Abstract
Although disialyl glycosphingolipids such as GD3 and GD2 have been considered to be associated with malignant tumours, whether branched-type disialyl glycosphingolipids show such an association is not well understood. We investigated the sialyltransferases responsible for the biosynthesis of DSGG (disialylgalactosylgloboside) from MSGG (monosialylgalactosylgloboside). Among six GalNAc:α2,6-sialyltransferases cloned to date, we focused on ST6GalNAc III, V and VI, which utilize sialylglycolipids as substrates. In vitro enzyme analyses revealed that ST6GalNAc III and VI generated DSGG from MSGG with Vmax/Km values of 1.91 and 4.16 respectively. Transfection of the cDNA expression vectors for these enzymes resulted in DSGG expression in a renal cancer cell line. Although both ST6GalNAc III and VI genes were expressed in normal kidney cells, the expression profiles of ST6GalNAc VI among 20 renal cancer cell lines correlated clearly with those of DSGG, suggesting that the sialyltransferase involved in the synthesis of DSGG in the kidney is ST6GalNAc-VI. ST6GalNAc-VI and DSGG were found in proximal tubule epithelial cells in normal kidney tissues, while they were downregulated in renal cancer cell lines and cancer tissues. All these findings indicated that DSGG was suppressed during the malignant transformation of the proximal tubules as a maturation arrest of glycosylation.
Keywords: globo-series, glycolipid, renal cancer, sialyltransferase
Abbreviations: asialo-BSM, bovine submaxillary asialomucin; BCIT, 5-bromo-4-chloro-3-indolyl phosphate; BSM, bovine submaxillary mucin; disialyl Lea, disialyl Lewis a; DSGG, disialylgalactosylgloboside; Gb3, globotriaosylceramide; Gb4, globotetraosylceramide; Gb5, β1,3-galactosyl Gb4; HRPTE cells, human renal proximal tubule epithelial cells; mAb, monoclonal antibody; MSGG, monosialylgalactosylgloboside; NBT, Nitrotetrazolium Blue chloride; NeuAc, N-acetylneuraminic acid; PNA, peptide–nucleic acid; RT, reverse transcription; sialyl Lc4, sialyl-lactotetraosylceramide, NeuAcα2,3Galβ1,3 GlcNAc β1,3Galβ1,4Glc-Cer; siglec, sialic acid-binding, immunoglobulin-like lectins
INTRODUCTION
Sialic acids are conjugated to the carbohydrate moieties of glycoproteins and glycosphingolipids, and modulate the characteristics of the whole molecules by changing properties such as their charge, binding affinity for ligands and stability [1]. Therefore, sialylation is one of the critical mechanisms for the regulation of various biological processes. In fact, inhibition of sialyltransferases [2] or gene targeting of sialyltransferases such as ST6Gal-I [3], ST8Sia-I [4], ST3Sia-V [5] and polysialo-synthases [6,7] revealed that sialylation of glycoproteins or glycosphingolipids is very important in tumour development, neuronal development, nerve repair, immunological processes and regulation of hormone sensitivity. So far, 20 mammalian sialyltransferase genes have been isolated and they can be classified into four families based on their acceptor substrates and types of linkages [8].
Among sialyl compounds, polysialo-structures, in particular, play important roles in neurogenesis [9] and the acquisition of malignant features during oncogenesis [10–13]. Disialyl glycosphingolipids such as GD3 and GD2 have been reported to be associated with malignant transformation, cancer invasion, metastasis, and prognosis [14–16]. Interactions of these disialyl structures with members of a lectin family, siglecs (sialic acid-binding, immunoglobulin-like lectins), are considered to be involved in the survival of cancer cells [17,18].
On the other hand, we have analysed the mechanisms for the synthesis of disialyl-gangliosides with α-structure, and isolated cDNAs for the responsible synthetic enzymes, such as ST6GalNAc-V [19] and ST6GalNAc-VI [20]. α-Series gangliosides consist of GD1α, GT1aα and GQ1bα, which contain NeuAc (N-acetylneuraminic acid) replaced by GalNAc with α2,6-linkage. ST6GalNAc-V is specifically expressed in the brain, whereas ST6GalNAc-VI is expressed in a wide range of tissues. Furthermore, we determined the sialyltransferase responsible for the synthesis of disialyl Lea (disialyl Lewis a) to be ST6GalNAc-VI [21]. This structure contains a branched-type disialyl structure on a lacto-core structure. This dual activity of ST6GalNAc-VI could be explained based on the similarity in the substrate structures, i.e. GM1b (NeuAcα2,3Galβ1,3 GalNAcβ1,4Galβ1,4Glc-Cer) and sialyl-Lc4 (sialyl-lactotetraosylceramide, NeuAcα2,3Galβ1,3 GlcNAc β1,3Galβ1,4Glc-Cer).
In the present study, we investigated the mechanism of the biosynthesis of DSGG (disialylgalactosylgloboside) and its expression in normal and cancerous kidney tissues. DSGG is a disialyl glycosphingolipid with a globo-series core structure, and has been detected in renal cancer cells [22]. It is thought to be synthesized from MSGG (monosialylgalactosylgloboside) by sialyltransferases. Among the six GalNAc:α2,6-sialyltransferases cloned to date, we focused on ST6GalNAc-III, -V and -VI, which utilize glycolipids as substrates. We have now identified the sialyltransferase involved in the synthesis of DSGG from MSGG in the kidney to be ST6GalNAc-VI, indicating that ST6GalNAc-VI is a unique sialyltransferase capable of synthesizing branched-type disialyl structures such as DSGG, disialyl Lea as well as α-series gangliosides with distinct core structures. Expression patterns of DSGG and ST6GalNAc VI in normal and cancerous kidney tissues indicated that they were expressed in the proximal tubule epithelial cells and downregulated during tumour transformation.
EXPERIMENTAL
Cell lines
Human renal cancer cell lines (SK-RC 1, SK-RC 6, SK-RC 7, SK-RC 17, SK-RC 29, SK-RC 35, SK-RC 39, SK-RC 44, SK-RC 45, Moroff and SK-RC 99) were established at the Memorial Sloan-Kettering Cancer Center (New York, NY, U.S.A.). Human renal cancer cell lines (Caki-1, VMRC-RCW, VMRC-RCZ, TUHR10TKB and TUHR14TKB) were obtained from the Human Science Research Resources Bank (Osaka, Japan). Human renal cancer cell lines (OS-RC-2, RCC10RGB, TUHR4TKB) were obtained from the Riken Cell Bank (Wako, Japan). A human renal cancer cell line, ACHN, was purchased from Dainihonseiyaku Co. Normal human renal proximal tubule epithelial cells were purchased from Sanko Junyaku Co. Mouse fibroblast L cells and all human renal cancer cell lines were grown in Dulbecco's modified Eagle's medium supplemented with 7.5% (v/v) fetal calf serum. HRPTE cells (normal human renal proximal tubule epithelial cells) were cultured in renal epithelial cell growth medium Bullet Kit™ (Sanko Junyaku Co.).
Isolation of human ST6GalNAc III
Isolation of cDNA clones was performed as described previously [23]. Briefly, RT (reverse transcription)-PCR using a human brain cDNA library as template was performed with the sense primer 5′-GAATGTGGGCTGGAGAGGTC-3′ and the antisense primer 5′-GCAGAGTCACCATCCACATC-3′ using TITANIUM™ Taq DNA Polymerase (Clontech) as follows; 95 °C for 3 min, 28 cycles of (95 °C for 0.5 min and 68 °C for 3 min), and 72 °C for 10 min. The RT-PCR amplified 1182 bp cDNA was subcloned into the pT-Adv vector using an AdvanTage™ Cloning Kit (Clontech).
