of MT and reconstitution with metal ions [6]. The crystal and solution structures of the mammalian cadmium/zinc protein or cadmium protein show 20 reduced cysteine residues bound to metal ions in two metal–thiolate clusters [7,8]. Functions of Zn–MT are based on reversible dissociation of its zinc ions and oxido-reduction (redox) of its sulfur ligands [9]. In experiments with the isolated protein, dissociation of the metal ions from MT under non-oxidative and oxidative conditions leads to the reduced (thionein, TR) and oxidized (thionin, TO) proteins respectively. However, it is unknown which species of the protein are produced under these conditions in vivo. Evidence for the existence of T came from studies of neoplastic cell lines [10] and rat tissues, in which commensurate amounts of MT and T were found by a differential modification assay [11]. This assay is based on the observation that zinc quenches the reactivity of cysteine residues with thiol-modifying agents, whereas removal of zinc from MT with chelating agents restores the reactivity. However, the assay cannot detect oxidized states of the protein because cysteine labelling with ABD-F (7-fluorobenz-2-oxa-1,3-diazole-4-sulfonamide) must be performed in the presence of the reducing agent TCEP [tris-(2-carboxyethyl)phosphine]. 6-IAF (6-iodoacetamidofluorescein) reacts with thiols in the absence of a reducing agent. When 6-IAF is employed in a double-differential modification assay in the absence or presence of EDTA and TCEP, all three species (MT, TR and TO) can be measured, thus distinguishing the reduced, oxidized and zinc-bound states of the cysteine side chain [12]. A partially oxidized disulfide-linked protein was isolated from mouse hearts that overexpress MT. Its amount increases when the heart is subjected to oxidative stress by the chemotherapeutic agent doxorubicin [13]. A full speciation and quantitative characterization of the redox state and metal load of the protein has yet to be achieved. When the differential modification assays are employed for speciation of MT in normal tissue, all three states of the protein are found in rat liver under normal physiological conditions. Mild oxidative stress, such as is generated by transformation of cells with the rasv12 oncogene, increases total and oxidized protein and available zinc in cultured cells. Thus it became possible to determine the zinc-binding capacity of MT (MT/TR ratio) and its redox state (TR/TO ratio). These measurements provide information on the availability of cellular zinc as a function of the cellular redox state, and vice versa, on the possible role of MT as a precursor of the TR/TO redox pair.
MATERIALS AND METHODS
Materials
Zn–MT and T were prepared from rabbit liver MT II (MT-2) (Sigma) [6]. ABD-F, FluoZin-3 AM (acetoxymethyl ester) and TCEP hydrochloride were purchased from Invitrogen. Trizma base (Tris), disodium salt of EDTA, TPEN [N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine], 1-hydroxypyridine-2-thione (pyrithione, sodium salt), NEM (N-ethylmaleimide), DTNB [5,5′-dithiobis(2-nitrobenzoic acid)], Zincon (sodium salt), glutathione disulfide (sodium salt), diethyl ether and anhydrous DMF (dimethylformamide) were from Sigma–Aldrich. Hepes (OmniPur) and nitric acid (OmniTrace Ultra) were from Merck KGaA. D-Mannitol, KCl, NaOH, boric acid and HCl were from Fisher. Sucrose was from Mallinkrodt. Chelex® 100 resin (100–200 mesh, sodium form) was from Bio-Rad.
Preparation of TO
TO was prepared by chemical oxidation of MT-2 (15 μM) with DTNB (150 μM) in 20 mM Tris/HCl and 20 mM EDTA, pH 8.0, at 25 °C under aerobic conditions for 20 min [12]. TO was purified by gel-filtration on a Sephadex G-25 (Amersham Biosciences) column (1.0 cm×25.0 cm), equilibrated and eluted by 5 mM Tris/HCl, pH 7.4. Absorbance readings at 412 nm yield the concentration of TNB (5-thio-2-nitrobenzoic acid) ions, while TO was detected at 220 nm and its concentration was determined by HPLC separation and fluorimetric analysis after its reduction with TCEP (see below). TO prepared under these conditions contains mostly intramolecular disulfides based on negligible absorbance at 325 nm, the wavelength at which intermolecular disulfides between MT and TNB absorb [14].
Sample preparation for HPLC and determination of MT, TR and TO
Buffers were treated overnight with Chelex® resin. An aliquot (approx. one-sixth) of a fresh rat liver was cut and homogenized on ice in 4 vol. of buffer (0.2 M mannitol, 50 mM sucrose, 10 mM KCl and 10 mM Hepes/Na+, pH 7.4) with 15 strokes in a Potter–Elvehjem homogenizer (Teflon pestle with a clearance of less than 0.1 mm). Homogenates were spun at 10000 g for 10 min at 4 °C. Acetonitrile was added to the supernatant to a final concentration of 40% (v/v). Samples were kept at room temperature (23 °C) for 10 min and spun at 18000 g for 10 min at 4 °C. For derivatization of thiols and determination of total MT (MT+T), 50 μl of sample was mixed with 5 μl of 0.5 M EDTA, pH 8.0, and 5 μl 0.3 M TCEP hydrochloride in 1.0 M Hepes, pH 7.4, 5 μl of ABD-F solution (40 mM ABD-F stock solution in anhydrous DMF; 20 mM for tissues other than liver) and 0.1 M borate buffer, pH 7.4, to yield a final volume of 100 μl [11]. After 6 min of incubation at 50 °C, samples were spun at 14000 g for 5 min. The supernatant was kept at 4 °C and analysed within hours. The same procedure without addition of EDTA was employed for analysis of T, the sum of TR and TO. MT is then calculated as the difference between the two measurements, i.e. (MT+T)−T=MT [11]. TO was determined by blocking all reduced thiols with NEM in the presence of EDTA. To this end, a third aliquot of the sample (50 μl) was mixed with 5 μl of 0.5 M EDTA, pH 8, 5 μl of 100 mM NEM and 30 μl of borate buffer (see above), incubated for 15 min at 37 °C, extracted ten times with 2 vol. of diethyl ether to remove the excess of NEM, and 20 μl of acetonitrile was added to compensate for the amount of acetonitrile lost during extraction. The sample was then derivatized with 2 mM ABD-F in the presence of 15 mM TCEP, incubated for 6 min at 50 °C, and kept at 4 °C for analysis within hours. Residual diethyl ether was removed before derivatization by blowing nitrogen gas over the sample for 2 min at 37 °C. Every sample was run in triplicate. The amount of TO was then used to determine TR, i.e. T−TO=TR.
