Abstract
Patients with congestive heart failure have impaired cognitive function, possibly caused by impaired global and regional cerebral blood flow. We hypothesized that biventricular pacing—simultaneous activation of the both the septum and the lateral wall of the left ventricle—would improve neurocognitive function and improve quality of life. Ten patients were examined before and after pacemaker implantation with standard measures of neurocognitive function. There were significant improvements in neurocognitive measures of attention (Digit Span: 50 ± 5 vs. 57 ± 7, p = 0.04) and information processing (Symbol Digit: 39 ± 9 vs. 49 ± 15, p = 0.04). There were also improvements in two psychosocial measure of quality of life: Left Ventricular Dysfunction-36 (13 ± 7 vs. 7 ± 5, p = 0.004) and Minnesota Living with Heart Failure Questionnaire (49 ± 25 vs. 23 ± 14, p = 0.02). These results translate into clinically significant functional benefits. We conclude that biventricular pacing improves cognitive and psychosocial outcomes, specifically in the domains of attention and speed of information processing.
Case presentation
A 75-year-old man with inoperable coronary artery disease presented with New York Heart Association (NYHA) functional class IV congestive heart failure (CHF), an ejection fraction of 25%, and a left bundle branch block morphology. He had had multiple episodes of syncope, including one while in the hospital on telemetry, thereby documenting sustained monomorphic ventricular tachycardia as the etiology. Despite this evidence, the patient refused implantation of a cardioverter defibrillator and left the hospital against medical advice. He appeared not to understand our explanation of the significance of ventricular arrhythmias and their relationship to mortality despite a college-level education.
Two weeks later, the patient returned to the hospital with a head injury caused by syncope and again left the hospital against medical advice. He was slow to respond verbally and belligerent when interacting with staff.
On the patient's next return to the hospital with syncope, his wife refused to take him home, and he finally consented to the implantation of a biventricular pacemaker with a cardiac defibrillator. During his follow-up visit to our outpatient clinic, we met a charming, completely compliant, educated man, which led to our hypothesis that biventricular pacing improves not only quality of life, exercise time, ejection fraction and multiple other measurable clinical parameters, but also cognition.
Background
Despite advances in pharmacologic therapy (1–4), the prognosis of patients with NYHA class III and IV CHF remains poor (5) and, therefore, innovative strategies for better management of this common clinical problem have been sought (6–9). Ventricular dysfunction is a hallmark of CHF and is frequently associated with ventricular conduction delays. Stevenson, et al reported that 30% of patients with CHF have intraventricular conduction disturbances severe enough to cause dyssynchronous ventricular contractions resulting in decreased cardiac output and ventricular filling time (10). Intraventricular conduction delays, usually left bundle branch blocks, may cause segments of the heart to contract at different times resulting in worsening mitral regurgitation, increased systolic ejection time, and a subsequent decrease in diastolic filling time. Technological advances in implantable intracardiac devices—implantable cardioverter defibrillators (ICDs) with biventricular pacing capabilities and biventricular pacemakers—now allow their use as a mainstay of CHF treatment in combination with aggressive medical therapy.
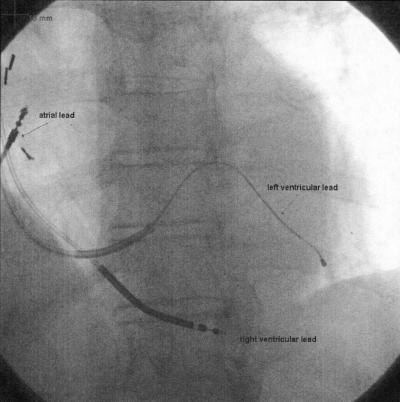
Cardiac resynchronization therapy (CRT), known alternatively as biventricular pacing, is the simultaneous pacing of the right and left ventricles, an approach that can improve symptoms and outcomes in some patients with CHF (Figure 1). CRT is an unique therapy for patients with CHF and mechanical dyssynchrony. Its therapeutic intent is to improve the mechanical efficiency of the heart by “resynchronizing” ventricular contraction. In multiple clinical trials, CRT has been shown to improve distance walked in six minutes, NYHA functional class, quality of life, ejection fraction, mortality and time to first hospitalization. We hypothesized that patient cognition would also measurably improve with biventricular pacing. The purpose of this study was to investigate changes in attention and cognitive function in CRT patients before and after implantation.