Flow cytometry
Cell surface expression of glycosphingolipids was analysed using a FACScalibur™ with Cell Quest™ version 3.1f software (Becton Dickinson) using mAbs (monoclonal antibodies). Anti-MSGG (RM1 and Raft2) were generated as described previously [24,25], and anti-DSGG (5F3, mouse IgM; cell culture supernatant at a dilution of 1:1) was established as described [22]. The cells were incubated with mAbs for 1 h on ice and stained with fluorescein isothiocyanate-conjugated goat anti-mouse IgM (ICN/Cappel) at a 1:200 dilution. Control samples were prepared using unreactive mAbs with same the Ig isotypes. Gb3 (globotriaosylceramide), Gb4 (globotetraosylceramide) and Gb5 (β1,3-galactosyl Gb4) were analysed as described previously [26,27]. Information about mAbs used in this study and their target structures are summarized in Table 1.
Table 1. Antibodies used in this study.
| Antigen | Structure | mAb | Reference |
|---|---|---|---|
| Gb3 | Galα1,4Galβ1,4Glcβ-Cer | 38.13 | [26] |
| Gb4 | GalNAcβ1,3Galα1,4Galβ1,4Glcβ-Cer | HIRO 34 | [26] |
| Gb5 | Galβ1,3GalNAcβ1,3Galα1,4Galβ1,4Glcβ-Cer | 5A3 | [27] |
| MSGG | Galβ1,3GalNAcβ1,3Galα1,4Galβ1,4Glcβ-Cer | RM1 | [24] |
| 3 | Raft2 | [25] | |
| | | |||
| NeuAcα2 | |||
| DSGG | Galβ1,3GalNAcβ1,3Galα1,4Galβ1,4Glcβ-Cer | 5F3 | [22] |
| 36 | |||
| | | | |||
| NeuAcα2 NeuAcα2 |
Substrates
GM2, GM1, GD3, GD1a, GD1b, GT1b, GA2, GA1, fetuin, asialofetuin, BSM (bovine submaxillary mucin), and asialo-BSM (bovine submaxillary asialomucin) were purchased from Sigma. Gb3, Gb4, Gb5, MSGG and DSGG were extracted from ACHN cells. The total lipid fraction was applied to DEAE-Sephadex to separate MSGG and DSGG. Neutral glycosphingolipids, Gb3, Gb4 and Gb5, were first eluted from the column by a solvent system of chloroform/methanol/water (30:60:8, by vol.) and separated by preparative TLC as described previously [28]. MSGG and DSGG were eluted from the DEAE-Sephadex by step-wise elution with sodium acetate (0.2, 0.25, 0.3, 0.45, 0.6 and 0.8 M) in a solvent system of chloroform/methanol/sodium acetate (30:60:8, by vol.). GM1b, sialyl Lc4, and sialyl nLc4 (see Table 2) were prepared as described previously [21].
Table 2. Determination of the kinetics parameters of hST6GalNAc III and VI toward MSGG.
Various acceptor substrates were incubated in the standard assay mixture using the membrane fraction of cells transfected with hST6GalNAc III and VI as enzyme sources. Each substrate was used at the concentration of 0.1 mM for glycolipids and 0.4 mg/ml for glycoproteins
| Relative rate (%)* | |||
|---|---|---|---|
| Acceptor | Structure | III | VI |
| Fetuin | NeuAcα2,3Galβ1,3GalNAc-Ser/Thr | 87 | 63 |
| NeuAcα2,3Galβ1,3(NeuAcα2,6)GalNAc-Ser/Thr | |||
| NeuAcα2,6(3)Galβ1,4GlcNAc-R† | |||
| Asialo-fetuin | 3.2 | 2.8 | |
| BSM | GlcNAcβ1,3(NeuAcα2,6)GalNAc-Ser/Thr | 0 | 0 |
| NeuAcα2,6GalNAc-Ser/Thr | |||
| Asialo-BSM | 0 | 0 | |
| GA2 | GalNAcβ1,4Galβ1,4Glcβ1-Cer | 0 | 0 |
| GA1 | Galβ1,3GalNAcβ1,4Galβ1,4Glcβ1-Cer | 0 | 0 |
| GM1b | NeuAcα2,3Galβ1,3GalNAcβ1,4Galβ1,4Glcβ1-Cer | 100 | 100 |
| GM2 | GalNAcβ1,4(NeuAcα2,3)Galβ1,4Glcβ1-Cer | 0 | 0 |
| GM1 | Galβ1,3GalNAcβ1,4(NeuAcα2,3)Galβ1,4Glcβ1-Cer | 0 | 0 |
| GD1a | NeuAcα2,3Galβ1,3GalNAcβ1,4(NeuAcα2,3)Galβ1,4Glcβ1-Cer | 3.8 | 7.8 |
| GD3 | NeuAcα2,8NeuAcα2,3Galβ1,4Glcβ1-Cer | 0 | 0 |
| GT1b | Gaβ1,3GalNAcβ1,4(NeuAcα2,8NeuAcα2,3)Galβ1,4Glcβ1-Cer | 2.4 | 8.7 |
| Gb3 | Galβ1,4Galβ1,4Glcβ1-Cer | 0 | 0 |
| Gb4 | GalNAcβ1,3Galβ1,4Galβ1,4Glcβ1-Cer | 0 | 0 |
| Gb5 | Galβ1,3GalNAcβ1,3Galβ1,4Galβ1,4Glcβ1-Cer | 0 | 0 |
| MSGG | NeuAcα2,3Galβ1,3GalNAcβ1,3Galβ1,4Galβ1,4Glcβ1-Cer | 29 | 46 |
| DSGG | NeuAcα2,3Galβ1,3GalNAcβ1,3(NeuAcα2,6)Galβ1,4Galβ1,4Glcβ1-Cer | 0 | 0 |
| Lc4 | Galβ1,3GlcNAcβ1,3Galβ1,4Glcβ1-Cer | 0 | 0 |
| Sialyl Lc4 | NeuAcα2,3Galβ1,3GlcNAcβ1,3Galβ1,4Glcβ1-Cer‡ | 6 | 33 |
| Sialyl nLc4 | NeuAcα2,3Galβ1,4GlcNAcβ1,3Galβ1,4Glcβ1-Cer‡ | 0 | 0 |
| Sialyl Lea | NeuAcα2,3Galβ1,3(Fucα1,4)GlcNAcβ1,3Galβ1,4Glcβ1-Cer | 0 | 0 |
*Relative rates are calculated as a percentage of the incorporated NeuAc relative to that for GM1b.
†R represents the remainder of the N-linked oligosaccharide chain.
‡Ceramide mimic -CH2(C14H29)2 was chemically bonded to the oligosaccharides instead of ceramide.
Preparation of membrane fraction
L cells (3×106) were plated in 10-cm diameter dishes at least 48 h prior to transfection experiments. Cells were transiently transfected with 6 μg of an expression plasmid using the DEAE-dextran method [29]. The cells were collected after 48 h and lysed in ice-cold PBS containing 1 mM PMSF using a nitrogen cavitation apparatus (Parr Instrument Co.) at 400 p.s.i. for 30 min at 4 °C. Nuclei were removed by low speed centrifugation at 140 g for 10 min, and the supernatant was centrifuged at 40000 rev./min in a TLS-5S rotor for 1 h at 4 °C. The pellet was resuspended in ice-cold 100 mM sodium cacodylate buffer, pH 7.0, and used as an enzyme source for the sialyltransferase assay described below.
Sialyltransferase assay
The sialyltransferase assay was performed in a reaction mixture containing 100 mM sodium cacodylate buffer, pH 6.0, 10 mM MgCl2, 0.3% Triton CF-54, 0.64 mM CMP-NeuAc (Sigma), 6000 dpm/μl CMP-[14C]NeuAc (Amersham Biosciences), 50 μg of membrane fraction as the enzyme source, 0.1 mM glycosphingolipids or 0.4 mg/ml of glycoproteins as acceptors in a total volume of 50 μl. The reaction mixture was incubated at 37 °C for 2 h. The products were isolated using a C18 Sep-Pak cartridge (Waters) and analysed by TLC with a solvent system of chloroform/methanol/0.2% CaCl2 (50:40:10, by vol.). The plate was exposed to an imaging plate for 12 h and the radioactivity on each plate was visualized with a BAS 2000 image analyser (Fuji). For kinetic analysis, the enzyme reactions were performed using various concentrations of acceptor substrates, 0–0.2 mM MSGG.