HPLC analysis
Samples were kept at 4 °C in the autosampler and analysed by separation on a reversed-phase C4 300 Å (1 Å=0.1 nm) Phenomenex Jupiter 5μ column (250 mm×4.6 mm) with an Alltech precolumn, using a Beckman Coulter System Gold HPLC with a Jasco Intelligent Fluorescence detector model 2020 at 25 °C (excitation and emission wavelengths: 384 and 512 nm; mobile phase: A, 5 mM Tris/HCl, pH 7.4; B, 50% 2-propanol in A; flow-rate: 1 ml/min; 90% A for 5 min, followed by a step gradient to 40% B for 7 min; 100% B for 5 min, and equilibration with 90% A). 32 Karat™ Software was used for chromatographic data acquisition and processing.
Cell culture and incubation
HOSE (human ovarian surface epithelial) T29 cells and the same cells transformed with an H-rasv12 oncogene (T29H) were obtained from Dr Xiaodong Cheng (University of Texas Medical Branch) [15]. Cells were maintained at 5% CO2 and 37 °C in a humidified atmosphere. Cells were cultured in DMEM (Dulbecco's modified Eagle's medium) with high glucose, L-glutamine and pyridoxine hydrochloride (Gibco) with 10% (v/v) foetal bovine serum, grade defined (Hyclone), 0.1 mg/ml penicillin G sodium and 0.1 mg/ml streptomycin sulfate. The cells, approx. 85% confluent, were scraped from the surface using a cell scraper with an 1.8 cm rubber blade (Falcon) or trypsinized [0.25% (v/v) trypsin solution without EDTA] and homogenized (same procedure as above).
Measurements of cellular available ‘free’ zinc
Cells at approx. 85% confluence were trypsinized, and detached cells were collected by centrifugation at 1000 g for 5 min, washed three times with cold DPBS (Dulbecco's PBS) without calcium and magnesium salts, and incubated with 1 μM FluoZin-3 AM for 30 min at 37 °C in the same buffer. Cells were spun at 1000 g for 5 min and washed three times with 10–15 vol. of DPBS. Cells were counted with a haemocytometer. Fluorescence (F) of 1×106 cells was measured with an SLM 8000 spectro-fluorimeter with ISS data acquisition and Vinci Multidimensional Fluorescence Spectroscopy software (25 °C, 492 nm excitation, 517 nm emission). Measurements are based on a two-point calibration by incubating the cells for 10 min with 200 μM TPEN to yield Fmin and then with 250 μM zinc sulfate and 100 μM pyrithione (sodium salt) to yield Fmax. Fractional saturation (FS) was calculated according to the equation: FS=(F−Fmin)/(Fmax−Fmin) and is a measure of the availability of free zinc.
Spectrophotometric assays
Transfer of zinc from Zn–MT to the chromophoric chelating agent Zincon was measured at 620 nm (Zincon assay). MT (1.2 μM) was mixed with 2.4 μM TO, 24 μM GSSG or 24 μM DTNB in the presence of 100 μM Zincon in 50 mM Hepes/Na+, pH 7.4. Total thiol groups of MT and TR were analysed by reaction with 1 mM DTNB in 50 mM Hepes/Na+, pH 7.4 [ϵ412 (TNB)=14150 M−1·cm−1] [16]. Total protein concentration of T29 cellular homogenates was measured with bicinchoninic acid (Pierce Biotechnology) using BSA as a standard [17]. Absorption readings were taken at 25 °C using a Beckman Coulter DU 800 spectrophotometer.
Measurement of total zinc
Cells (2–6×106) were washed with DPBS, collected by centrifugation at 1000 g for 5 min, homogenized with 4 vol. of homogenization buffer (see above) without adding acetonitrile. An aliquot of the extract was set aside for total protein analysis. The homogenate was digested by adding 100 μl of concentrated nitric acid and incubating at 80 °C until all the liquid evaporated. The residue was dissolved in 50 μl of nitric acid and diluted to 3 ml with Milli-Q water (Millipore Corp.). Samples were analysed by flame atomic absorption spectrophotometry (PerkinElmer 5100 instrument).
RESULTS
Differential labelling of MT and T
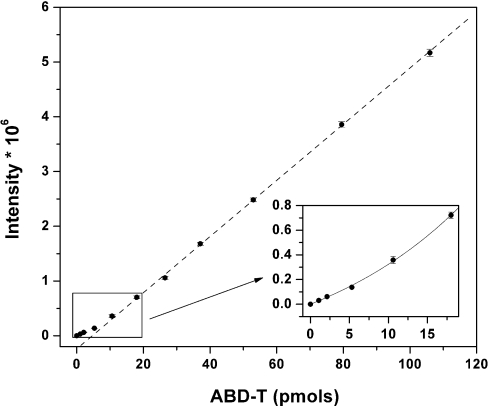
Chemical modification is a common approach to investigate the role of cysteine residues in protein structure and function [18]. For MT, differential labelling distinguishes between the metal-bound thiolates in MT and the free thiols in T [11]. Strong chelating agents release zinc from its co-ordination environments in proteins. When cysteine is a ligand, unbound cysteine generally has a higher reactivity towards reactive probes such as alkylating agents. This differential chemical reactivity was employed in studies of MT [11]. The fluorigenic agent ABD-F reacts with T in the presence of the reducing agent TCEP, whereas, in the absence of EDTA, its reactivity with MT is very low. TCEP reduces disulfides rapidly. The use of this phosphine-based reducing agent is critical for the labelling of T. The most common reducing agents are thiols. They react with ABD-F or bind zinc ions and thus interfere with the analysis. Zinc binding to TCEP is very weak [19]. At pH 7.4 and 50 °C in the presence of EDTA, the reactions of all three isoforms, MT-1, MT-2 and MT-3, with ABD-F are complete within 5–6 min, yielding a derivative ABD-T (ABD-F-modified TR) with strong fluorescence at 512 nm when excited with 384 nm light [11]. The fluorescence spectra and elution times of the derivatives of T and MT+EDTA are identical, indicating that the same labelling product is obtained in both instances. The fluorimetric HPLC assay is exquisitely sensitive with a detection limit below 10 pmol [11]. At concentrations below 20 pmol (M)T, the relationship between peak area and the amount of (M)T is non-linear (Figure 1).
Figure 1. Calibration curve for the total amount of MT and the fluorescence of the ABD-T derivative.
The curve was obtained by serial dilutions of purified Zn-MT and modification with ABD-F in the presence of TCEP and EDTA. Protein concentrations are based on spectrophotometric titrations of thiols in MT with DTNB. Fluorescence intensity is a linear function of concentration over a wide range, but, below 20 pmol, the response is non-linear (inset).
