Abstract
PPO (protoporphyrinogen IX oxidase) catalyses the flavin-dependent six-electron oxidation of protogen (protoporphyrinogen IX) to form proto (protoporphyrin IX), a crucial step in haem and chlorophyll biosynthesis. The apparent Km value for wild-type tobacco PPO2 (mitochondrial PPO) was 1.17 μM, with a Vmax of 4.27 μM·min−1·mg−1 and a catalytic activity kcat of 6.0 s−1. Amino acid residues that appear important for substrate binding in a crystal structure-based model of the substrate docked in the active site were interrogated by site-directed mutagenesis. PPO2 variant F392H did not reveal detectable enzyme activity indicating an important role of Phe392 in substrate ring A stacking. Mutations of Leu356, Leu372 and Arg98 increased kcat values up to 100-fold, indicating that the native residues are not essential for establishing an orientation of the substrate conductive to catalysis. Increased Km values of these PPO2 variants from 2- to 100-fold suggest that these residues are involved in, but not essential to, substrate binding via rings B and C. Moreover, one prominent structural constellation of human PPO causing the disease variegate porphyria (N67W/S374D) was successfully transferred into the tobacco PPO2 background. Therefore tobacco PPO2 represents a useful model system for the understanding of the structure–function relationship underlying detrimental human enzyme defects.
Keywords: enzyme kinetics, haem biosynthesis, protoporphyrin, protoporphyrinogen IX oxidase, stopped-flow fluorescence, variegate porphyria
Abbreviations: INH, 4-bromo-3-(5′-carboxy-4′-chloro-2′-fluorophenyl)-1-methyl-5-trifluoromethyl-pyrazol; Ni-IDA, Ni2+-iminodiacetic acid; PPO, protoporphyrinogen IX oxidase; PPO2, mitochondrial PPO; proto, protoporphyrin IX; protogen, protoporphyrinogen IX; VP, variegate porphyria
INTRODUCTION
PPO (protoporphyrinogen IX oxidase; EC 1.3.3.4) catalyses the oxygen-dependent aromatization of protogen (protoporphyrinogen IX) to proto (protoporphyrin IX) [1] (Scheme 1). It is the last common step of tetrapyrrole biosynthesis for the formation of haems and chlorophylls. Initially, the enzyme was characterized from yeast [2] and mammalian liver [3,4]. It catalyses the six-electron oxidation of protogen using a flavin cofactor and molecular oxygen as terminal electron acceptor. PPO is described as a soluble monomer in Bacillus subtilis [5], as a membrane-associated monomer in bovine [6] or as a homodimer in Myxococcus xanthus and humans [7–9]. In eukaryotes, PPO is associated with the outer surface of the inner mitochondrial membrane [10], while the plant enzyme is additionally located in chloroplasts [11,12].
Scheme 1. Reaction scheme of PPO.
Protogen is oxidized to proto by molecular oxygen with the generation of hydrogen peroxide. Hydrogen atoms eliminated during the reaction are written in bold letters.
In humans, partial PPO deficiency causes a disease known as VP (variegate porphyria; OMIN™ accession number 176200) that is inherited as an autosomal dominant trait displaying incomplete penetrance [13]. The biochemical abnormalities found in VP patients include overproduction and increased excretion of porphyrins and porphyrin precursors. VP manifests clinically with photosensitivity and acute attacks, which includes various neuropsychiatric symptoms [14]. In South Africa, VP is common due to a founder effect, where mainly one amino acid exchange from Arg59 to tryptophan causes the disease [15–18]. However, there are various additional mutations known to cause VP [19].
In plants, two isoforms of PPO, namely PPO1 (plastidic PPO) and PPO2 (mitochondrial PPO), have been found [11,20,21]. They share an amino acid sequence identity of less than 30%. Both enzymes operate as homodimers and use FAD as a cofactor. Recently, the crystal structure of tobacco (Nicotiana tabacum) PPO2 with the bound inhibitor INH [4-bromo-3-(5′-carboxy-4′-chloro-2′-fluorophenyl)-1-methyl-5-trifluoromethyl-pyrazol] and non-covalently bound FAD has been solved [22]. This crystal structure is the only currently available structural information for eukaryotic PPO at the atomic level. The structure of tobacco PPO2 revealed a loosely associated dimer, each monomer consisting of a membrane-, an FAD- and a substrate-binding domain respectively. The inhibitor INH was found to be located in the active site domain and consists of a phenyl- and pyrazol-ring system. Even though the FAD is not covalently bound to the enzyme it was found in close proximity to the co-crystallized inhibitor in the active site cavity. A potential reaction mechanism was proposed based on the fact that the substrate has only limited space to rotate in the active site. The six-electron oxidation of protogen to proto is supposedly catalysed via three steps. Three times the FAD cofactor is reduced by two electrons from the tetrapyrrole ring and reoxidized by molecular oxygen, which in turn is reduced to H2O2. The reduction starts at the C-20 atom of the tetrapyrrole via enamine-imine tautomerization and H rearrangements [23].