TLC-immunostaining
TLC-immunostaining was performed as described previously [30]. MSGG and DSGG were detected using anti-MSGG mAb RM1 and anti-DSGG mAb 5F3 respectively, at a 1:1 dilution as a primary antibody. Briefly, after chromatography of the glycolipids, the TLC plate was heat-blotted onto a PVDF membrane using a TLC Thermal Blotter™ (ATTO). The membrane was incubated with the mAb for 1 h, washed, and incubated with biotinylated horse anti-mouse IgM at a 1:200 dilution for 30 min. The antibody binding was visualized with an ABC-PO Kit™ (Vector Laboratories) and HRP-1000™ (Konica).
Transfection for flow cytometric analysis and immunofluorescence assay
Caki-1 cells in a 6-cm diameter dish (Falcon) were transiently transfected with 4 ml (1 μg/ml) of pcDNA3.1, pcDNA3.1-hST6GalNAc III and pcDNA3.1-hST6GalNAc VI using Lipofectamine 2000™ (Invitogen) according to the manufacturer's instructions.
Immunofluorescence assay
Caki-1 cells were cultured on glass cover slips in 24-well plates and incubated at 37 °C for 24 h and transiently transfected with expression vectors as described above. After 48 h, the cells were fixed with cold acetone for 10 min, air-dried, and processed for indirect immunofluorescence analysis as described [31]. They were immunostained with mouse anti-DSGG mAb 5F3 and FITC-conjugated goat anti-mouse IgM, then observed using an ORCA-ER-1394 imaging system (Hamamatsu Photonics).
Immunohistochemical staining of tissue sections with mAbs RM1 and 5F3
Frozen sections of 4 μm thicknesses were prepared from surgical specimens. Sections were blocked with normal goat serum for 2 h. After blocking of endogenous biotin using the Vector Biotin Blocking Kit™ (Vector Laboratories), sections were incubated with a primary antibody for 2 h. Immunoreactivity was detected using the Vector alkaline phosphatase-conjugated ABC kit™ (Vector Laboratories) and stained with BCIT (5-bromo-4-chloro-3-indolyl phosphate)/NBT (Nitrotetrazolium Blue chloride).
Quantitative real-time RT-PCR
The quantitative RT-PCR was performed using the following primer sets: hST6GalNAc III (human ST6GalNAc III) forward, 5′-TGCTGGTTGTGCGTCTTGTA-3′ (nucleotides 86–105 in the coding sequence) and reverse, 5′-GGCCGCCTGTATGTGTAGGA-3′ (nucleotides 191–172); hST6GalNAc VI forward, 5′-TCAGCAGTGTTCGTGATCCT-3′ (nucleotides 21–40) and reverse, 5′-GAAGTGGAGCATCACTGACG-3′ (nucleotides 188–169) using the SYBR® green PCR kit (Applied Biosystems) in a DNA Engine Opticon continuous fluorescence detection system (MJ Research). All samples were quantified by the comparative cycle threshold (Ct) method for relative quantification of gene expression and normalized to GAPDH (glyceraldehydes-3-phosphate dehydrogenase) expression levels.
In situ hybridization
Expression of hST6GalNAc III and VI were detected by in situ hybrdization using PNA (peptide–nucleic acid) probes and the GenPoint™ system kit (DAKO). Frozen sections of non-tumour kidney and tumour lesions were mounted on RNAase-free glass slides. The sections were treated with TBS (Tris-buffered saline) and then digested with 2000 fold-diluted Proteinase K solution for 10 min. Hybridization was performed at 37 °C for 90 min with hybridization solution containing 1 ng/ml FITC-conjugated PNA probe. The antisense probe of hST6GalNAc III (FITC–AAACGATGCCATTGCCAT), the sense probe of hST6GalNAc III (FITC–ATGGCAATGGCATCGTTT), the antisense probe of hST6GalNAc VI (FITC–CGTCAAATTGCCGCATGC), or the sense probe of hST6GalNAc VI (FITC–GCATGCGGCAATTTGACG) was used. The slides were washed with stringent wash solution at 55 °C for 30 min and incubated with an alkaline phosphatase-labelled anti-FITC antibody. Visualization was performed with BCIT/NBT.
RESULTS
Typing of globo-series glycosphingolipids in renal cancer cell lines
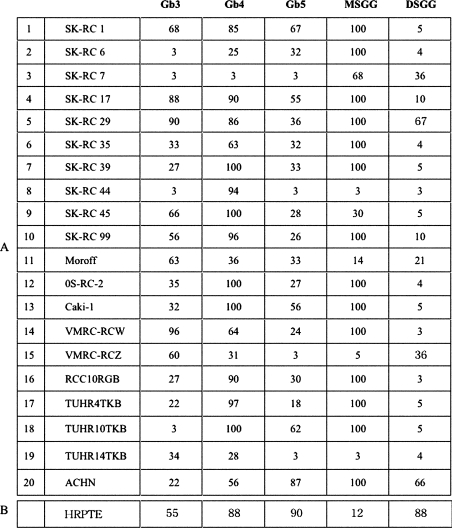
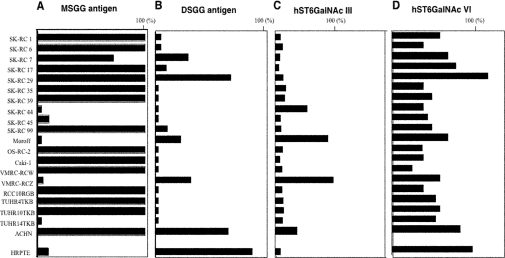
Expression of globo-series glycosphingolipids in 20 renal cancer cell lines were analysed by flow cytometry (Figure 1). High expression of Gb3 and Gb4 was detected in the renal cancer cell lines and the normal HRPTE cells. High expression levels of Gb5 were also detected in the normal HRPTE cells, whereas its expression was low in the renal cancer cell lines. In contrast, the expression of MSGG was low in the normal HRPTE cells, but high in the renal cancer lines. On the other hand, the expression of DSGG was high in the normal HRPTE cells, whereas low level expression was detected in a few cancer cell lines. Generally, renal cancer cell lines showed high expression of globo-series glycolipids, although a few lines expressed only low levels of any structures in the series. This may correlate with their slower growth rates compared with other lines with definite expression (results not shown).
Figure 1. Expression of globo-series glycosphingolipids analysed by flow cytometry.
Twenty renal cancer cell lines and HRPTE cells were examined by flow cytometry using antibodies as listed in Table 1. Expression levels were classified and are presented as percentage of cells showing more intensity than the individual controls in the histograms.
Preparation of globo-series glycosphingolipids
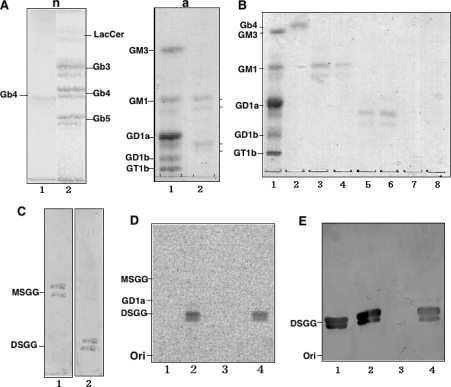
To prepare acceptor substrates, neutral and acidic glycosphingolipids obtained from ACHN cells were separated from the total glycosphingolipids by DEAE-Sephadex ion-exchange column chromatography. The neutral glycosphingolipids detected were lactosylceramide, Gb3, Gb4 and Gb5, and the acidic fraction showed two major components by HP-TLC (Figure 2A). Acidic glycosphingolipids were further separated by step-wise elution by gradually increasing concentrations of sodium acetate (Figure 2B). Mono- and di-sialo fractions were detected in lanes 3 and 4 and in lanes 5 and 6 of Figure 2(B) respectively. The presence of MSGG and DSGG in mono- and di-sialo fractions was immunologically ascertained using TLC-immunostaining with anti-MSGG and anti-DSGG antibodies respectively (Figure 2C).
Figure 2. Preparation of globo-series glycosphingolipids from ACHN cells and determination of sialyltransferase activity of hST6GalNAc III/V/VI toward MSGG.