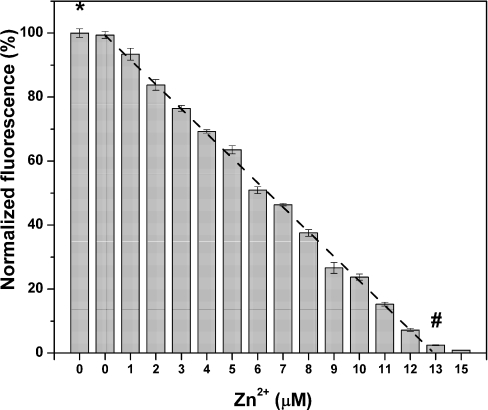
Titration of TR with zinc ions and analysis of the remaining T (ABD-F modification without EDTA) demonstrates a stoichiometric reaction between apoprotein and zinc from one to seven zinc ions. The reactivity of TR is quenched completely when the stoichiometry of Zn/TR reaches seven (Figure 2). The same amount of TR is measured in the presence and absence of EDTA (first and second bar), demonstrating that the preparation of T does not contain zinc. The concentration of T based on titration with zinc sulfate is in excellent agreement with the concentration of thiols obtained from an assay of T with DTNB (Figure 2). These data demonstrate that the amount of TR can be determined reliably over the entire range of MT/TR ratios.
Figure 2. Titration of TR with zinc sulfate.
Final concentrations: 1.83 μM T, 0.1 M sodium borate, pH 7.4, 2 mM ABD-F, 15 mM TCEP and 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 and 15 μM Zn(II). The first bar (*) represents the average fluorescence of T labelled in the presence of 25 mM EDTA. Data are given as percentages of fluorescence relative to that of T modified in the absence of added zinc (n=3). The concentration of T (1.83 μM) based on the titration end point (#) is in excellent agreement with the concentration obtained from the DTNB assay (1.85 μM).
Differential labelling of TR and TO
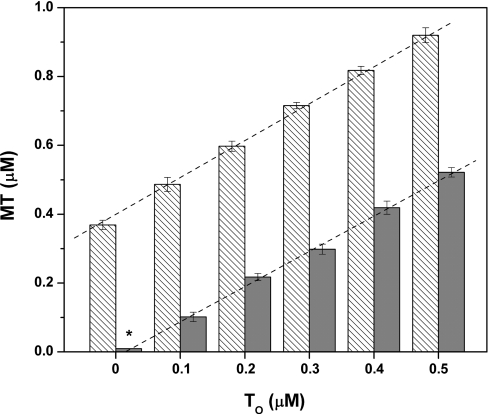
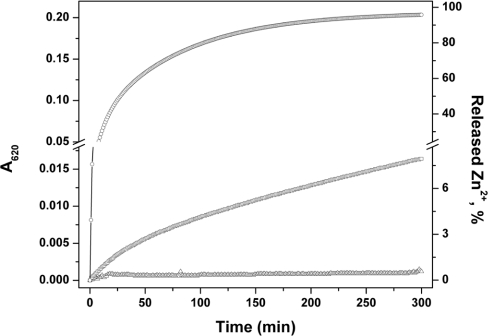
Chemical modification of free thiols in the presence of a reductant such as TCEP does not allow determination of the original oxidation state of the thiols in the protein. The differential labelling in the presence and absence of EDTA measures total protein (MT+TR+TO) and total apoprotein (TR+TO). By difference calculation, one obtains the amount of MT. To determine the amount of oxidized protein (thionin, TO), a double-differential labelling procedure was developed. Free cysteines were first alkylated with NEM in the presence of EDTA, any remaining disulfides were reduced with TCEP, the excess of NEM was extracted with diethylether, and then the newly formed free cysteines were labelled with ABD-F. Mixtures of MT and various amounts of chemically fully oxidized TO were analysed in the absence of EDTA (Figure 3). Zn–MT used for this experiment was stored at −20 °C in nitrogen gas-saturated 20 mM Tris/HCl, pH 7.4, at micromolar concentrations for a few months and contained approx. 2±1% disulfides (Figure 3, asterisk). Stepwise addition of chemically oxidized protein (TO) by increments of 0.1 μM increased the total protein concentration by exactly 0.1 μM. Thus the oxidized protein could be determined quantitatively in the presence of MT. The Zincon assay was used to test whether TO reacts with MT and releases zinc. Thiol/disulfide exchange occurs between MT and DTNB or glutathione disulfide with concomitant zinc release (Figure 4), but TO does not release any zinc within 5 h (Figure 4).
Figure 3. Titration of MT with chemically oxidized TO.
MT was analysed together with TO in the presence of EDTA (hatched bars). MT (0.37 μM) was mixed with 0.1–0.5 μM TO. TO (grey bars) was analysed in the presence of MT after incubation with NEM in the presence of EDTA. Approx. 2% of the oxidized product was detected in MT stored at −20 °C at pH 7.4 for several months (*).
Figure 4. Kinetics of zinc release from MT by disulfides.
MT (1.2 μM) was incubated with 100 μM Zincon in 50 mM Hepes/Na+, pH 7.4, in the presence of 24 μM DTNB (○), 24 μM GSSG (□) or 2.4 μM TO (△). Zinc release was followed spectrophotometrically by measuring the formation of the Zn–Zincon complex at 620 nm (25 °C).