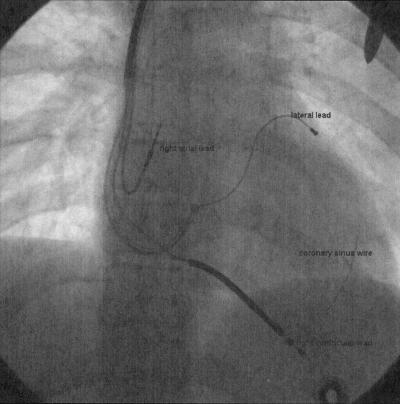
Fig. 1.
Fluoroscopic image of a heart with a biventricular pacing system implanted. Specifically visualized are the right atrial, right ventricular and left ventricular (coronary sinus) leads. Also apparent is the guidewire inserted in the coronary sinus over which the lead is advanced for placement.
Methods
We assessed memory, attention, processing speed, quality of life and depression in 10 patients before and after implantation of a biventricular pacemaker. The cognitive measures used included the Hopkins Verbal Learning Test (HVLT) for memory and the Digit Span Subtest of the Wechsler Adult Intelligence Test-III (WAIS-III) for attention (Figure 2). We also assessed processing speed using the Symbol Digit Modalities Subtest of the WAIS-III (Figure 3). At the same time, psychosocial measures of cardiac quality of life and depression were taken using the Minnesota Living with Heart Failure Questionnaire (MLHFQ), the Left Ventricular Dysfunction-36 and the Center for Epidemiological Studies Depression Scale (CES-D).
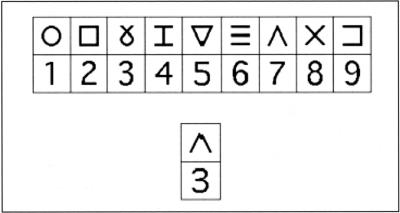
Fig. 2.
Example of Symbol Digit trials. First line indicates the symbols and corresponding digits, while the second line indicates an incorrect coding by the subject.
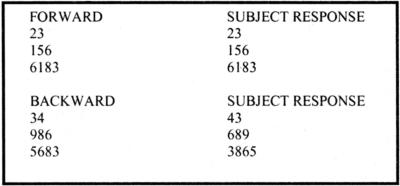
Fig. 3.
Example of Digit Span in which subjects are required to repeat back series of increasing digits introduced to them in an auditory manner.
Implantation Techniques
Standard atrial and ventricular pacing leads were placed in both the right atrium and the right ventricle. To achieve ventricular resynchronization, a specially designed lead was advanced into a lateral or posterolateral branch of the coronary sinus to pace the left ventricle. To achieve accurate lead placement, retrograde venography was performed by inserting commercially-available balloon-tipped catheters into the coronary sinus. The balloon was temporarily inflated to occlude flow in the coronary sinus, and contrast was injected to opacify the left ventricular venous system. Images were captured as a “roadmap” in multiple views; generally, 30 degrees right anterior oblique, 30 degrees left anterior oblique and anteroposterior to best define the major venous structures and their branches (Figure 4). The balloon catheter was then removed, and a guide catheter was inserted into the coronary sinus os. Next, the coronary sinus pacing lead was advanced into a lateral venous branch of the coronary sinus, and threshold testing was performed (Figure 5). All leads were then connected to the pulse generator—either a pacemaker or an ICD.
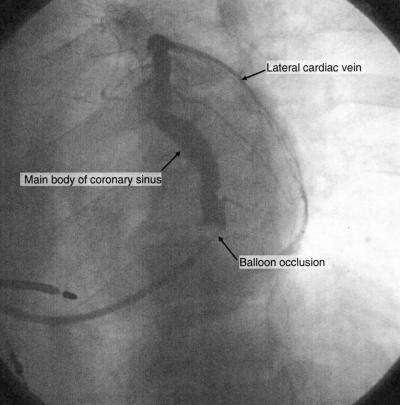
Fig. 4.
Fluoroscopic image of a coronary sinus venogram in the LAO projection during balloon occlusion that demonstrates the main body of the coronary sinus, as well as a lateral branch that wraps around the left ventricle.
Fig. 5.
Fluoroscopic image of a left ventricular lead in a mid lateral branch of the coronary sinus. This site is ideal for biventricular pacing.
Results
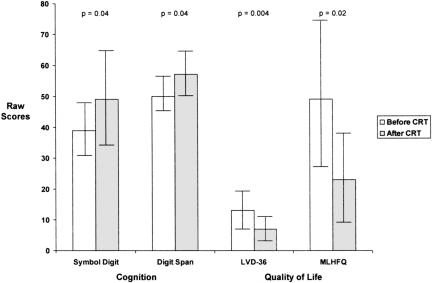
Ten patients had complete data sets and were willing to participate in a one-hour battery of tests at the University of Florida. Their demographic information is seen in Table 1, their cognitive and quality of life test results in Figure 6.