(A) Extraction of neutral (n) and acidic (a) glycolipids from ACHN cells. n, Orcinol-sulfuric acid staining of the neutral fraction. Lane 1, Gb4; lane 2, neutral glycolipid fraction. a, Resorcinol staining of the acidic fraction. Lane 1, bovine brain gangliosides as standards; lane 2, acidic fraction. (B) Purification of MSGG and DSGG. Acidic glycolipids were eluted by step-wise elution with sodium acetate in a solvent system of chloroform/methanol/sodium acetate (30:60:8, by vol., lanes 3–10). Lane 1, ganglioside marker; lane 2, Gb4; lane 3, 0.2 M sodium acetate fraction; lane 4, 0.25 M; lane 5, 0.3 M; lane 6, 0.45 M; lane 7, 0.6 M; and lane 8, 0.8 M. (C) TLC-immunostaining. Lane 1, MSGG (eluted by 0.2 and 0.25 M sodium acetate) was immunostained with mAb RM1. Lane 2, DSGG (eluted by 0.3 and 0.45 M sodium acetate) was immunostained with mAb 5F3. (D) The sialyltransferase assay was performed using the membrane fraction with MSGG as an acceptor substrate as described in the Materials and methods section, and the products were analysed by TLC and autofluorography. Lane 1, products with membrane fractions from L cells transfected with vector control; lane 2, hST6GalNAcIII; lane 3, hST6GalNAc V; lane 4, hST6GalNAc VI. (E) TLC-immunostaining of the enzyme products was performed with mAb 5F3. Lane 1, DSGG (2.5 μg); lanes 2–4, are essentially the same as in (D) except that bands were detected with mAb 5F3.
hST6GalNAc III and VI synthesize DSGG
The enzyme activity to synthesize DSGG from MSGG was analysed using the extracts from cells transfected with hST6GalNAc-III, V or VI expression vectors. The results showed that hST6GalNAc III and VI generated two bands at the migration site of DSGG. hST6GalNAc V did not produce any new products in the TLC/autofluorogram (Figure 2D). TLC-immunostaining with an anti-DSGG antibody, 5F3, showed that both hST6GalNAc III and VI synthesized DSGG (Figure 2E).
The results of substrate specificity analyses of hST6GalNAc III and VI using various glycoproteins and glycolipids are summarized in Table 2A. Some of the data were essentially the same as reported in [21] and [23], but they were confirmed in this study using the identical conditions. Both enzymes showed activity toward fetuin, MSGG and sialyl Lc4 as well as GM1b. The activity of hST6GalNAc VI toward MSGG and sialyl Lc4 was higher than that of hST6GalNAc III. When kinetic parameters toward MSGG were roughly compared, under the assumption that there were equivalent expression levels of hST6GalNAc III and VI, there was no marked difference in Km, although the Vmax value of hST6GalNAc VI was about 2-fold higher than that of hST6GalNAc III (results not shown).
Synthesis of DSGG in cultured cells transfected with cDNA expression vectors
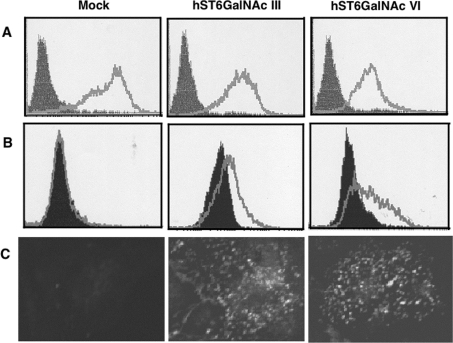
To investigate whether DSGG is synthesized in vivo, expression vector encoding hST6GalNAc III or hST6GalNAc VI cDNA was transfected into Caki-1 cells, in which a large amount of the precursor, MSGG, was detected with no expression of DSGG. The results of FACS analysis (Figures 3A and 3B) and immunofluorescence analysis (Figure 3C) are shown. Both hST6GalNAc III and VI could induce the production of DSGG and reduce that of MSGG, suggesting that both of these enzymes had enzymatic activity that could synthesize DSGG from MSGG in the cultured cells.
Figure 3. Expression of DSGG in Caki-1 cells transfected with hST6GalNAc III and VI cDNA.
(A and B) Flow cytometric patterns of Caki-1 cells transiently transfected with empty pcDNA3.1 vector (Mock), pcDNA3.1-hST6GalNAc III or VI. The expression of MSGG (A) and DSGG (B) in Caki-1 cells was detected with mAbs RM1 and 5F3 at a 1:1 dilution (cell culture supernatant) respectively. (C) Microscopic fluorescence images of Caki-1 cells transiently transfected with empty pcDNA3.1 vector, pcDNA3.1-hST6GalNAc III or VI. The cells were fixed with paraformaldehyde and permeabilized with 0.1% Triton X-100 and then processed for indirect immunofluorescence analysis as described in the Materials and methods section. Original images were obtained at 400× magnification.
Expression of the hST6GalNAc III and hST6GalNAc VI genes in various tissues
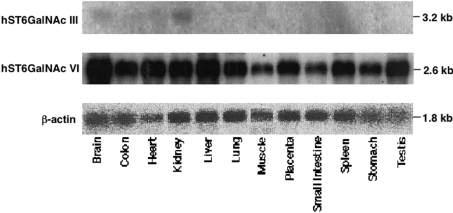
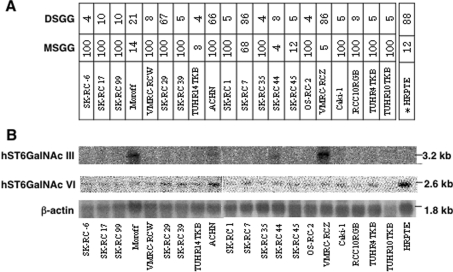
To determine the expression patterns of the hST6GalNAc III and ST6GalNAc VI genes, Northern blot analysis was performed in various human tissues (Figure 4) and human renal cancer cell lines (Figure 5). The expression of the hST6GalNAc III gene was restricted to the brain and kidney, where low expression levels of the gene were observed. The hST6GalNAc VI gene was widely expressed in all tissues.
Figure 4. Expression patterns of the hST6GalNAc III and VI genes in human tissues.
Membrane filters with 2 μg of poly(A)+ RNA from 12 major tissues were probed with 32P-labelled hST6GalNAc III or VI full-length cDNA as described in the Materials and methods section. The same filters were probed with human β-actin cDNA as a loading control after removing the original radioactivity.
Figure 5. Expression patterns of the hST6GalNAc III and VI genes in the renal cancer cell lines.
(A) The expression of MSGG and DSGG in the 20 renal cancer cell lines and HRPTE cells as analysed by flow cytometry in Figure 1 are summarized as percentage positive cells for comparison. The expression level was classified into five groups based on the percentage of positive cells. (B) Expression patterns of the hST6GalNAc III and VI genes in renal cancer cell lines. Northern blots with 15 μg of total RNA were probed with 32P-labelled hST6GalNAc III or VI full-length cDNAs. The same filters were probed with human β-actin cDNA after removing the radioactivity.
Expression of MSGG/DSGG and human ST6GalNAc III and ST6GalNAc VI genes in human renal cancer cell lines
The expression of MSGG/DSGG in 20 renal cancer cell lines was analysed by flow cytometry as shown in Figure 1 and summarized in Figure 5(A). The expression of MSGG was low in the normal HRPTE cells, although high expression was observed in the majority of the renal cancer cell lines. On the other hand, the expression of DSGG was rather high in the normal HRPTE cells, whereas only low level expression was detected in a few cancer cell lines.
In the cultured epithelial cells of the proximal kidney tubules, the expression of hST6GalNAc III mRNA was low, while hST6GalNAc VI gene expression was high. The expression of hST6GalNAc III could be detected in only approx. 20% of the cancer cell lines, although human ST6GalNAc VI gene expression was detected in the majority of the cancer cell lines examined (Figure 5B). However, the expression levels of hST6GalNAc VI in all, except one, of the cancer cell lines were lower than that in the epithelial cells of the proximal kidney tubule. The DSGG expression pattern in FACScan almost paralleled that of the ST6GalNAc VI gene (Figure 6). However, in several cases, there is little difference in ST6GalNAc VI expression, whereas DSGG is significantly different among cells. The concentration of MSGG is also important in order to explain the high level of DSGG. Consequently, these results suggested that ST6GalNAc VI plays a major role in the synthesis of DSGG in the kidney, and that ST6GalNAc VI is capable of utilizing various acceptors with at least three different cores, resulting in the synthesis of branched-type disialyl compounds.