Using a model based on the solved crystal structure of tobacco PPO2, we have generated 14 variant proteins carrying amino acid residue exchanges in the corresponding positions of the active site domain. The mutant variant proteins were kinetically characterized by steady-state and stopped-flow fluorescence spectrometry. Furthermore, the amino acid residues responsible for the most common cause of VP in humans were replaced and analysed on the basis of the crystal structure of PPO2 from tobacco.
EXPERIMENTAL
Materials
Proto was purchased from Sigma–Aldrich (Hamburg, Germany). Tween 20 was obtained from Carl Roth GmbH (Karlsruhe, Germany). The remaining chemicals were purchased from Sigma–Aldrich unless specified otherwise. Mutagenesis experiments were performed using the QuikChange® site-directed mutagenesis kit (Stratagene, Heidelberg, Germany). Oligonucleotides were purchased from Biomers.net GmbH (Ulm, Germany). S·Tag Rapid Assay kit was from Novagen (Darmstadt, Germany). Cells were disrupted using a sonotrode from Bandelin (Berlin, Germany). Protino® Ni-IDA (Ni2+-iminodiacetic acid) resin was from Machery-Nagel (Düren, Germany).
Site-directed mutagenesis of tobacco PPO2 gene
The N. tabacum PPO2 gene expression vector pET32a-PPO2(M1-K224Q) was described previously [22]. The plasmid was used as a template for site-directed mutagenesis. Primers to generate N67R, N67W, R98A, R98E, R98K, L356N, L356V, L372N, L372V, S374D, F392E and F392H respectively were as follows.
N67R: 5′-GGGATGAAGGGGCACGTACTATGACTG-3′ and 5′-CAGTCATAGTACTTGCCCCTTCATCCC-3′; N67W: 5′-GGGATGAAGGGGCATGGACTATGACTG-3′ and 5′-CAGTCATAGTCCATGCCCCTTCATCCC-3′; R98A: 5′-CCACTTTCACAAAACAAGGCCTACATTGCCAGAAATGG-3′ and 5′-CCATTTCTGGCAATGTAGGCCTTGTTTTGTGAAAGTGG-3′; R98E: 5′-CCACTTTCACAAAACAAGGAGTACATTGCCAGAAATGG-3′ and 5′-ATTTCTGGCAATGTACTCCTTGTTTTGTGAAAGTGG-3′; R98K: 5′-CCACTTTCAAAACAAGAAGTACATTGCCAGAAATGG-3′ and 5′-CCATTTCTGGCAATGACTTTGTTGTGAAAGTGG-3′; L356N: 5′-GGTGGGGTTAATGTACCTTCCAAGGAGC-3′ and 5′-GCTCCTTGGAAGTACATTACCCAAAGCCC-3′; L356V: 5′-GGGCTTTGGGGTTGTTGTACCTTCCAAGGAGC-3′ and 5′-GCTCCTTGGAAGGTACAACAACCCCAAAGCCC-3′; L372N: 5′-GACACTAGGCACCAACTTCTCTTCTATGATGTTTCC-3′ and 5′-GGAAACATCATAGAAGAGAAGTTGGTGCCTAGTGTC-3′; L372V: 5′-GACACTAGGCACCGTCTTCTCTTCTATGATGTTTCC-3′ and 5′-GGAAACATCATAGAAGAGAAGACGGTGCCTAGTGTC-3′; S374D: 5′-GACACTAGGCACCCTCTTCGATTCTATGATGTTTCC-3′ and 5′-GGAAACATCATAGAATCGAAGAGGGTGCCTAGTGTC-3′; F392E: 5′-CTCTATACTACTGAGGTTGGTGGAAGCC-3′ and 5′-GGCTTCCACCAACCTCAGTAGTATAGAG-3′; F392H: 5′-CTCTATACTACTCATGTTGGTGGAAGCC-3′ and 5′-GGCTTCCACCAACATGAGTAGTATAGAG-3′. Individual mutations were combined to generate the double-mutation-carrying variants N67R/S374D and N67W/S374D respectively. For all introduced mutations, overall PPO2 gene integrity was verified by DNA sequence determination.