Metal load and redox states of MT in rat liver
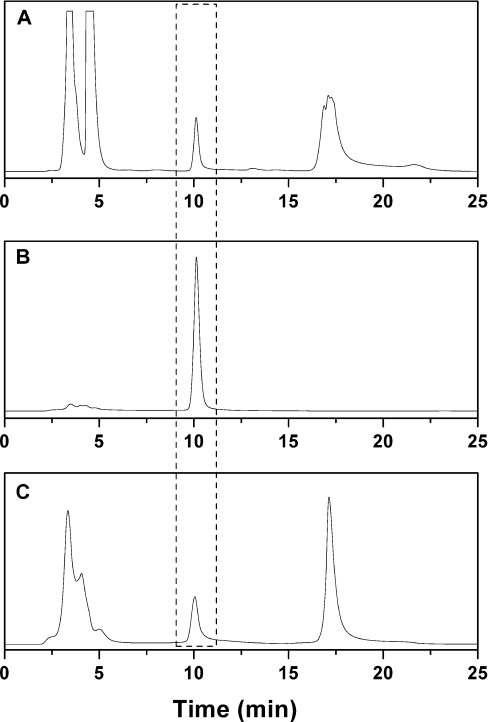
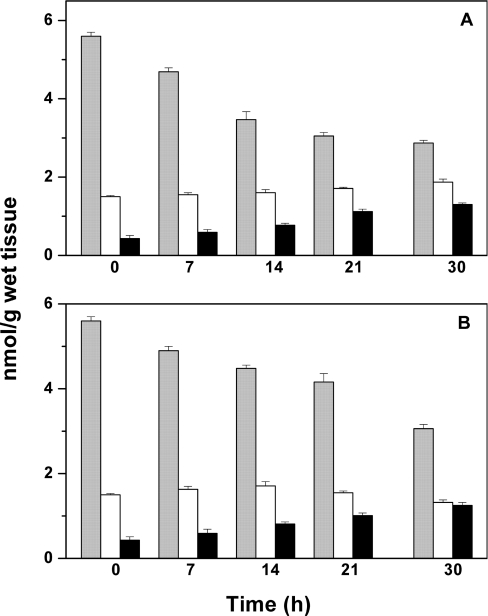
Livers contain relatively large amounts of MT and therefore are used frequently for MT isolation and characterization. It is mandatory to employ fresh liver for the assay as the distribution of species changes during storage of tissue. When rat liver homogenates are kept on ice, MT decreases and T increases [11]. In order to determine whether the oxidation state of the protein changes in this process, a liver homogenate, prepared immediately after the animal was killed, was analysed with the double-differential labelling assay to determine MT, TR and TO. The chromatographic peak of ABD-T from liver homogenates (Figure 5A) is well separated from those of the ABD derivatives of low-molecular-mass thiols, which like MT are not precipitated with acetonitrile [20]; it also occurs at the same retention time and with the same peak shape as purified MT (Figure 5B). Double-differential labelling (with NEM and ABD-F) demonstrates the presence of the oxidized protein in rat liver besides MT and TR (Table 1). TO, 0.4±0.1 nmol/g of wet tissue, amounts to 27% of total apoprotein (TO+TR) and to approx. 7% of total metallothionein (MT+TO+TR). The homogenate was kept on ice and analysed again after 7, 14, 21 and 30 h (Table 1 and Figure 6A). An aliquot of the tissue was also kept on ice and analysed after the same periods of time (Table 1 and Figure 6B). When the liver (homogenate) was kept on ice for 30 h, the total concentration of MT decreases to 51% (55%) of its initial value, while that of the total apoprotein (TO+TR) increases from 27 to 65% (43%) (Figure 6 and Table 1). In both cases, the concentration of TO increases linearly to three times the initial value (black bars in Figure 6). The reduced form, TR (difference between white and black bars) decreases in the intact tissue from the beginning, but a substantial amount of TR exists after 30 h, suggesting that, in the intact tissue, redox metabolism can maintain the apoprotein in the reduced form. In the extract, however, no TR is left after 30 h (Table 1).
Figure 5. Chromatograms for the analyses of total MT by the ABD-F labelling method.
(A) Rat liver homogenate, (B) purified MT and (C) HOSE cells (the same cells transformed with the oncogene h-rasv12 gave identical results). The broken lines identify the peak corresponding to ABD-T.
Table 1. Metal load and redox states of MT in rat liver.
| Sample | Total MT (nmol/g of tissue) | T/total MT (%) | MT (nmol/g of tissue) | TR (nmol/g of tissue) | TO (nmol/g of tissue) |
|---|---|---|---|---|---|
| Liver 0 h | 5.6±0.1 | 27 | 4.1±0.1 | 1.1±0.1 | 0.4±0.1 |
| Liver 7 h | 4.7±0.1 | 33 | 3.1±0.1 | 1.0±0.1 | 0.6±0.1 |
| Liver 14 h | 3.5±0.2 | 46 | 1.9±0.3 | 0.8±0.1 | 0.8±0.1 |
| Liver 21 h | 3.0±0.1 | 56 | 1.3±0.1 | 0.6±0.1 | 1.1±0.1 |
| Liver 30 h | 2.9±0.1 | 65 | 1.0±0.2 | 0.6±0.1 | 1.3±0.2 |
| Extract 7 h | 4.9±0.1 | 33 | 3.3±0.2 | 1.0±0.2 | 0.6±0.1 |
| Extract 14 h | 4.5±0.1 | 38 | 2.8±0.2 | 0.9±0.1 | 0.8±0.1 |
| Extract 21 h | 4.2±0.2 | 38 | 2.6±0.2 | 0.6±0.1 | 1.0±0.1 |
| Extract 30 h | 3.1±0.1 | 43 | 1.7±0.2 | 0.1±0.1 | 1.3±0.1 |
Figure 6. Stability of MT in rat liver.
Tissue was homogenized and labelled as described in the Materials and methods section. (A) Liver. (B) Homogenate. The liver was kept on ice for the same period of time and analysed in the same way as the homogenate. Three assays allow determination of MT+TR+TO (total metallothionein) (grey bars) (i), TR+TO (total apoprotein) (white bars) (ii), and TO (black bars) (iii). Concentrations of individual species are obtained by subtracting bars (ii) from (i) (MT) or (iii) from (i) (TR) (see Table 1).
MT and zinc availability in human ovarian surface epithelial cells
Homogenates of HOSE cells analysed in the same way also demonstrate a well-separated peak at the same retention time as in the liver homogenate (Figure 5C). Transformation of HOSE T29 cells with the Ras oncogene generates the cell line T29H with increased oxidative stress and an antioxidant response [15]. Therefore these cell lines were employed for an investigation of whether or not oxidative stress affects the amount of TO and zinc release. In the ras-transformed cells, the concentration of total apoprotein is slightly higher (27% of total protein) than in the control T29 cells (20%), while the concentration of TO is about three times higher (Table 2). Differences in the concentrations of MT and TR are not statistically significant.
Table 2. Metal load and redox states of MT in normal T29 and transformed T29H cells.
| Cell line | Total MT (nmol/mg of protein) | T/total MT (%) | TO/total MT (%) | MT/TR | MT (nmol/mg of protein) | TR (nmol/mg of protein) | TO (nmol/mg of protein) |
|---|---|---|---|---|---|---|---|
| T29H | 0.30±0.04 | 27±6 | 20±7 | 11.0±1.3 | 0.22±0.04 | 0.02±0.01 | 0.06±0.02 |
| T29 | 0.25±0.03 | 20±8 | 8±4 | 6.7±0.7 | 0.20±0.04 | 0.03±0.01 | 0.02±0.01 |
Oxidative or nitrosative stress releases zinc from proteins with zinc–thiolate (Zn–SCys) co-ordination environments. Therefore it was tested whether or not the more oxidized redox state of MT in T29H cells is associated with an increase in the availability of cellular zinc. The available free zinc was measured by loading the cells with a membrane-permeable fluorescent zinc-chelating agent and determining its saturation with zinc after proper calibration of fluorescence [21]. The fractional saturation of the dye, a measure of free zinc (see the Materials and methods section), was 25% higher in ras-transformed cells than in the control cells, corresponding to 8.9±0.7% saturation of the dye with zinc. Whereas available free zinc increases, the total zinc concentration in both cells is the same (4.1±0.2 compared with 4.2±0.4 nmol of Zn/mg of protein for transformed and control cells respectively).