Figure 6. Correlation between the expression levels of globo-series glycosphingolipids and those of α2,6-sialyltransferase genes in renal cancer cell lines.
Surface expression of MSGG and DSGG was analysed by flow cytometry as described in the legend for Figure 3, and percentage positive rates are presented in (A) and (B) respectively. Percentages of cells present in the areas with higher fluorescence intensity compared with the right edge of the controls' peaks in the individual histograms were calculated and are presented as percentage positive. The expression levels of hST6GalNAc III (C) and hST6GalNAc VI (D) from the Northern blot bands shown in Figure 3 are presented as relative intensities, which were measured using the NIH Image program and are presented as a ratio to intensity of the β-actin bands. The expression level of hST6GalNAc III in VMRC-RCZ was regarded as 100% in (C). The expression level of hST6GalNAc VI in normal HRPTE cells was regarded as 100% in (D).
Immunohistochemistry of MSGG and DSGG
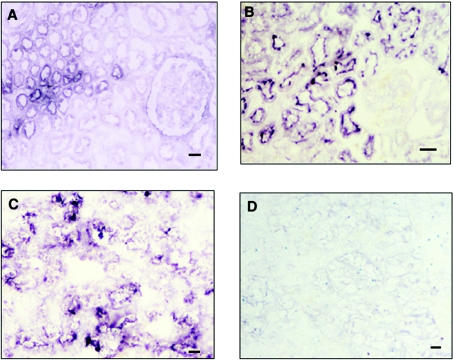
Immunohistological staining of MSGG and DSGG in normal kidney and cancer tissues from the same patients was performed. In normal kidney tissues, only a faint staining of MSGG was detected in the epithelium of proximal kidney tubule, while marked expression was detected in the epithelium of distal tubule and collecting tubule (Figure 7A). DSGG expression was detected only in the epithelium of the proximal kidney tubule (Figure 7B). MSGG was detected throughout the entire cancer tissues with strong intensity (Figure 7C), whereas DSGG was stained very weakly (Figure 7D).
Figure 7. Imunohistochemical staining of normal human kidney and renal cancer tissues from the same cases.
(A) Normal kidney section stained by mAb RM1 (100× magnification). (B) Normal kidney section stained by mAb 5F3 (200× magnification). (C) Cancer section stained by mAb RM1 (100× magnification). (D) Cancer section stained by mAb 5F3 (100× magnification). Scale bar: 40 μm.
Quantitative real-time RT-PCR
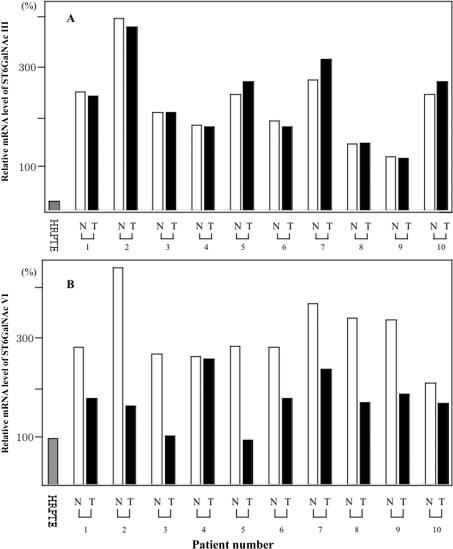
To clarify the mRNA expression of hST6GalNAc III and VI in the normal HRPTE cells, non-tumour kidney and tumour tissues, quantitative real-time RT-PCR was performed as a preliminary analysis. The expression level of hST6GalNAc VI in the normal HRPTE cells was considered as 100% (Figure 8B). The expression level of hST6GalNAc III was not different between the non-tumour kidney and tumour tissues (Figure 8A). In contrast, the expression level of hST6GalNAc VI was lower in the tumour tissues compared with those in the non-tumour kidney tissues in nine out of ten patients (Figure 8B). In fact, the difference in the expression levels between the tumour samples and the non-tumour kidney samples was statistically significant at P<0.001, although those of hST6GalNAc III were almost equivalent (P=0.458).
Figure 8. Quantitative real-time RT-PCR analysis of mRNA levels of hST6GalNAc III and VI in non-tumour kidney tissues and tumour tissues.
mRNA levels of hST6GalNAc III (A) and hST6GalNAc VI (B). The amount of expression of hST6GalNAc VI in HRPTE was regarded as 100%. N, non-tumour kidney tissues; T, tumour tissues. Each pair of non-tumour kidney and tumour tissues is from the same case.
In situ hybridization
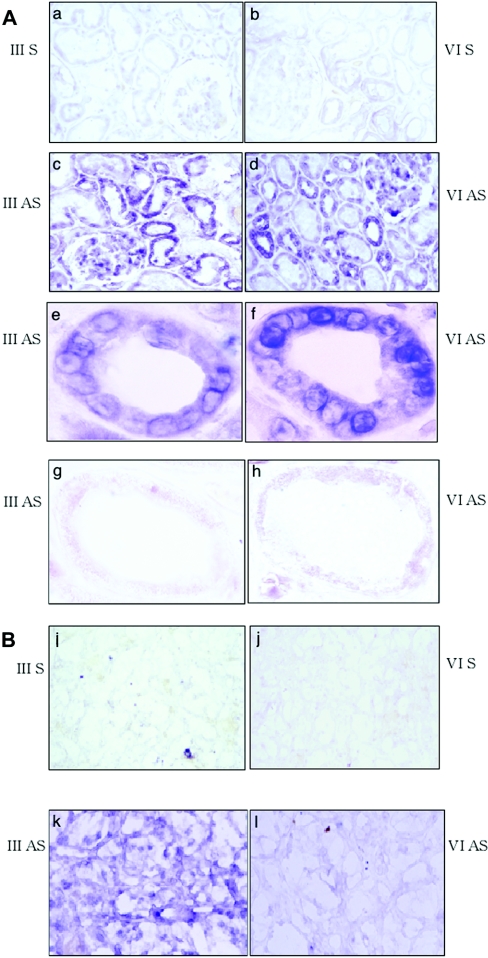
To identify the expression sites of hST6GalNAc III and VI in normal kidney tissues and cancer tissues, in situ hybridization using PNA probes was performed. Strong expression of both hST6GalNAc III and VI was observed in the epithelium of the proximal tubules and their marginal expression was also found in the distal tubules and collecting tubules (Figure 9A). In the sections of cancer tissues, the expression of hST6GalNAc III was generally high, whereas that of hST6GalNAc VI was scarcely detected (Figure 9B) corresponding to the poor staining of DSGG (Figure 7).
Figure 9. In situ hybridization using PNA probe for hST6GalNAc III/VI in non-tumour kidney and tumour sections.
(A) Non-tumour kidney sections. Panel a, sense probe of hST6GalNAc III. Panel b, sense probe of hST6GalNAc VI. Panel c, anti-sense probe of hST6GalNAc III. Panel d, anti-sense probe of hST6GalNAc VI. Panels e and f, the proximal tubule shown by higher magnification of panels c and d respectively. Panels g and h, the distal tubule shown by higher magnification of c and d respectively. (B) Tumour sections. Panel i, sense probe of hST6GalNAc III. Panel j, sense probe of hST6GalNAc VI. Panel k, anti-sense probe of hST6GalNAc III. Panel l, anti-sense probe of hST6GalNAc VI. Original magnifications are as follows, panels a–d, 200×; panels e–h, 1,000×; and panels i–l, 200×. S, sense; AS, antisense.