Recombinant production and purification of N. tabacum PPO2 and its variants
Recombinant proteins were produced using the Escherichia coli strain BL21(DE3)RIL (Stratagene) containing the original or mutated pET32a-PPO2 plasmids. Cells were grown in LB (Luria–Bertani) medium at 37 °C under vigorous aeration. When the cultures reached a D578 of 0.7, protein production was induced by the addition of 250 μM isopropyl-1-thio-β-D-galactopyranoside. Cells were further cultivated overnight at 25 °C and 150 rev./min, harvested, washed with buffer A (10 mM Tris/HCl, pH 8.0, 300 mM NaCl, 10 mM MgCl2 and 0.05% Triton X-100) and resuspended in a minimal volume of buffer A. Cells were broken by sonication (Bandelin HD 2070, 0.5 s sound, 0.5 s paused, MS73 tip, 70% amplitude) and the cell-free extract was cleared by centrifugation at 66000 rev./min for 45 min using a Ti70 rotor (Beckman). Protein integrity was verified via Western-blot analysis. Recombinant PPO2 was purified to apparent homogeneity by Protino® Ni-IDA resin (Machery-Nagel) as outlined in the manufacturer's instructions. PPO concentrations were determined via the S·Tag Rapid Assay kit. Protein purification and integrity were analysed by MS and SDS/PAGE as outlined previously [24]. The overall protein folding was analysed by CD spectroscopy using a Jasco J810 CD spectropolarimeter as described previously [25,26]. The flavin content of each mutant enzyme was determined spectroscopically as outlined previously and is given in Table 1 [9,27].
Table 1. Kinetic parameters of wild-type and mutant PPO2.
The determination of kinetic constants was performed as described in the Experimental section. The Michaelis–Menten constant Km, the kcat and the kcat/Km values for the N. tabacum PPO2 were determined from substrate velocity plots by measuring the constant velocity formation of proto from protogen over the substrate range 1–25 μM. Values were determined by computerized Lineweaver–Burk iterative curve fitting (SigmaPlot 8.0 Enzyme Kinetics v1.1). The FAD content of wild-type and mutant PPO2 were determined spectroscopically. The FAD content was calculated using standard FAD (Sigma). The standard error of the results shown was between 5 and 10%. n.d., Not detectable.
| Protein variant | Km (μM) | kcat (s−1) | kcat/Km (μM−1·s−1) | FAD content (mol/mol of subunit) |
|---|---|---|---|---|
| Wild-type | 1.17 | 6 | 5.1 | 0.63 |
| Substrate binding ring A | ||||
| F392H | n.d. | n.d. | n.d. | 0.58 |
| F392E | 11.20 | 11 | 1.0 | 0.60 |
| Substrate binding ring B | ||||
| L356N | 11.0 | 38 | 3.4 | 0.55 |
| L356V | 7.30 | 300 | 41.1 | 0.62 |
| L372N | 16.40 | 7 | 0.4 | 0.60 |
| L372V | 103 | 597 | 5.8 | 0.53 |
| Substrate binding ring C | ||||
| R98K | 2.6 | 37 | 14.4 | 0.50 |
| R98E | 12.50 | 34 | 2.7 | 0.51 |
| R98A | 8.30 | 365 | 44 | 0.57 |
| VP | ||||
| S374D | 10.9 | 208 | 18.5 | 0.55 |
| N67R | 97 | 97 | 1.0 | 0.61 |
| N67W | n.d. | n.d. | n.d. | 0.48 |
| N67R/S374D (‘human PPO’) | 1.4 | 111 | 79.4 | 0.65 |
| N67W/S374D (‘human VP’) | 21 | 12 | 0.57 | 0.38 |
PPO assays
The substrate protogen was produced as described previously [28,29]. PPO2 activity was monitored using a continuous assay via the detection of proto fluorescence. Stopped-flow kinetic experiments were performed under aerobic conditions using a Jasco 810 stopped-flow spectrometer and data obtained were analysed with Biokine Acquire Kinetics (Jasco, Überlingen, Germany). Averages of three to five individual transients were analysed. Protogen was dissolved in 50 mM Mes buffer (pH 6.0), 1% (v/v) Triton X-100 and 20 mM EDTA. Protogen solution and PPO2 preparations were mixed in a 6:1 ratio in a total volume of 300 μl and fluorescence resulting from proto formation was continuously recorded via excitation at 409 nm and emission measurement above 450 nm. Approximately 8000 measurement points per assay were recorded. Proto concentrations were calculated using an appropriate calibration curve. The percentage of substrate consumed was less than 5%.