DISCUSSION
Three species and three states of metallothionein
Metallothionein with seven bivalent metal ions as determined from structural analyses in crystals and solution [7,8] is only one of several physiologically significant forms of the protein. The apoform, thionein, was detected by both invasive and non-invasive methods in neoplastic cell lines [10], different tissues [11] and cells that overexpress ‘metallothionein’ [22]. The results of the present study confirm and amplify the observation of a third form with oxidized cysteine sulfurs [12]. Because of this multiplicity, the term ‘metallothionein’ is ambiguous. Therefore ‘MT’ will be used in this Discussion whenever the state of the protein in vivo is not defined. The differential modification assays [11,12] determine three states of thiols: free, metal-bound and disulfide. It is unknown whether the three states detected by these assays in tissues and cells are identical with the three defined species that can be prepared in vitro: MT with seven zinc ions and two species of the apoprotein, fully reduced (thionein, TR) and fully oxidized (thionin, TO). The differential modification assays cannot distinguish between one partially oxidized species and a mixture of two species, one reduced and one oxidized. However, the known properties of isolated MT provide aid in interpreting the results obtained with the differential modification assays. TR and TO form a redox pair, whose ratio depends on the cellular redox potential. Therefore a physiological condition under which only either fully reduced or fully oxidized apoprotein exists is most unlikely. TR binds zinc ions with high co-operativity [23]. Hence stable intermediates with different metal loads and free cysteines are not thought to exist for MT-1 and MT-2, and MT and TR can coexist. Whether TO can also coexist with MT and/or TR was unknown. Disulfides release zinc from MT [24] and therefore disulfide-bridged TO could potentially react with MT and release zinc. However, TO does not release zinc from MT (Figure 4), indicating that MT and TO can coexist as well. Intermediate states between TR and TO with a defined number of disulfides were also never observed. At present, there is no technique to study the interaction between TR and TO. Until resolution of this issue becomes possible, the interpretation is favoured that minimally two species exist, the holoprotein and the apoprotein with either only the apoprotein or both species being partially oxidized. The characteristics of MT-3 provide additional information about the chemical activities of MT. MT-3 is a growth-inhibitory factor, a biological activity that it does not share with MT-1/2. It binds more than seven metal ions with apparently less co-operativity than MT-1/2 [25]. Evidence has been found for a partially oxidized and partially metallated species [26]. In a mixed copper/zinc species of MT-3, one zinc atom in the C-terminal domain is redox-labile, generating a disulfide-bridged Cu3Zn3 form from the Cu3Zn4 form. However, the different characteristics of MT-3 would seem to preclude drawing conclusions as to the states of MT-1/2.
Oxidized forms of ‘MT’ were observed under conditions of cadmium and copper overload [27–29], both conditions that elicit considerable oxidative stress. By direct isolation and mass spectrometric characterization, disulfides in MT were detected in mouse hearts that overexpress MT [13]. The amount of oxidized protein increases when the heart is subjected to oxidative stress with the anticancer drug doxorubicin [13]. Although this work demonstrates the presence of oxidized protein under this specific condition, quantification of either the oxidation state or the metal load was not achieved. Because a single species was not found, the authors conclude that disulfides are formed randomly in the C-terminal domain of the protein during oxidation [13]. Structurally, disulfide formation in metallothionein/thionein is potentially extremely complex, although one could argue that the proximity of cysteines in the clusters of MT provides a blueprint for the preferred formation of certain disulfides [9]. With 20 cysteines, 654729075 species with different intramolecular disulfide bonds could form [30]. To complicate matters further, an intermolecular disulfide in MT-1 has been characterized [31]. Thus, while the molecular speciation is more complex, our results demonstrate that the protein does not occur in just the one state known from structural studies, but instead in at least three different states that differ in metal load and oxidation of cysteine sulfur ligands.
Redox control of MT/T
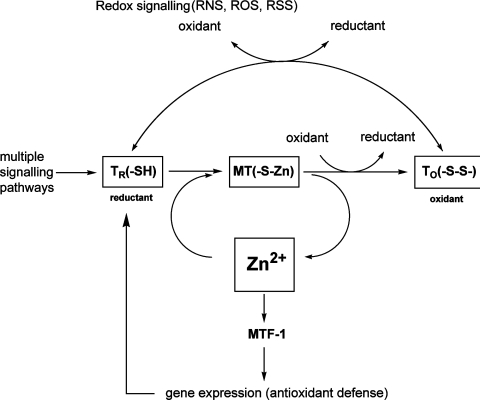
Zinc release by oxidative signalling puts MT at a nodal point of zinc and redox signalling (Scheme 1). MT transduces redox signals to zinc signals and vice versa [32,33]. Reactive species such as nitric oxide and peroxide release zinc from MT and increase the amount of available free zinc. Free zinc is a potent signal that affects cellular signalling with multiple effects on the physiology of a cell, expresses either pro-antioxidant or pro-oxidant functions [34] and binds to MTF-1 (metal-response-element-binding transcription factor-1), activating transcription of genes involved in regulating cellular zinc homoeostasis and antioxidant responses [35].
Scheme 1. MT-dependent zinc and redox regulation in cellular signalling.
Major cellular signalling pathways lead to the induction of TR. The zinc binding of its thiols is redox-dependent, allowing redox control of the availability of cellular zinc for multiple cellular functions. Thus, depending on the redox state and the level of oxidative stress of reactive oxygen, nitrogen and sulfur species (ROS, RNS and RSS respectively), TR either binds zinc to form MT or is oxidized to TO, which does not bind zinc. Reactive species formed under oxidative stress can also convert MT into TO directly and release zinc. Released zinc binds to MTF-1 and induces the transcription of genes involved in antioxidant defence, including TR. In this manner, zinc can control the reducing capacity of TR, a potent cellular reductant owing to its 20 thiols, at both the protein and gene level.
Multiple inducers of T link the regulation of this protein to major cellular signalling pathways. In this way, the cellular signalling network exerts control over T, which in turn controls the availability of zinc for gene expression in a redox-sensitive manner. Owing to its chelating and reducing capacity, T can either quench zinc signals or participate in the reduction of redox-active disulfides/sulfenic acids in proteins. Seen in this light, MT, i.e. the zinc-bound form, is the precursor of a thiol/disulfide-based redox system. Because of this redox link and the potent action of intracellularly released zinc in the picomolar range [21], it is by no means straightforward to distinguish between zinc and a redox signal, suggesting that wherever oxidative signalling is involved, the possibility of zinc regulation should be investigated.