DISCUSSION
A number of tumour-associated carbohydrate antigens have been identified using mAbs [32]. Besides sialyl Lewis x and sialyl Lewis a, disialyl glycosphingolipids such as GD3 and GD2 have been found to be useful markers for malignant melanomas and neuroblastomas respectively. On the other hand, branched- type disialyl structures are not as abundant, except for α-series gangliosides, which were found in some animal brain tissues [33]. Also, these structures have never been found in or discussed with respect to malignant transformation. Besides α-series gangliosides, disialyl Lea [34] and disialyl T [35] have also been reported. Disialyl Lea in serum was reported to increase in patients with colon cancers and pancreatic cancers [34].
Among the six members of the ST6GalNAc family cloned to date, ST6GalNAc I and II mainly utilize asialo-structures in O-glycans [36], whereas ST6GalNAc III–VI are considered to be involved in the synthesis of disialyl-structures in α-series gangliosides and O-glycans. [19,20,37,38]. However, it was demonstrated for the first time by our group that some of these enzymes could catalyse the synthesis of branched-type disialyl structures, i.e., disialyl Lc4, leading to the synthesis of disialyl Lea [21]. This result indicated that some ST6GalNAc family members could synthesize disialyl structures onto lacto-core acceptors as well as ganglio-core and O-glycans.
In the present study, it was demonstrated that these enzymes could also synthesize branched-type disialyl structures with a globo-core, such as DSGG. In particular, it was demonstrated that ST6GalNAc VI could synthesize all these disialyl compounds containing various core structures in vivo, whereas the majority of sialyltransferases utilize relatively restricted acceptors. More over, ST6GalNAc VI gene is expressed in a wide variety of tissues in both humans and mice, and the expression levels in tissues are fairly high as indicated by the intensity of bands in Northern blotting or real time RT-PCR (results not shown). Consequently, these disialyl structures such as disialyl Lea, disialyl Lc4, DSGG and GalNAc–disialyl Lc4 might play more important roles than previously expected in various tissues where this gene is expressed.
In kidney tissues and renal cancers, many characteristic antigens have been defined by autologous typing [39] and with neo-expression of viral K-ras and H-ras oncogenes [40]. Composition analysis of glycosphingolipids in renal cancers revealed increased gangliosides in some cases [41]. Fukushi et al. [42] reported the expression of disialyl Lc4 in many tumour cells based on studies using a specific mAb. This structure was first defined by Smith and Ginsburg [43]. Later, a derivative of disialyl Lc4, GalNAc-disialyl Lc4, was shown to be a renal cancer-associated antigen [44,45]. These disialoganglioside antigens in renal cancer tissues were reported to be correlated with the metastatic potential [44]. However, most characteristic glycolipid profiles expressed in normal and malignant kidney tissues compared with other tissues are of globo-series [46]. Not only Gb3 and Gb4, but also extended globo-series structures are also enriched in the normal kidney and renal cancers [22]. MSGG was reported to be expressed in many renal cancer tissues, and DSGG was expressed in normal kidney and in about half the samples of renal cancers examined [22]. However, quantitative changes of DSGG in renal cancer tissues, and the implications of MSGG and/or DSGG expression in renal cancers have not been examined. Based on the identification of the genes responsible for the synthesis of DSGG, we analysed the expression patterns of MSGG/DSGG and ST6GalNAc VI gene in normal kidney tissues, and also in renal cancer tissues, although the case number was not high enough and the results should be considered as preliminary data.
As expected from the expression patterns of globo-series glycolipids as analysed by flow cytometry (Figure 1), many renal cancer cell lines expressed MSGG. This corresponded that MSGG was widely distributed in normal kidney tissues including proximal tubules. On the other hand, DSGG was expressed only in the proximal tubules, and not in the distal tubules and collecting tubules. However, many renal cancer cell lines and cancer tissues expressed low or no DSGG, whereas renal cancers have been believed to derive from proximal tubule epithelial cells. Consequently, ST6GalNAc VI that was shown to be responsible for the synthesis of DSGG should be downregulated during the transformation of normal proximal tubule epithelial cells. Comparison of ST6GalNAc VI mRNA between normal and cancerous tissues of 10 patients clearly demonstrated the downregulation of ST6GalNAc VI in renal cancer tissues, leading to the ‘maturation arrest’ of the globo-series glycolipids. When compared with the DSGG staining in renal cancer tissues in Figure 7(D), mRNA levels of ST6GalNAc VI appeared to be fairly high in the tumours as well as in the normal tissues. This might imply that action of ST6GalNAc VI as a synthetic enzyme is not very efficient. These results suggest the roles of MSGG/DSGG in normal and malignant kidney tissues, and the function analyses are now on-going in our laboratories.
Analyses of siglec proteins revealed that siglec-7 recognizes disialyl structures [17]. Siglec-7 reacts with tandem disialyl structures such as GD3 and GD1b, and the reaction induces modulation of natural killer cell activity [47]. Moreover, Siglec-7 also recognizes branched-type disialyl structures, i.e. DSGG, disialyl Lc4 and GalNAc-disialyl Lc4 [48], suggesting that clump formation based on the aggregation of blood mononuclear cells and tumour cells due to the interaction of these molecules might cause embolism of microvasculature and tumour metastasis. If so, the fact that only few cases in renal cancer cell lines and cancer tissues showed sustained expression of ST6GalNAc VI and high levels of DSGG seemed controversial, given the expected biological function of DSGG as described above. Identification of physiological ligand molecules for MSGG and/or DSGG is essential to clarify these issues, and remains to be investigated. Availability of the genes that encode the enzymes which synthesize these disialyl structures would certainly contribute to understanding the biological function of the enzymatic products, or to classify the renal cancers depending on the phenotye of globo-series glycolipids. This would help to predict prognosis, and might give insights into the development of novel therapeutic approaches to cancers.
Online data
Acknowledgments
We thank Dr S. Hakomori (Pacific Northwest Research Institute, Seattle, WA, U.S.A.) and Dr A. Suzuki (Riken Frontier Research Systems, Wako, Japan) for kindly providing mAbs (mAbs RM1 and 5F3, and mAb 5A3 respectively). We also thank Dr M. Uchikawa (Tokyo Blood Center, Japanese Red Cross, Tokyo, Japan) and Dr J. Wiels (Institut Gustare, Rousy, France) for providing mAbs HIRO 34 and 38.13 respectively. We acknowledge Ms. T. Mizuno and Ms. Y. Nakayasu for excellent technical assistance. This study was supported by Grants-in-Aid for Scientific Research of Priority Areas (14082102) from the Ministry of Education, Culture, Science Sports and Technology, and by a grant from the CREST (Clinical Research Enhancement through Supplemental Training) programme of the Japan Science and Technology Agency. This study was also partly supported by NEDO (New Energy and Industrial Technology Development Organization). Ganglioside nomenclature was based on that of Svennerholm [49].