Determination of kinetic constants
The Michaelis–Menten constant (Km), the maximal velocity Vmax and the catalytic constant kcat were determined from substrate velocity plots by measuring the constant velocity formation of proto from protogen over a substrate range 1–25 μM and a time course of up to 2 h at 20 °C. The initial reaction rates were linear and values were determined by computerized Lineweaver–Burk iterative curve fitting using SigmaPlot 8.0 Enzyme Kinetics v1.1. For the calculation of the catalytic efficiency kcat, the maximal velocity Vmax was divided by the corresponding enzyme concentration.
RESULTS AND DISCUSSION
Active site architecture of N. tabacum PPO2
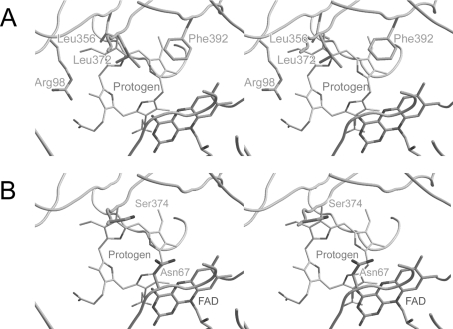
The crystal structure of tobacco PPO2 revealed a close insight into the three-dimensional structure of the enzyme [22]. Modelling of the protogen into the active site of tobacco PPO2 is based on the first model proposed by Koch et al. [22]. Arg98, Phe392, Leu372 and Leu356 co-ordinated the inhibitor INH which was co-crystallized within the active site of PPO2. These four amino acid residues were found highly conserved throughout the PPO family. Ring A is co-ordinated via aromatic stacking with Phe392 (Figure 1A) [22]. The side chains of Leu356 and Leu372 interact hydrophobically with ring B. The side chain of Arg98 forms a salt bridge to the propionate carboxy group of ring C. Additionally ring C might be stabilized by flipping and subsequent stacking of the conserved residue Phe353. The dome conformation of the protogen is probably stabilized by the carbonyl oxygen of the highly conserved Gly175.
Figure 1. Active site cavity of PPO2.
(A) Active site of tobacco PPO2. Depicted are the investigated residues Arg98, Leu356, Leu372 and Phe392. Phe392 is shown to co-ordinate ring A of the protogen. Leu356 and Leu372 are stacking protogen ring B from the ‘back’ and the ‘front’, and Arg98 is supposed to co-ordinate the propionate carboxylate group of the protogen ring C. (B) VP mutants of tobacco PPO2. Asn67 and Ser374 which play an important role in human VP, are depicted.
Experimental determination of amino acid residues important for substrate binding
Based on the proposed model, we functionally investigated the molecular basis of substrate co-ordination and its catalytic implications. We constructed an appropriate set of mutant PPO2 genes, isolated the respective mutant protein variants and determined their kinetic parameters via fluorescence stopped-flow spectrometry in comparison with wild-type PPO2. We used the strategy of introducing conservative and non-conservative amino acid exchanges for residues of interest in order to evaluate their chemical contribution to substrate binding and catalysis. Obtained results are listed in Table 1. The apparent Km value of purified wild-type PPO2 protein for its substrate was 1.17 μM with a kcat value of 6.0 s−1. The determined kinetic parameters were within the range of values measured for PPOs from other organisms, e.g. Km=1.0 μM and kcat=0.0031 s−1 for B. subtilis [30]; Km=0.1 μM for Saccharomyces cerevisiae [31]; Km=2.8 μM for Aquifex aeolicus [32] and Km=0.85 μM and either kcat=0.175 s−1 [7] or kcat=5.95 s−1 [9] respectively for Homo sapiens. The FAD content of PPO2 was 0.63 mol of FAD/subunit PPO2. All PPO2 mutant enzymes revealed a similar FAD content, the only exception being the double mutant N67W/S374D, which is discussed in detail below (Table 1).