In contrast with the MT disulfides that were found in the overexpressed protein and in MT from the oxidatively stressed heart [13], the present paper reports both the presence of TO under normal physiological conditions in the liver and in cultured cells and an increase of TO under a different paradigm of oxidative stress. When compared with untransformed cells, concentrations of TO are higher in HOSE (T29) cells transformed with an oncogenic allele of H-rasv12. Such transformed cell lines serve as a model for investigating cellular signalling of individual oncogenes [36]. Transformation with the Ras oncogene causes a sustained production of superoxide ions [37] and elicits an antioxidant response [15]. The results demonstrate that the change of the TR/TO ratio is accompanied by an increase in the availability of zinc. Thus, in addition to disulfide- and NO-induced release of zinc from MT [38–40], superoxide ions also change the availability of zinc, demonstrating that ‘MT’ participates in other oxidative signalling pathways. Superoxide ions could effect these changes by reacting with MT or TR, or with both. Reaction with either TR or MT will change the availability of zinc because the MT/TR ratio determines the metal-binding capacity of ‘MT’. It is now appreciated that minute changes of the availability of zinc have profound physiological effects. Thus the free zinc concentration in intestinal epithelial cells fluctuates in the picomolar range [21]. In cardiomyocytes of normal rats, it is 520 pM while it is 870 pM in those of diabetic rats [41]. The results suggest that two redox reactions, which may involve the same or different redox pairs, control the redox state of ‘MT’ and hence the availability of zinc from ‘MT’ (Scheme 2). One redox reaction is the oxidation of MT and the other is the conversion between TO and TR. The identification of TR as a cofactor for methionine sulfoxide reductase in a reaction coupled to thioredoxin supports the physiological functions of a TR/TO-based redox system [42].
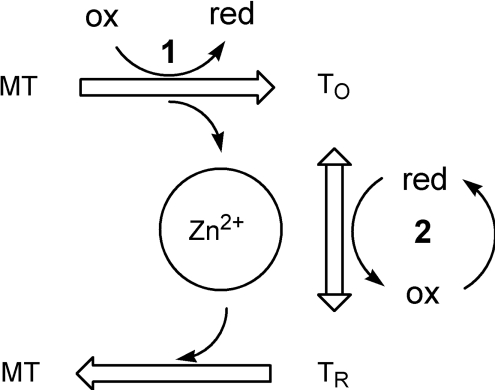
Scheme 2. Redox biochemistry of MT.
MT, the zinc-loaded form, is oxidized to TO by redox couple 1. The oxidation releases zinc, thus increasing the available zinc, which can affect multiple cellular processes. Redox couple 2, which can be different from redox couple 1, effects conversion between TO and TR and can also modulate the availability of zinc. ox, oxidation; red, reduction.
Interaction of MT with other essential metal ions
Usually, MT contains only trace amounts of other transition metals [2]. Because of the kinetic lability of zinc in MT in exchange reactions with other metal ions, it is unknown whether these metal ions were taken up during isolation or were already present in MT in vivo. Under specific conditions, copper accumulates in MT [4]. MTs containing either seven ferrous ions [43] or 12 cupric ions [44] can be prepared in vitro. Whether or not MT binds iron in vivo under conditions of iron overload has not been investigated. Iron(II) binds significantly less tightly to MT than zinc, which in turn binds less tightly than copper(I). The relative affinities of these metal ions for sulfur ligands are well established from inorganic thiolate complexes [45–47]. Therefore, for iron MT to form in vivo, either a large excess of iron to displace zinc from MT or a virtual absence of zinc would be required. It was suggested that autophagocytosis of MT may lead to its binding of iron in lysosomes, suppressing peroxidative reactions initiated by free iron [48]. Although there is evidence for autophagocytosis of oxidized forms of MT [4,29], it is unclear whether or not oxidized MT would be reduced in lysosomes to restore its chelating ability because recent evidence suggests an oxidative environment in lysosomes [49]. However, MT is taken up by endocytosis and releases its metal in lysosomes for translocation to the cytosol [50]. If metal release occurs by a non-oxidative mechanism in lysosomes, and if there is a relative large excess of iron compared with other metal ions that bind more strongly to T, then T could serve a purpose in iron binding. A function of MT/T in iron metabolism in cellular compartments that are rich in iron may deserve further investigations. In any event, MT exerts its antioxidant properties via at least two mechanisms, the binding of redox-active metal ions and the reducing and radical-scavenging capacity of its 20 cysteine ligands.
In conclusion, the results support the hypothesis that changes of the redox state of ‘MT’ modulate the availability of zinc and possibly other coupled redox reactions [32]. The state of the protein can be determined in terms of its zinc load (MT/TR ratio) and its oxidation state (TR/TO ratio). The presence of more than one species of ‘MT’ in tissues and cells supports dynamic functions of the protein at the crossroads of metal (zinc) and redox metabolism.
Acknowledgments
This work was supported by the National Institutes of Health Grant GM 065388 to W.M. and a sponsored research agreement with NeuroBioTex, Inc., Galveston, TX, U.S.A. We thank Dr V.M. Sadagopa Ramanujam, Associate Professor, Department of Preventive Medicine and Community Health, The University of Texas Medical Branch, for metal analyses by atomic absorption spectrophotometry (supported by the Human Nutrition Research Facility) and Dr Wen-Hao Yu for discussions, and Dr Xiaodong Cheng for providing us with the HOSE cell lines.