References
- 1.Varki A. Sialic acids as ligands in recognition phenomena. FASEB J. 1997;11:248–255. doi: 10.1096/fasebj.11.4.9068613. [DOI] [PubMed] [Google Scholar]
- 2.Drinnan N. B., Halliday J., Ramsdale T. Inhibitors of sialyltransferases: potential roles in tumor growth and metastasis. Mini. Rev. Med. Chem. 2003;3:501–517. doi: 10.2174/1389557033487881. [DOI] [PubMed] [Google Scholar]
- 3.Hennet T., Chui D., Paulson J. C., Marth J. D. Immune regulation by the ST6Gal sialyltransferase. Proc. Natl. Acad. Sci. U.S.A. 1998;95:4504–4509. doi: 10.1073/pnas.95.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okada M., Itoh M., Haraguchi M., Okajima T., Inoue M., Oishi H., Matsuda Y., Iwamoto T., Kawano T., Fukumoto S., et al. b-Series ganglioside deficiency exhibits no definite changes in the neurogenesis and the sensitivity to Fas mediated apoptosis but impairs regeneration of the lesioned hypoglossal nerve. J. Biol. Chem. 2002;277:1633–1636. doi: 10.1074/jbc.C100395200. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita T., Hashiramoto A., Haluzik M., Mizukami H., Beck S., Norton A., Kono M., Tsuji S., Daniotti J. L., Werth N., et al. Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc. Natl. Acad. Sci. U.S.A. 2003;100:3445–3449. doi: 10.1073/pnas.0635898100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angata K., Long J. M., Bukalo O., Lee W., Dityatev A., Wynshaw-Boris A., Schachner M., Fukuda M., Marth J. D. Sialyltransferase ST8Sia-II assembles a subset of polysialic acid that directs hippocampal axonal targeting and promotes fear behavior. J. Biol. Chem. 2004;279:32603–32613. doi: 10.1074/jbc.M403429200. [DOI] [PubMed] [Google Scholar]
- 7.Eckhardt M., Bukalo O., Chazal G., Wang L., Goridis C., Schachner M., Gerardy-Schahn R., Cremer H., Dityatev A. Mice deficient in the polysialyltransferase ST8SialV/PST-1 allow discrimination of the roles of neural cell adhesion molecule protein and polysialic acid in neural development and synaptic plasticity. J. Neurosci. 2000;20:5234–5244. doi: 10.1523/JNEUROSCI.20-14-05234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harduin-Lepers A., Vallejo-Ruiz V., Krzewinski-Recchi M. A., Samyn-Petit B., Julien S., Delannoy P. The human sialyltransferase family. Biochimie. 2001;83:727–737. doi: 10.1016/s0300-9084(01)01301-3. [DOI] [PubMed] [Google Scholar]
- 9.Brocco M., Pollevick G. D., Frasch A. C. Differential regulation of polysialyltransferase expression during hippocampus development: implications for neuronal survival. J. Neurosci. Res. 2003;74:744–753. doi: 10.1002/jnr.10781. [DOI] [PubMed] [Google Scholar]
- 10.Dall'Olio F., Chiricolo M. Sialyltransferases in cancer. Glycoconj. J. 2001;18:841–850. doi: 10.1023/a:1022288022969. [DOI] [PubMed] [Google Scholar]
- 11.Furukawa K., Akagi T., Nagata Y., Yamada Y., Shimotohno K., Cheung N. K., Shiku H., Furukawa K. GD2 ganglioside on human T-lymphotropic virus type I-infected T cells: possible activation of β-1,4-N-acetyl-galactosaminyltransferase gene by p40tax. Proc. Natl. Acad. Sci. U.S.A. 1993;90:1972–1976. doi: 10.1073/pnas.90.5.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okada M., Furukawa K., Yamashiro S., Yamada Y., Haraguchi M., Horibe K., Kato K., Tsuji Y., Furukawa K. High expression of ganglioside α-2,8-sialyltransferase (GD3 synthase) gene in adult T-cell leukemia cells unrelated to the gene expression of human T-lymphotropic virus type I. Cancer Res. 1996;56:2844–2848. [PubMed] [Google Scholar]
- 13.Yoshida S., Fukumoto S., Kawaguchi H., Sato S., Ueda R., Furukawa K. Ganglioside G(D2) in small cell lung cancer cell lines. Cancer Res. 2001;61:4244–4252. [PubMed] [Google Scholar]
- 14.Thurin J., Thurin M., Herlyn M., Elder D. E., Steplewski Z., Clark W. H., Jr, Koprowski H. GD2 ganglioside biosynthesis is a distinct biochemical event in human melanoma tumor progression. FEBS Lett. 1986;208:17–22. doi: 10.1016/0014-5793(86)81522-8. [DOI] [PubMed] [Google Scholar]
- 15.Kuo C. T., Bostick P. J., Irie R. F., Morton D. L., Conrad A. J., Hoon D. S. Assessment of messenger RNA of b1→4-N-acetylgalactosaminyl-transferase as a molecular marker for metastatic melanoma. Clin. Cancer Res. 1998;4:411–418. [PubMed] [Google Scholar]
- 16.Nakano J., Raj B. K., Asagami C., Lloyd K. O. Human melanoma cell lines deficient in GD3 ganglioside expression exhibit altered growth and tumorigenic characteristics. J. Invest. Dermatol. 1996;107:543–548. doi: 10.1111/1523-1747.ep12582802. [DOI] [PubMed] [Google Scholar]
- 17.Angata T., Varki A. Siglec-7: a sialic acid-binding lectin of the immunoglobulin superfamily. Glycobiology. 2000;10:431–438. doi: 10.1093/glycob/10.4.431. [DOI] [PubMed] [Google Scholar]
- 18.Nicoll G., Ni J., Liu D., Klenerman P., Munday J., Dubock S., Mattei M. G., Crocker P. R. Identification and characterization of a novel siglec, siglec-7, expressed by human natural killer cells and monocytes. J. Biol. Chem. 1999;274:34089–34095. doi: 10.1074/jbc.274.48.34089. [DOI] [PubMed] [Google Scholar]
- 19.Okajima T., Chen H. H., Ito H., Kiso M., Tai T., Furukawa K., Urano T., Furukawa K. Molecular cloning and expression of mouse GD1α/GT1aα/GQ1bα synthase (ST6GalNAc VI) gene. J. Biol. Chem. 2000;275:6717–6723. doi: 10.1074/jbc.275.10.6717. [DOI] [PubMed] [Google Scholar]
- 20.Okajima T., Fukumoto S., Ito H., Kiso M., Hirabayashi Y., Urano T., Furukawa K. Molecular cloning of brain-specific GD1α synthase (ST6GalNAc V) containing CAG/glutamine repeats. J. Biol. Chem. 1999;274:30557–30562. doi: 10.1074/jbc.274.43.30557. [DOI] [PubMed] [Google Scholar]
- 21.Tsuchida A., Okajima T., Furukawa K., Ando T., Ishida H., Yoshida A., Nakamura Y., Kannagi R., Kiso M., Furukawa K. Synthesis of disialyl Lewis a (Lea) structure in colon cancer cell lines by a sialyltransferase, ST6GalNAc VI, responsible for the synthesis of α-series gangliosides. J. Biol. Chem. 2003;278:22787–22794. doi: 10.1074/jbc.M211034200. [DOI] [PubMed] [Google Scholar]
- 22.Ito A., Saito S., Masuko T., Oh-Eda M., Matsuura T., Satoh M., Nejad F. M., Enomoto T., Orikasa S., Hakomori S. I. Monoclonal antibody (5F3) defining renal cell carcinoma-associated antigen disialosyl globopentaosylceramide (V3NeuAcIV6 NeuAcGb5), and distribution pattern of the antigen in tumor and normal tissues. Glycoconj. J. 2001;18:475–485. doi: 10.1023/a:1016281002344. [DOI] [PubMed] [Google Scholar]
- 23.Tsuchida A., Ogiso M., Nakamura Y., Kiso M., Furukawa K., Furukawa K. Molecular cloning and expression of human ST6GalNAc III: restricted tissue distribution and substrate specificity. J. Biochem. 2005;138:237–243. doi: 10.1093/jb/mvi124. [DOI] [PubMed] [Google Scholar]
- 24.Satoh M., Handa K., Saito S., Tokuyama S., Ito A., Miyao N., Orikasa S., Hakomori S. Disialosy galactosylgloboside as an adhesion molecule expressed on renal cell carcinoma and its relationship to metastatic potential. Cancer Res. 1996;156:1932–1938. [PubMed] [Google Scholar]