TABLE 1.
Demographic Characteristics of Patients Analyzed
| Demographics | Mean |
|---|---|
| Age (years) | 52 ± 11* |
| Sex (male) | 87% |
| Ethnicity (Caucasian) | 100% |
Data presented as mean ± standard deviation.
Fig. 6.
Measures of cognition and quality of life before and after cardiac resynchronization therapy. MLHFQ = Minnesota Living with Heart Failure Questionnaire. A decrease in score on the MLHFQ indicates improvement in quality of life. Data are depicted as means and standard deviations (error bars).
No difference was demonstrated in verbal memory (HVLT) before and after CRT. However, there was significant improvement in processing speed (Symbol Digit; t[10] = −2.569, p = 0.04) when measured three months after CRT as compared to previous. The average t score increased from 39 ± 9 to 49 ± 15. There was also significant improvement in attention processing (Digit Span; t[10] = −2.398, p = 0.04) three months after CRT when compared to before (50 ± 5 vs. 57 ± 7).
Quality of life also improved after three months as measured by the LVD-36; t[8] = 4.127, p = 0.004 (13 ± 7 vs. 7 ± 5) and the Minnesota Living with Heart Failure Questionnaire; t[8] = 3.129, p = 0.015 (49 ± 25 to 23 ± 14) (decreased scores on quality of life measures indicate improvement). When depression was evaluated, it did not change with the addition of CRT because the majority of participants did not meet criteria for depression at baseline in this small sample.
Discussion
About 70–80% of patients who receive CRT respond positively (11–12). The topic of response versus nonresponse is complex (13) with both clinical and echocardiographic parameters typically used for assessment. Other parameters considered are clinical symptoms such as dyspnea, ability to walk and quality of life (which are subjective), as well as rates of hospitalization and mortality (which can be more reliably measured). Echocardiographic measurements suggesting response include improvement in left ventricular ejection fraction and evidence of reverse left ventricular remodeling (decrease in left ventricular systolic and diastolic diameters and volumes) (14). Our data suggest that another objective measurement of response to CRT is improvement in cognition.
Decline in cognitive functioning is a recognized outcome in patients with heart disease (15–16). Large scale epidemiological studies—for example, the Framingham Heart Study (17)—demonstrate that there are cognitive domains that are differentially affected by decreased cerebral perfusion and atherosclerotic changes, specifically attention and processing speed. Areas of the brain such as the hippocampus, periventricular white matter areas, and watershed areas are known to be susceptible to hypoperfusion (14). Another factor effecting perfusion in the brain is longstanding hypertension in older adults who undergo cardiac procedures. Pre- and post-coronary artery bypass grafting (CABG) studies demonstrate short-term decline (one to three months) in areas such as attention, new learning and psychomotor abilities (18).
In a large review of cognitive impairment in CHF (similar to many who receive BiV ICDs), Clark and McDougall demonstrated etiologies similar to those in CABG patients with cognitive dysfunction (19). Most patients with significant CHF have intermittent cerebral hypoperfusion. Low cardiac output states result in chronic cerebral ischemia which leads to mild-to-moderate changes in subtle aspects of cognition. Reviews of the transplant literature also point to a reversal of cognitive complaints three to six months after successful heart transplantation (20). Similarly, studies examining cognitive abilities in patients with left ventricular assist devices (LVADs) demonstrate that individuals perform better on measures of attention and short-term memory after the device implantation, further enforcing the relationship between increasing cardiac output and cognitive function (21).
A more objective test of global cognitive changes in patients is the use of P300 evoked potentials. P300 evoked potentials are the result of activation of a large cortical network captured by EEG during an auditory task and are commonly used in patients with metabolic disorders and patients undergoing open heart surgery and heart transplantation to demonstrate areas of increased regional cerebral blood flow. Zimper and colleagues (22) used P300 evoked potentials to show cognitive changes in patients after LVAD implantation. This study entailed neurocognitive EEG testing prior to implant, eight weeks after implant and twelve weeks after implant. Results of the study demonstrated that patients had improvement in neurocognitive functioning eight and twelve weeks after LVAD implantation, though the direct mechanism of this change remains to be examined.
Summary
Preliminary evidence demonstrates that CRT improves both cognition and quality of life within three months of treatment. Tasks that tap into attentional domains and processing speed appear to become easier (fewer errors) for patients after CRT. Are our patients smarter because of this therapy? Probably not. But they certainly seem more able to pay attention and to process information more quickly, both of which contribute to improvement in quality of life.