References
- 1.Margoshes M., Vallee B. L. A cadmium protein from equine kidney cortex. J. Am. Chem. Soc. 1957;79:4813. [Google Scholar]
- 2.Kägi J. H. R., Vallee B. L. Metallothionein: a cadmium and zinc-containing protein from equine renal cortex. J. Biol. Chem. 1961;236:2435–2442. [PubMed] [Google Scholar]
- 3.Kägi J. H. R. Evolution, structure, and chemical activity of class I metallothioneins: an overview. In: Suzuki K. T., Imura N., Kimura M., editors. Metallothionein III. Switzerland: Birkhäuser; 1993. pp. 29–55. [Google Scholar]
- 4.Ryden L., Deutsch H. F. Preparation and properties of the major copper-binding component in human fetal liver: its identification as metallothionein. J. Biol. Chem. 1978;253:519–524. [PubMed] [Google Scholar]
- 5.Johnson G. F., Morell A. G., Stockert R. J., Sternlieb I. Hepatic lysosomal copper protein in dogs with an inherited copper toxicosis. Hepatology. 1981;1:243–248. doi: 10.1002/hep.1840010309. [DOI] [PubMed] [Google Scholar]
- 6.Vašák M. Metal removal and substitution in vertebrate and invertebrate metallothioneins. Methods Enzymol. 1991;205:452–458. doi: 10.1016/0076-6879(91)05130-n. [DOI] [PubMed] [Google Scholar]
- 7.Robbins A. H., McRee D. E., Williamson M., Collett S. A., Xuong N. H., Furey W. F., Wang B. C., Stout C. D. Refined crystal structure of Cd, Zn metallothionein at 2.0 Å resolution. J. Mol. Biol. 1991;221:1269–1293. [PubMed] [Google Scholar]
- 8.Arseniev A., Schultze P., Wörgötter E., Braun W., Wagner G., Vašák M., Kägi J. H. R., Wüthrich K. Three-dimensional structure of rabbit liver [Cd7]metallothionein-2a in aqueous solution determined by nuclear magnetic resonance. J. Mol. Biol. 1988;201:637–657. doi: 10.1016/0022-2836(88)90644-4. [DOI] [PubMed] [Google Scholar]
- 9.Maret W., Vallee B. L. Thiolate ligands in metallothionein confer redox activity on zinc clusters. Proc. Natl. Acad. Sci. U.S.A. 1998;95:3478–3482. doi: 10.1073/pnas.95.7.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pattanaik A., Shaw C. F., III, Petering D. H., Garvey J., Kraker A. J. Basal metallothionein in tumors: widespread presence of apoprotein. J. Inorg. Biochem. 1994;54:91–105. doi: 10.1016/0162-0134(94)80023-5. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y., Maret W., Vallee B. L. Differential fluorescence labeling of cysteinyl clusters uncovers high tissue levels of thionein. Proc. Natl. Acad. Sci. U.S.A. 2001;98:5556–5559. doi: 10.1073/pnas.101123298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haase H., Maret W. A differential assay for the reduced and oxidized states of metallothionein and thionein. Anal. Biochem. 2004;333:19–26. doi: 10.1016/j.ab.2004.04.039. [DOI] [PubMed] [Google Scholar]
- 13.Feng W., Benz F. W., Cai J., Pierce W. M., Kang Y. J. Metallothionein disulfides are present in metallothionein-overexpressing transgenic mouse heart and increase under conditions of oxidative stress. J. Biol. Chem. 2006;281:681–687. doi: 10.1074/jbc.M506956200. [DOI] [PubMed] [Google Scholar]
- 14.Savas M. M., Shaw C. F., Petering D. H. The oxidation of rabbit liver metallothionein-II by 5,5′-dithiobis(2-nitrobenzoic acid) and glutathione disulfide. J. Inorg. Biochem. 1993;52:235–249. doi: 10.1016/0162-0134(93)80028-8. [DOI] [PubMed] [Google Scholar]
- 15.Young T. W., Mei F. C., Yang G., Thompson-Lanza J. A., Liu J., Cheng X. Activation of antioxidant pathways in ras-mediated oncogenic transformation of human surface ovarian epithelial cells revealed by functional proteomics and mass spectrometry. Cancer Res. 2004;64:4577–4584. doi: 10.1158/0008-5472.CAN-04-0222. [DOI] [PubMed] [Google Scholar]
- 16.Eyer P., Worek F., Kiderlen D., Sinko G., Stuglin A., Simeon-Rudolf V., Reiner E. Molar absorption coefficients for the reduced Ellman reagent: reassessment. Anal. Biochem. 2003;312:224–227. doi: 10.1016/s0003-2697(02)00506-7. [DOI] [PubMed] [Google Scholar]
- 17.Smith P. K., Krohn R. J., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M., Fujimoto E. K., Goeke N. M., Olson G. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 18.Riordan J. F., Sokolovsky M. Chemical approaches to the study of enzymes. Acc. Chem. Res. 1971;4:353–360. [Google Scholar]
- 19.Krężel A., Bujacz G. D., Latajka R., Bal W. Coordination properties of tris(2-carboxyethyl)phosphine, a newly introduced thiol reductant, and its oxide. Inorg. Chem. 2003;42:1994–2003. doi: 10.1021/ic025969y. [DOI] [PubMed] [Google Scholar]
- 20.Beattie J. H., Richards M. P., Self R. Separation of metallothionein isoforms by capillary zone electrophoresis. J. Chromatogr. 1993;632:127–135. doi: 10.1016/0021-9673(93)80035-7. [DOI] [PubMed] [Google Scholar]
- 21.Krężel A., Maret W. Zinc-buffering capacity of a eukaryotic cell at physiological pZn. J. Biol. Inorg. Chem. 2006;11:1049–1062. doi: 10.1007/s00775-006-0150-5. [DOI] [PubMed] [Google Scholar]
- 22.St. Croix C. M., Wasserloos K. J., Dineley K. E., Reynolds I. J., Leviatan E. S., Pitt B. R. Nitric oxide-induced changes in intracellular zinc homeostasis are mediated by metallothionein/thionein. Am. J. Physiol. Lung Cell Mol. Physiol. 2002;282:L185–L192. doi: 10.1152/ajplung.00267.2001. [DOI] [PubMed] [Google Scholar]
- 23.Gehrig P. M., You C. H., Dallinger R., Gruber C., Brouwer M., Kägi J. H. R. Electrospray ionization mass spectrometry of zinc, cadmium, and copper metallothioneins: evidence for metal-binding cooperativity. Protein Sci. 2000;9:395–402. doi: 10.1110/ps.9.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maret W. Oxidative metal release from metallothionein via zinc-thiol/disulfide interchange. Proc. Natl. Acad. Sci. U.S.A. 1994;91:237–241. doi: 10.1073/pnas.91.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palumaa P., Eriste E., Njunkova O., Pokras L., Jörnvall H., Sillard R. Brain-specific metallothionein-3 has higher metal-binding capacity than ubiquitous metallothioneins and binds metals noncooperatively. Biochemistry. 2002;41:6158–6163. doi: 10.1021/bi025664v. [DOI] [PubMed] [Google Scholar]