The crystal structure revealed that ring A of the inhibitor INH is kept in position by aromatic stacking with Phe392, which stabilizes the electron transfer over the ring during the reaction. An amino acid exchange to Glu392 in the variant F392E was tolerated within the active site cavity and resulted in inferior binding of the substrate protogen (Km=11.2 μM). However, once the substrate protogen was bound, its conversion was catalysed sufficiently (kcat=11 s−1). The tetrapyrrole ring is made positive by the hydride abstraction at the N-5 atom of FAD. Thus the negative charge of the introduced glutamate might stabilize the ring and catalysis may proceed equally fast. In this case, only the stabilization of the substrate by aromaticity is lowered and so its binding is weaker. Additionally, the PPO2 variant F392H was found to be virtually inactive. Determination of reliable kinetic parameters was not possible. Histidine at a pH below its pK at 6.1 is positively charged. Here, at a pH of 6.0, this holds true for most histidine molecules of PPO2. The positive charge of the histidine ring at this position leads to repulsion and obstructs substrate binding; consequently, reaction kinetics collapsed (Figure 1A). For detailed kinetic parameters of the mutant proteins see Table 1.
In the model for substrate binding deduced from the PPO2–INH crystal structure, ring B of protogen is sandwiched between the conserved residues Leu356 and Leu372. Both amino acid residues were mutated to either valine or asparagine respectively. In both cases the conservative amino acid exchange to valine enhanced catalysis up to 100-fold (kcat=300 and 597 s−1 respectively). However, in the case of an amino acid exchange of Leu372 to valine, binding of the substrate protogen was 100-fold reduced (Km=103 μM). In contrast with that, the PPO2 variants L356N and L372N showed an only slightly inferior binding capacity compared with the wild-type and revealed comparable catalytic PPO2 activities (see Table 1). Valine possesses comparable hydrophobic features to leucine. However, the carbon backbone of the side chain is shorter than that of leucine. Most likely the appropriate location of the hydrophobic residue in relation to the co-ordinated substrate ring is essential, explaining the increasing Km values of L356V and especially L372V. Once the substrate is bound, catalysis is found to be enhanced for both mutants. Most likely the smaller side chain of valine better accommodates the necessary complex arrangement of the substrate during catalysis. On the other hand, asparagine obviously provides the necessary side-chain length and chemical character to supplement for leucine in both positions (Figure 1A). The next amino acid residue potentially involved in substrate binding to be investigated was Arg98. It was deduced from the N. tabacum PPO2 crystal structure that Arg98 provides the counter charge for the carboxylate group of the inhibitor which most likely mimics the propionate group of ring C of protogen. The PPO2 variants R98K, R98E and R98A were chosen to evaluate the contribution of this positively charged amino acid. While the conservative exchange R98K improved enzyme activity (kcat=37 s−1), the non-conservative exchange R98E resulted in inferior substrate binding (Km=12.5 μM). Most likely the introduced negative charge prohibited the ionic interaction necessary for the stabilization of ring C. In agreement with this assumption, the replacement of Arg98 with the non-polar amino acid alanine resulted in an enzyme variant (R98A) with an 8-fold increased Km value (Figure 1A). Interestingly, all three variants in position 98 showed an increased kcat value, especially variant R98A (kcat=309 s−1), indicating improved turnover in the absence of ionic interaction between enzyme and substrate. Nevertheless, the overall response of PPO2 towards the amino acid exchanges introduced in position 98 is nicely reflected by the kcat and Km values.
In summary, by measuring enzyme activity of mutant PPO2s we were able to verify that the well-conserved residues Arg98, Phe392, Leu356 and Leu372 are functionally involved in substrate co-ordination within the active site.