Limitations
Our sample size is small with little diversity. Additionally, all of our patients were CRT responders by classic definitions.
Clinical Implications
CHF patients report memory complaints that may be better explained by attention limitations. As patient education efforts are dependent on attention, it is critical to include spouses/family in educational programs, particularly prior to implantation of a device. The literature is replete with evidence that patients don't remember what we tell them, that is, they only recall about 50% of the information given to them. That may also be why they don't do everything we ask of them. CRT offers our patients the potential for heightened attention to detail and more rapid processing of information.
ACKNOWLEDGMENTS
The authors wish to thank Neha K. Dixit, M.S. for data collection and statistical analysis and Lisa A. Hamilton, M.A. for editorial assistance and manuscript preparation.
DISCUSSION
Mackowiak: Baltimore: Very nice, thank you. I realized this might be difficult to do because of blinding problems, but with regard to the question of were these improvements in cognition clinically significant, did you make any effort to question family members or spouses to see if they noted a change as a result of the intervention?
Conti: Gainesville: That is a good question. The psychology people are the ones who actually did the questioning, so I wasn't there, but they routinely include family. What they tell me is that this is about a 90 minute battery of tests that the patients have to undergo and that these numbers translate into clinical significance, but we should go back and ask the families. That is a good idea.
Friesinger: Nashville: Thank you very much, Jamie. What data do you have, at least in the correlative sense, that might tell us about this improved functional status? Like information about ejection fraction or wall motion abnormality or other hemodynamic things?
Conti: That is a good question too. We have 20 patients in this study right now. We are going back and getting together the before and after echos. We have an echo immediately prior to the BiV pacer implantation and then an echo three months out. We haven't completely evaluated that data yet. But, you know, in populations, in all the big studies of BiV pacing, ejection fraction increases; blood pressure increases; cardiac output increases. You know, I am not sure if with 20 patients that we are going to see a huge difference, but in populations, we certainly do.
Friesinger: The other thing, because we in cardiology have a tendency to overuse, if not abuse these highly technical procedures, you commented about wide QRS and left bundle branch blockage and indication for use of this technique. Would you comment what the thinking is about what kinds of patients are going to benefit from this technique?
Conti: Certainly. The patients that have an indication for this kind of device are the patients with heart failure with an ejection fraction of less than 35% and a left bundle branch block. There is also some data that patients with right bundle branch block will benefit as well, but it's not as well done as the left bundle information. These patients should be either class III or class IV congestive heart failure, and they can be either ischemic or non-ischemic. Initially, people thought the non-ischemics got better more, but that hasn't really born out. So, ejection fraction less than 35%, left bundle, class III or IV heart failure, we can get these devices into about 95% of those people transvenously. About 80 to 85% actually feel better, but I mean the people that do get better, it's a miracle. I mean some of these people go from being bedridden to being able to go out and walk a half mile or a mile, which is a total change in their quality of life.
Hillis: Dallas: Do you have any control data on patients with class IV heart failure who are managed with intensive medical therapy, including frequent visits like these patients had, who did not have ICD implant?
Conti: No, we didn't do that. We used the patient as their own control. We examined them before the biventricular. It wasn't the ICD that we think so much changed their cognition. It was the biventricular pacing–pacing the septum and the left ventricular wall to come in at the same time.
Hillis: It would be awfully nice to put in the ICD and not turn it on for a few months. Thought about doing that?
Conti: If I thought that was ethical, I would.
Hillis: I'm not sure its unethical. I mean you're saying its unethical if you are convinced that your therapy is what's causing the effect. Maybe the therapy has nothing to do with it.
Conti: The cognition part, you know, I am pretty convinced about the cognition part. It was just based on my one patient and with this data too. But no, not turning it on now, biventricular pacing for heart failure is an established therapy and I'm not sure anymore that you could justify implanting it and not turning it on.
Hillis: But it would be awfully nice, I think, to see a group of patients who are undergoing the same intensive medical attention in therapy who don't have this intervention to see if their cognitive function changes over a course of three months.
Conti: I agree.
Branch: Atlanta: It was implied, but I wanted to ask explicitly—would you use the cognitive testing to select people for the biventricular pacing? In other words, if someone's cognitive function was very sharp, is that a reason not to go ahead with this, and if you find that it is abnormal, is that an indication to go ahead with this?
Conti: No. I'm sorry if I implied that. I think that this is an additional benefit to the standard benefits that people get with biventricular pacing. It's well established that biventricular pacing increases quality of life, increases exercise time, increases ejection fraction, and increases blood pressure and cardiac output. I think this is in addition to that.