- 25.Katagiri Y. U., Ohmi K., Katagiri C., Sekino T., Nakajima H., Ebata T., Kiyokawa N., Fujimoto J. Prominent immunogenicity of mono-sialosyl galactosylgloboside, carrying a stage-specific embryonic antigen-4 (SSEA-4) epitope in the ACHN human renal tubular cell line: a simple method for producing monoclonal antibodies against detergent-insoluble microdomains/raft. Glycoconj. J. 2001;18:347–353. doi: 10.1023/a:1013673300717. [DOI] [PubMed] [Google Scholar]
- 26.Iwamura K., Furukawa K., Uchikawa M., Sojka B. N., Kojima Y., Wiels J., Shiku H., Urano T., Furukawa K. The blood group P1 synthase gene is identical to the Gb3/CD77 synthase gene. A clue to the solution of the P1/P2/p puzzle. J. Biol. Chem. 2003;278:44429–44438. doi: 10.1074/jbc.M301609200. [DOI] [PubMed] [Google Scholar]
- 27.Marcus D. M., Gilbert S., Sekine M., Suzuki A. Monoclonal antibodies that bind to galactosylgloboside (SSEA-3 antigen) Arch. Biochem. Biophys. 1988;262:620–625. doi: 10.1016/0003-9861(88)90414-6. [DOI] [PubMed] [Google Scholar]
- 28.Furukawa K., Chait B. T., Lloyd K. O. Identification of N-glycolylneuraminic acid-containing gangliosides of cat and sheep erythrocytes. 252Cf fission fragment ionization mass spectrometry in the analysis of glycosphingolipids. J. Biol. Chem. 1988;263:14939–14947. [PubMed] [Google Scholar]
- 29.Nagata Y., Yamashiro S., Yodoi J., Lloyd K. O., Shiku H., Furukawa K. Expression cloning of β1, 4 N-acetylgalactosaminyltransferase cDNAs that determine the expression of GM2 and GD2 gangliosides. J. Biol. Chem. 1992;267:12082–12089. [PubMed] [Google Scholar]
- 30.Kojima Y., Fukumoto S., Furukawa K., Okajima T., Wiels J., Yokoyama K., Suzuki Y., Urano T., Ohta M., Furukawa K. Molecular cloning of globotriaosylceramide/CD77 synthase, a glycosyltransferase that initiates the synthesis of globo-series glycosphingolipids. J. Biol. Chem. 2000;275:15152–15156. doi: 10.1074/jbc.M909620199. [DOI] [PubMed] [Google Scholar]
- 31.Furukawa K., Yokoyama K., Sato T., Wiels J., Hirayama Y., Ohta M., Furukawa K. Expression of the Gb3/CD77 synthase gene in megakaryoblastic leukemia cells: implication in the sensitivity to verotoxins. J. Biol. Chem. 2002;277:11247–11254. doi: 10.1074/jbc.M109519200. [DOI] [PubMed] [Google Scholar]
- 32.Hakomori S. Cancer-associated glycosphingolipid antigens: their structure, organization, and function. Acta Anat. (Basel) 1998;161:79–90. doi: 10.1159/000046451. [DOI] [PubMed] [Google Scholar]
- 33.Taki T., Hirabayashi Y., Ishikawa H., Ando S., Kon K., Tanaka Y., Matsumoto M. A ganglioside of rat ascites hepatoma AH 7974F cells. Occurrence of a novel disialoganglioside (GD1α) with a unique N-acetyl-neuraminosyl (α2-6)-N-acetylgalactosamine structure. J. Biol. Chem. 1986;261:3075–3078. [PubMed] [Google Scholar]
- 34.Kannagi R., Kitahara A., Itai S., Zenita K., Shigeta K., Tachikawa T., Noda A., Hirano H., Abe M., Shin S. Quantitative and qualitative characterization of human cancer-associated serum glycoprotein antigens expressing epitopes consisting of sialyl or sialyl-fucosyl type 1 chain. Cancer Res. 1988;48:3856–3863. [PubMed] [Google Scholar]
- 35.Harduin-Lepers A., Stokes D. C., Steelant W. F., Samyn-Petit B., Krzewinski-Recchi M. A., Vallejo-Ruiz V., Zanetta J. P., Auge C., Delannoy P. Cloning, expression and gene organization of a human Neu5Acα2–3Galβ1–3GalNAc α2,6-sialyltransferase: hST6GalNAcIV. Biochem. J. 2000;352:37–48. [PMC free article] [PubMed] [Google Scholar]
- 36.Marcos N. T., Pinho S., Grandela C., Cruz A., Samyn-Petit B., Harduin-Lepers A., Almeida R., Silva F., Morais V., Costa J., et al. Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialyl-Tn antigen. Cancer Res. 2004;64:7050–7057. doi: 10.1158/0008-5472.CAN-04-1921. [DOI] [PubMed] [Google Scholar]
- 37.Sjoberg E. R., Kitagawa H., Glushka J., van Halbeek H., Paulson J. C. Molecular cloning of a developmentally regulated N-acetylgalactosamine α2,6-sialyl-transferase specific for sialylated glycoconjugates. J. Biol. Chem. 1996;271:7450–7459. doi: 10.1074/jbc.271.13.7450. [DOI] [PubMed] [Google Scholar]
- 38.Lee Y. C., Kaufmann M., Kitazume-Kawaguchi S., Kono M., Takashima S., Kurosawa N., Liu H., Pircher H., Tsuji S. Molecular cloning and functional expression of two members of mouse NeuAcα2,3Galβ1,3GalNAc GalNAcα2,6- sialyltransferase family, ST6GalNAc III and IV. J. Biol. Chem. 1999;274:11958–11967. doi: 10.1074/jbc.274.17.11958. [DOI] [PubMed] [Google Scholar]
- 39.Ueda R., Shiku H., Pfreundschuh M., Takahashi T., Li L. T., Whitmore W. F., Oettgen H. F., Old L. J. Cell surface antigens of human renal cancer defined by autologous typing. J. Exp. Med. 1979;150:564–579. doi: 10.1084/jem.150.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nanus D. M., Ebrahim S. A., Bander N. H., Real F. X., Pfeffer L. M., Shapiro J. R., Albino A. P. Transformation of human kidney proximal tubule cells by ras-containing retroviruses. Implications for tumor progression. J. Exp. Med. 1989;169:953–972. doi: 10.1084/jem.169.3.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karlsson K. A., Samuelsson B. E., Schersten T., Steen G. O., Wahlqvist L. The sphingolipid composition of human renal carcinoma. Biochim. Biophys. Acta. 1974;337:349–355. doi: 10.1016/0005-2760(74)90110-6. [DOI] [PubMed] [Google Scholar]
- 42.Fukushi Y., Nudelmanm E., Levery S. B., Higuchi T., Hakomori S. A novel disialo-ganglioside (IV3NeuAcIII6NeuAcLc4) of human adenocarcinoma and the monoclonal antibody (FH9) defining this disialosyl structure. Biochemistry. 1986;25:2859–2866. doi: 10.1021/bi00358a018. [DOI] [PubMed] [Google Scholar]
- 43.Smith D. F., Ginsburg V. Antibodies against sialyloligosaccharides coupled to protein. J. Biol. Chem. 1980;255:55–59. [PubMed] [Google Scholar]
- 44.Ito A., Levery S. B., Saito S., Satoh M., Hakomori S. A novel ganglioside isolated from renal cell carcinoma. J. Biol. Chem. 2001;276:16695–16703. doi: 10.1074/jbc.M011791200. [DOI] [PubMed] [Google Scholar]
- 45.Saito S., Levery S. B., Salyan M. E., Goldberg R. I., Hakomori S. Common tetrasaccharide epitope NeuAcα2→3Galβ1→3(Neu-Acα2→6)GalNAc, presented by different carrier glycosylceramides or O-linked peptides, is recognized by different antibodies and ligands having distinct specificities. J. Biol. Chem. 1994;269:5644–5652. [PubMed] [Google Scholar]
- 46.Sekine M., Suzuki M., Inagaki F., Suzuki A., Yamakawa T. A new extended globoglycolipid carrying the stage specific embryonic antigen-1 (SSEA-1) determinant in mouse kidney. J. Biochem. 1987;101:553–562. doi: 10.1093/jb/101.3.553. [DOI] [PubMed] [Google Scholar]
- 47.Nicoll G., Avril T., Lock K., Furukawa K., Bovin N., Crocker P. R. Ganglioside GD3 expression on target cells can modulate NK cell cytotoxicity via siglec-7-dependent and -independent mechanisms. Eur. J. Immunol. 2003;33:1642–1648. doi: 10.1002/eji.200323693. [DOI] [PubMed] [Google Scholar]
- 48.Ito A., Handa K., Withers D. A., Satoh M., Hakomori S. Binding specificity of siglec7 to disialogangliosides of renal cell carcinoma: possible role of disialogangliosides in tumor progression. FEBS Lett. 2001;504:82–86. doi: 10.1016/s0014-5793(01)02734-x. [DOI] [PubMed] [Google Scholar]
- 49.Svennerholm L. Chromatographic separation of human brain gangliosides. J. Neurochem. 1963;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.