VP
The dominantly inherited genetic disorder VP in humans results from impaired PPO activity as a consequence of an amino acid residue exchange. In more than 94% of the VP cases in South Africa, Arg59 is mutated to tryptophan [16]. The residue corresponding to human Arg59 in the tobacco PPO2 is Asn67. It is positioned on a longer loop between a β-strand and an α-helix exactly between the FAD-binding and substrate-binding sites (Figure 1B). The residue is conserved in all known mitochondrial and plastidic plant PPO amino acid sequences. We started to mimic the situation of the human enzyme on the basis of the related crystal structure of PPO2 from tobacco by mutating Asn67 to arginine. The obtained mutant N67R was found to be reasonably active. Even though the mutation resulted in inferior substrate binding, the catalytic efficiency was acceptable. This observation is in agreement with the findings of Maneli et al. [9], who underlined the importance of a hydrophilic, favourably positively charged amino acid at position Arg59 in the human enzyme. Replacing Asn67 with tryptophan abolished enzyme activity. The bulky nature of an aromatic tryptophan in position Asn67 results in impaired enzyme activity. Thus the enzymatic defect responsible for most VP cases in humans can be transferred on to tobacco PPO2. The spatial structure of PPO2 from tobacco can be used as a model for human PPO. However, in the tobacco PPO2 crystal structure, the residue opposite Asn67 is Ser374 (Figure 1B). The corresponding residue in human PPO is Asp349, conserved in all known animal PPOs. Asp349 is likely to form a salt bridge with Arg59, which would sterically fit in this area of the protein [22]. In order to adapt tobacco PPO2 further towards the human counterpart, we first mutated Ser374 to aspartate. The S374D single mutant showed reduced substrate binding capacity with a significantly increased kcat value (kcat=208 s−1). Subsequently, we mutated Asn67 of S374D to arginine to reconstruct the assumed salt bridge Asp374/Arg67 in the human enzyme for our tobacco VP model. The resulting N67R/S374D double mutant showed an almost wild-type level Km value (Km=1.4). We were able to obtain a PPO2 variant with wild-type-like substrate recognition and even improved catalytic properties (kcat=111 s−1) by a reconstruction of the salt bridge in the tobacco enzyme. These results argue for the existence of a salt bridge linking Arg59 and Asp349 in the human enzyme as well. The double mutation N67W/S374D, mimicking VP in the human enzyme, significantly reduced enzyme activity. The Km value was increased by a factor of 20, while the kcat/Km value decreased 10-fold. Obviously, the reduced FAD content of 0.38 mol of FAD/subunit for the PPO2 variant N67W/S473D, representing approx. 60% of the wild-type enzyme content, does not account for the observed decrease in enzyme activity. Similar observations were made previously for the human R59W mutant enzyme [9]. Most importantly, in contrast with most other generated mutant enzymes, this PPO2 variant showed an increased Km with a parallel decreased kcat compared with the double mutant N67R/S374D. Both amino acids neighbouring Asp349 (Ser374 in PPO2) in the human enzyme have previously been characterized. A mutation of Trp348 of the human enzyme to cysteine resulted in a wild-type-like Km value and a 10-fold reduced kcat [9]. A mutation of Ser350 to proline abolished enzyme activity [18]. These observations underscore that the structural integrity of this region is important for enzyme activity.
Consequently, the mutant tobacco PPO2 enzymes generated, the single as well as the double mutants, may serve as a model for further investigations towards the structural understanding of the disease-causing situation in the human PPO.
Conclusions
We conclude from our investigation that it is most likely that all four rings of the protogen substrate of PPO2 are being co-ordinated during substrate binding by tobacco PPO2. Ring A is stacked by Phe392, ring B is sandwiched in between Leu356 and Leu372, and the propionate carboxylate of ring C is bound to Arg98. Furthermore, ring D might be stacked against the A ring of the iso-alloxazine moiety of the FAD. Most of the generated PPO2 variants were characterized by an increase in Km and kcat values. One may conclude that tobacco PPO2 was optimized during evolution towards a stringent substrate recognition and discrimination, and not towards catalytic turnover.
The results obtained for the VP-type enzyme variants showed that the enzymatic defect responsible for causing VP in humans can be mimicked with the tobacco PPO2 model.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (JA 470-7-3).