- 26.Roschitzki B., Vašák M. Redox labile site in a Zn4 cluster of Cu4, Zn4- metallothionein-3. Biochemistry. 2003;42:9822–9828. doi: 10.1021/bi034816z. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki K. T., Yamamura M. Changes of metal contents and isometallothionein levels in rat tissues after cadmium loading. Biochem. Pharmacol. 1980;29:2407–2412. doi: 10.1016/0006-2952(80)90342-1. [DOI] [PubMed] [Google Scholar]
- 28.Hanaichi T., Kidokoro R., Hayashi H., Sakamoto N. Electron probe X-ray analysis on human hepatocellular lysosomes with copper deposits: copper binding to a thiol-protein in lysosomes. Lab. Invest. 1984;51:592–597. [PubMed] [Google Scholar]
- 29.Lerch K., Johnson G. F., Grushoff P. S., Sternlieb I. Canine hepatic lysosomal copper protein: identification as metallothionein. Arch. Biochem. Biophys. 1985;243:108–114. doi: 10.1016/0003-9861(85)90778-7. [DOI] [PubMed] [Google Scholar]
- 30.Jänicke R., Rudolph R. Folding proteins. In: Creighton T. E., editor. Protein Structure: A Practical Approach. Oxford: IRL Press; 1989. pp. 191–223. [Google Scholar]
- 31.Zangger K., Shen G., Oz G., Otvos J. D., Armitage I. M. Oxidative dimerization in metallothionein is a result of intermolecular disulphide bonds between cysteines in the α-domain. Biochem. J. 2001;359:353–360. doi: 10.1042/0264-6021:3590353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maret W. Zinc and sulfur: a critical biological partnership. Biochemistry. 2004;43:3301–3309. doi: 10.1021/bi036340p. [DOI] [PubMed] [Google Scholar]
- 33.Maret W. Zinc coordination environments in proteins as redox sensors and signal transducers. Antioxid. Redox Signaling. 2006;8:1419–1441. doi: 10.1089/ars.2006.8.1419. [DOI] [PubMed] [Google Scholar]
- 34.Hao Q., Maret W. Imbalance between pro-oxidant and pro-antioxidant functions of zinc in disease. J. Alzheimer's Dis. 2005;8:161–170. doi: 10.3233/jad-2005-8209. [DOI] [PubMed] [Google Scholar]
- 35.Andrews G. K. Cellular zinc sensors: MTF-1 regulation of gene expression. Biometals. 2001;14:223–237. doi: 10.1023/a:1012932712483. [DOI] [PubMed] [Google Scholar]
- 36.Liu J., Yang G., Thompson-Lanza J. A., Glassman A., Hayes K., Patterson A., Marquez R. T., Auersperg N., Yu Y., Hahn W. C., et al. A genetically defined model for human ovarian cancer. Cancer Res. 2004;64:1655–1663. doi: 10.1158/0008-5472.can-03-3380. [DOI] [PubMed] [Google Scholar]
- 37.Irani K., Xia Y., Zweier J. L., Sollott S. J., Der C. J., Fearon E. R., Sudaresan M., Finkel T., Golschmidt-Clermont P. J. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y., Irie Y., Keung W. M., Maret W. S-Nitrosothiols react preferentially with zinc thiolate clusters of metallothionein III through transnitrosation. Biochemistry. 2002;41:8360–8367. doi: 10.1021/bi020030+. [DOI] [PubMed] [Google Scholar]
- 39.Pearce L. L., Gandley R. E., Han W., Wasserloos K., Stitt M., Kanai A. J., McLaughlin M. K., Pitt B. R., Levitan E. S. Role of metallothionein in nitric oxide signaling as revealed by a green fluorescent fusion protein. Proc. Natl. Acad. Sci. U.S.A. 2000;97:477–482. doi: 10.1073/pnas.97.1.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kröncke K.-D., Fehsel K., Schmid T., Zenke F. T., Dasting I., Wesener J. R., Bettermann H., Breunig K. D., Kolb-Bachofen V. Nitric oxide destroys zinc–sulfur clusters inducing zinc release from metallothionein and inhibition of the zinc finger-type yeast transcription activator LAC9. Biochem. Biophys. Res. Commun. 1994;200:1105–1110. doi: 10.1006/bbrc.1994.1564. [DOI] [PubMed] [Google Scholar]
- 41.Ayaz M., Turan B. Selenium prevents diabetes-induced alterations in [Zn2+]i and metallothionein level of rat heart via restoration of cell redox cycle. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H1071–H1081. doi: 10.1152/ajpheart.00754.2005. [DOI] [PubMed] [Google Scholar]
- 42.Sagher D., Brunell D., Hejtmancik J. F., Kantorow M., Brot N., Weissbach H. Thionein can serve as a reducing agent for the methionine sulfoxide reductases. Proc. Natl. Acad. Sci. U.S.A. 2006;103:8656–8661. doi: 10.1073/pnas.0602826103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Good M., Vašák M. Iron(II)-substituted metallothionein: evidence for existence of iron–thiolate clusters. Biochemistry. 1986;25:8353–8356. doi: 10.1021/bi00374a003. [DOI] [PubMed] [Google Scholar]
- 44.Stillman M. J. Metallothioneins. Coord. Chem. Rev. 1995;144:461–511. [Google Scholar]
- 45.Leussing D. L., Jayne J. Mononuclear and polynuclear complex formation between iron(II) and 2,3-dimercapto-1-propanol. J. Phys. Chem. 1962;66:426–429. [Google Scholar]
- 46.Leussing D. L., Tischer T. N. Mononuclear and polynuclear complex formation by manganese(II) and zinc(II) ions with 2,3-dimercapto-1-propanol: the behavior of the Er function with mercaptide. J. Am. Chem. Soc. 1961;83:65–70. [Google Scholar]
- 47.Krężel A., Leśniak W., Jeżowska-Bojczuk M., Młynarz P., Brasuń J., Kozłowski H., Bal W. Coordination of heavy metals by dithiothreitol, a commonly used thiol group protectant. J. Inorg. Biochem. 2001;84:77–88. doi: 10.1016/s0162-0134(00)00212-9. [DOI] [PubMed] [Google Scholar]
- 48.Baird S. K., Kurz T., Brunk U. T. Metallothionein protects against oxidative stress-induced lysosomal destabilization. Biochem. J. 2006;394:275–283. doi: 10.1042/BJ20051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Austin C. D., Wen X., Gazzard L., Nelson C., Scheller R. H., Scales S. J. Oxidizing potential of endosomes and lysosomes limits intracellular cleavage of disulfide-based antibody-drug conjugates. Proc. Natl. Acad. Sci. U.S.A. 2005;102:17987–17992. doi: 10.1073/pnas.0509035102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hao Q., Hong S.-H., Maret W. Lipid raft-dependent endocytosis of metallothionein in HepG2 cells. J. Cell. Physiol. 2007;210:428–435. doi: 10.1002/jcp.20874. [DOI] [PubMed] [Google Scholar]