Branch: Thank you.
Conti: Thanks.
REFERENCES
- 1.MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure. Lancet. 1999;353:2001–7. [PubMed] [Google Scholar]
- 2.Cohn JN, Johnson G, Ziesche S, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med. 1991;325:303–10. doi: 10.1056/NEJM199108013250502. [DOI] [PubMed] [Google Scholar]
- 3.Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–8. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 4.Pitt B, Zannad F, Remme WJ, et al. for the Randomized Aldactone Evaluation Study Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 5.Hunt SA, Baker DW, Chin MH, et al. American College of Cardiology/American Heart Association guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the International Society for Heart and Lung Transplantation; endorsed by the Heart Failure Society of America. Circulation. 2001;104:2996–3007. doi: 10.1161/hc4901.102568. [DOI] [PubMed] [Google Scholar]
- 6.Abraham WT, Fisher WG, Smith AL, et al. MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–53. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 7.Bristow MR, Saxon LA, Boehmer J, et al. Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 8.Moss AJ, Zareba W, Hall WJ, et al. Multicenter Automatic Defibrillator Implantation II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 9.Bardy GH, Lee KL, Mark DB, et al. Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 10.Stevenson W, Stevenson LW, Middlekauf HR, et al. Improving survival for patients with advanced heart failure: a study of 737 consecutive patients. J Am Coll Cardiol. 1995;26:1417–23. doi: 10.1016/0735-1097(95)00341-x. [DOI] [PubMed] [Google Scholar]
- 11.Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–53. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 12.Bax JJ, Van der Wall EE, Schalij MJ. Correspondence: Cardiac resynchronization therapy for heart failure. N Engl J Med. 2002;347:1803–4. doi: 10.1056/NEJM200211283472216. [DOI] [PubMed] [Google Scholar]
- 13.Aranda JM, Jr, Woo GW, Schofield RS, et al. Management of heart failure after cardiac resynchronization therapy: integrating advanced heart failure therapy with optimal device function. J Am Coll Cardiol. 2005;46:2193–8. doi: 10.1016/j.jacc.2005.03.078. [DOI] [PubMed] [Google Scholar]
- 14.Abildstrom H, Hogh P, Sperling B, et al. Cerebral blood flow and cognitive dysfunction after coronary surgery. Ann Thorac Surg. 2002;73:1174–8. doi: 10.1016/s0003-4975(01)03618-9. [DOI] [PubMed] [Google Scholar]
- 15.Wolfe R, Worrall-Carter L, Foister K, et al. Assessment of cognitive function in heart failure patients. Eur J Cardiovasc Nurs. 2006;5:158–64. doi: 10.1016/j.ejcnurse.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Petrucci RJ, Truesdell KC, Carter A, et al. Cognitive dysfunction in advanced heart failure and prospective cardiac assist device patients. Ann Thorac Surg. 2006;81:1738–44. doi: 10.1016/j.athoracsur.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Peeters A, Mamun AA, Willekens F, et al. A cardiovascular life history. A life course analysis of the original Framingham Heart Study cohort. Eur Heart J. 2002;23:458–66. doi: 10.1053/euhj.2001.2838. [DOI] [PubMed] [Google Scholar]
- 18.Selnes OA, McKhann GM, Guy MM, et al. Cognitive and neurobehavioral dysfunction after cardiac bypass procedures. Neurol Clin. 2006;24:133–45. doi: 10.1016/j.ncl.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Clark AP, McDougall G. Cognitive impairment in heart failure. Dimens Crit Care Nurs. 2006;25:93–100. doi: 10.1097/00003465-200605000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Grimm M, Yeganehfar W, Laufer G, et al. Cyclosporine may affect improvement of cognitive brain function after successful cardiac transplantation. Circulation. 1996;94:1339–45. doi: 10.1161/01.cir.94.6.1339. [DOI] [PubMed] [Google Scholar]
- 21.Zimpfer D, Kilo J, Czerny M, et al. Neurocognitive deficit following aortic valve replacement with biological/mechanical prosthesis. Eur J Cardiothorac Surg. 2003;23:544–51. doi: 10.1016/s1010-7940(02)00843-6. [DOI] [PubMed] [Google Scholar]
- 22.Zimpfer D, Wieselthaler G, Czerny M, et al. Neurocognitive function in patients with ventricular assist devices: a comparison of pulsatile and continuous blood flow devices. ASAIO J. 2006;52:24–7. doi: 10.1097/01.mat.0000191334.51375.7e. [DOI] [PubMed] [Google Scholar]