References
- 1.Brenner D. A., Bloomer J. R. The enzymatic defect in variegate porphyria. Studies with human cultured skin fibroblasts. N. Engl. J. Med. 1980;302:765–769. doi: 10.1056/NEJM198004033021401. [DOI] [PubMed] [Google Scholar]
- 2.Poulson R., Polglase W. J. The enzymic conversion of protoporphyrinogen IX to protoporphyrin IX. Protoporphyrinogen oxidase activity in mitochondrial extracts of Saccharomyces cerevisiae. J. Biol. Chem. 1975;250:1269–1274. [PubMed] [Google Scholar]
- 3.Poulson R. The enzymic conversion of protoporphyrinogen IX to protoporphyrin IX in mammalian mitochondria. J. Biol. Chem. 1976;251:3730–3733. [PubMed] [Google Scholar]
- 4.Dailey H. A., Karr S. W. Purification and characterization of murine protoporphyrinogen oxidase. Biochemistry. 1987;26:2697–2701. doi: 10.1021/bi00384a007. [DOI] [PubMed] [Google Scholar]
- 5.Hansson M., Hederstedt L. Cloning and characterization of the Bacillus subtilis hemEHY gene cluster, which encodes protoheme IX biosynthetic enzymes. J. Bacteriol. 1992;174:8081–8093. doi: 10.1128/jb.174.24.8081-8093.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siepker L. J., Ford M., de Kock R., Kramer S. Purification of bovine protoporphyrinogen oxidase: immunological cross-reactivity and structural relationship to ferrochelatase. Biochim. Biophys. Acta. 1987;913:349–358. doi: 10.1016/0167-4838(87)90146-4. [DOI] [PubMed] [Google Scholar]
- 7.Dailey T. A., Dailey H. A. Human protoporphyrinogen oxidase: expression, purification, and characterization of the cloned enzyme. Protein Sci. 1996;5:98–105. doi: 10.1002/pro.5560050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dailey T. A., Dailey H. A. Expression, purification, and characteristics of mammalian protoporphyrinogen oxidase. Methods Enzymol. 1997;281:340–349. doi: 10.1016/s0076-6879(97)81041-5. [DOI] [PubMed] [Google Scholar]
- 9.Maneli M. H., Corrigall A. V., Klump H. H., Davids L. M., Kirsch R. E., Meissner P. N. Kinetic and physical characterisation of recombinant wild-type and mutant human protoporphyrinogen oxidases. Biochim. Biophys. Acta. 2003;1650:10–21. doi: 10.1016/s1570-9639(03)00186-9. [DOI] [PubMed] [Google Scholar]
- 10.Deybach J. C., da Silva V., Grandchamp B., Nordmann Y. The mitochondrial location of protoporphyrinogen oxidase. Eur. J. Biochem. 1985;149:431–435. doi: 10.1111/j.1432-1033.1985.tb08943.x. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs J. M., Jacobs N. J. Protoporphyrinogen oxidation, an enzymatic step in heme and chlorophyll synthesis: partial characterization of the reaction in plant organelles and comparison with mammalian and bacterial systems. Arch. Biochem. Biophys. 1984;229:312–319. doi: 10.1016/0003-9861(84)90157-7. [DOI] [PubMed] [Google Scholar]
- 12.Dailey H. A. Terminal steps of haem biosynthesis. Biochem. Soc. Trans. 2002;30:590–595. doi: 10.1042/bst0300590. [DOI] [PubMed] [Google Scholar]
- 13.Kappas A. S. S., Galbraith R. A., Nordmann Y. New York: McGraw-Hill; 1995. The Metabolic and Molecular Basis of Inherited Diseases. [Google Scholar]
- 14.Mustajoki P. Variegate porphyria. Twelve years' experience in Finland. Q. J. Med. 1980;49:191–203. [PubMed] [Google Scholar]
- 15.Dean G. Screening tests for porphyria. Lancet. 1971;1:86–87. doi: 10.1016/s0140-6736(71)90820-8. [DOI] [PubMed] [Google Scholar]
- 16.Meissner P. N., Dailey T. A., Hift R. J., Ziman M., Corrigall A. V., Roberts A. G., Meissner D. M., Kirsch R. E., Dailey H. A. A R59W mutation in human protoporphyrinogen oxidase results in decreased enzyme activity and is prevalent in South Africans with variegate porphyria. Nat. Genet. 1996;13:95–97. doi: 10.1038/ng0596-95. [DOI] [PubMed] [Google Scholar]
- 17.Warnich L., Kotze M. J., Groenewald I. M., Groenewald J. Z., van Brakel M. G., van Heerden C. J., de Villiers J. N., van de Ven W. J., Schoenmakers E. F., Taketani S., et al. Identification of three mutations and associated haplotypes in the protoporphyrinogen oxidase gene in South African families with variegate porphyria. Hum. Mol. Genet. 1996;5:981–984. doi: 10.1093/hmg/5.7.981. [DOI] [PubMed] [Google Scholar]
- 18.Roberts A. G., Puy H., Dailey T. A., Morgan R. R., Whatley S. D., Dailey H. A., Martasek P., Nordmann Y., Deybach J. C., Elder G. H. Molecular characterization of homozygous variegate porphyria. Hum. Mol. Genet. 1998;7:1921–1925. doi: 10.1093/hmg/7.12.1921. [DOI] [PubMed] [Google Scholar]
- 19.von und zu Fraunberg M., Timonen K., Mustajoki P., Kauppinen R. Clinical and biochemical characteristics and genotype-phenotype correlation in Finnish variegate porphyria patients. Eur. J. Hum. Genet. 2002;10:649–657. doi: 10.1038/sj.ejhg.5200860. [DOI] [PubMed] [Google Scholar]
- 20.Lermontova I., Kruse E., Mock H. P., Grimm B. Cloning and characterization of a plastidal and a mitochondrial isoform of tobacco protoporphyrinogen IX oxidase. Proc. Natl. Acad. Sci. U.S.A. 1997;94:8895–8900. doi: 10.1073/pnas.94.16.8895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beale S. I. Enzymes of chlorophyll biosynthesis. Photosynth. Res. 1999;60:43–73. [Google Scholar]
- 22.Koch M., Breithaupt C., Kiefersauer R., Freigang J., Huber R., Messerschmidt A. Crystal structure of protoporphyrinogen IX oxidase: a key enzyme in haem and chlorophyll biosynthesis. EMBO J. 2004;23:1720–1728. doi: 10.1038/sj.emboj.7600189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips J. D., Whitby F. G., Kushner J. P., Hill C. P. Structural basis for tetrapyrrole coordination by uroporphyrinogen decarboxylase. EMBO J. 2003;22:6225–6233. doi: 10.1093/emboj/cdg606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breckau D., Mahlitz E., Sauerwald A., Layer G., Jahn D. Oxygen-dependent coproporphyrinogen III oxidase (HemF) from Escherichia coli is stimulated by manganese. J. Biol. Chem. 2003;278:46625–46631. doi: 10.1074/jbc.M308553200. [DOI] [PubMed] [Google Scholar]
- 25.Layer G., Grage K., Teschner T., Schunemann V., Breckau D., Masoumi A., Jahn M., Heathcote P., Trautwein A. X., Jahn D. Radical S-adenosylmethionine enzyme coproporphyrinogen III oxidase HemN: functional features of the [4Fe-4S] cluster and the two bound S-adenosyl-L-methionines. J. Biol. Chem. 2005;280:29038–29046. doi: 10.1074/jbc.M501275200. [DOI] [PubMed] [Google Scholar]
- 26.Randau L., Schauer S., Ambrogelly A., Salazar J. C., Moser J., Sekine S., Yokoyama S., Soll D., Jahn D. tRNA recognition by glutamyl-tRNA reductase. J. Biol. Chem. 2004;279:34931–34937. doi: 10.1074/jbc.M401529200. [DOI] [PubMed] [Google Scholar]
- 27.Ohno Y., Buescher E. S., Roberts R., Metcalf J. A., Gallin J. I. Reevaluation of cytochrome b and flavin adenine dinucleotide in neutrophils from patients with chronic granulomatous disease and description of a family with probable autosomal recessive inheritance of cytochrome b deficiency. Blood. 1986;67:1132–1138. [PubMed] [Google Scholar]
- 28.Labbe P., Camadro J. M., Chambon H. Fluorometric assays for coproporphyrinogen oxidase and protoporphyrinogen oxidase. Anal. Biochem. 1985;149:248–260. doi: 10.1016/0003-2697(85)90502-0. [DOI] [PubMed] [Google Scholar]
- 29.Layer G., Moser J., Heinz D. W., Jahn D., Schubert W. D. Crystal structure of coproporphyrinogen III oxidase reveals cofactor geometry of radical SAM enzymes. EMBO J. 2003;22:6214–6224. doi: 10.1093/emboj/cdg598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corrigall A. V., Siziba K. B., Maneli M. H., Shephard E. G., Ziman M., Dailey T. A., Dailey H. A., Kirsch R. E., Meissner P. N. Purification of and kinetic studies on a cloned protoporphyrinogen oxidase from the aerobic bacterium Bacillus subtilis. Arch. Biochem. Biophys. 1998;358:251–256. doi: 10.1006/abbi.1998.0834. [DOI] [PubMed] [Google Scholar]
- 31.Camadro J. M., Thome F., Brouillet N., Labbe P. Purification and properties of protoporphyrinogen oxidase from the yeast Saccharomyces cerevisiae. Mitochondrial location and evidence for a precursor form of the protein. J. Biol. Chem. 1994;269:32085–32091. [PubMed] [Google Scholar]
- 32.Wang K. F., Dailey T. A., Dailey H. A. Expression and characterization of the terminal heme synthetic enzymes from the hyperthermophile Aquifex aeolicus. FEMS Microbiol. Lett. 2001;202:115–119. doi: 10.1111/j.1574-6968.2001.tb10789.x. [DOI] [PubMed] [Google Scholar]