Abstract
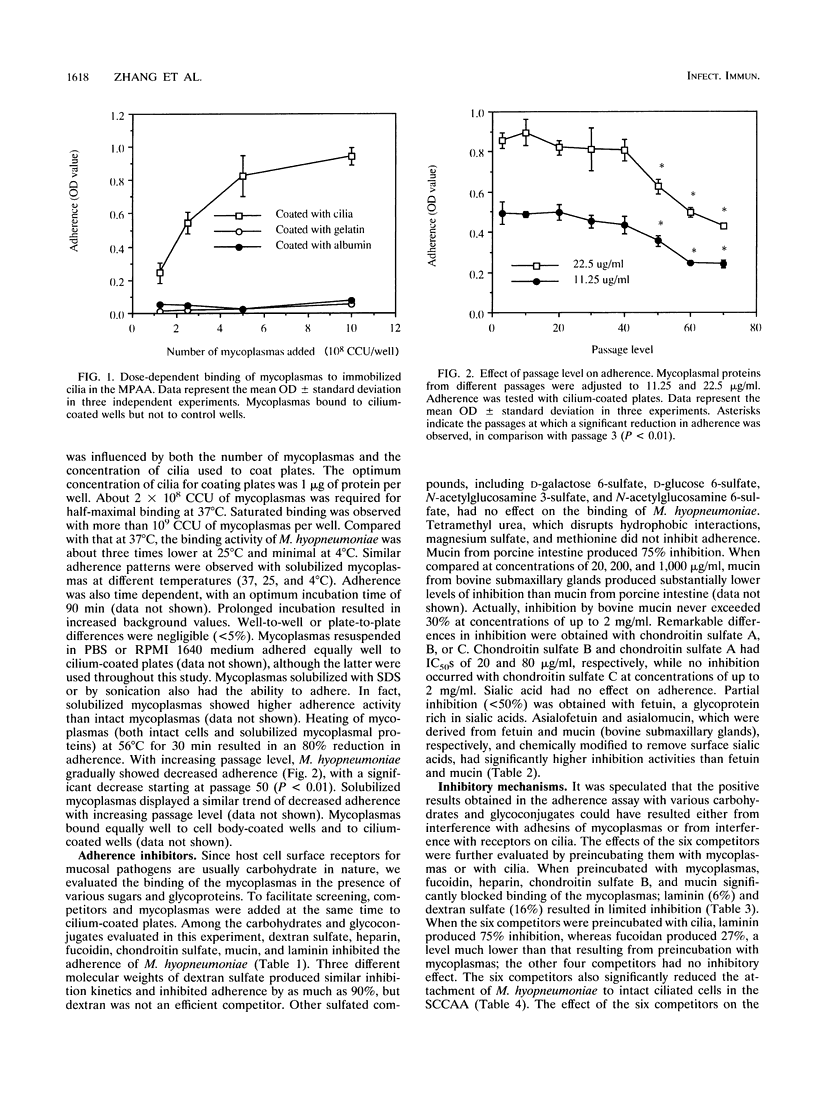
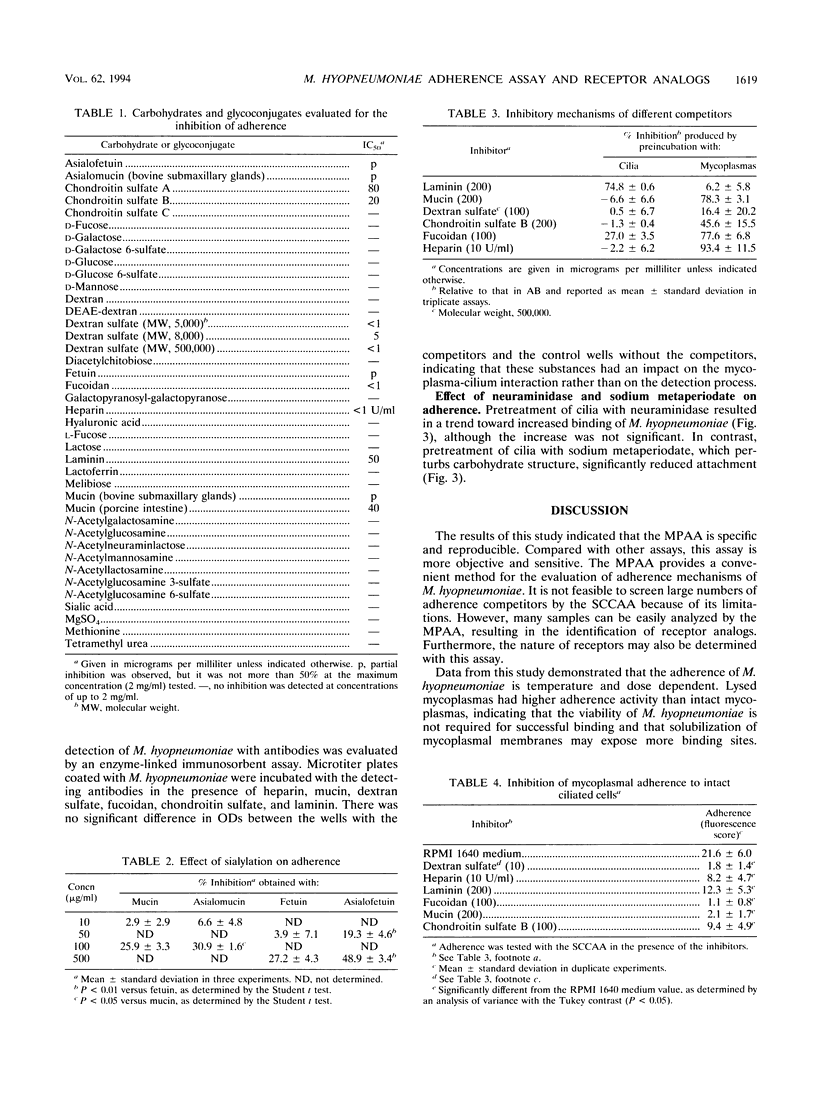
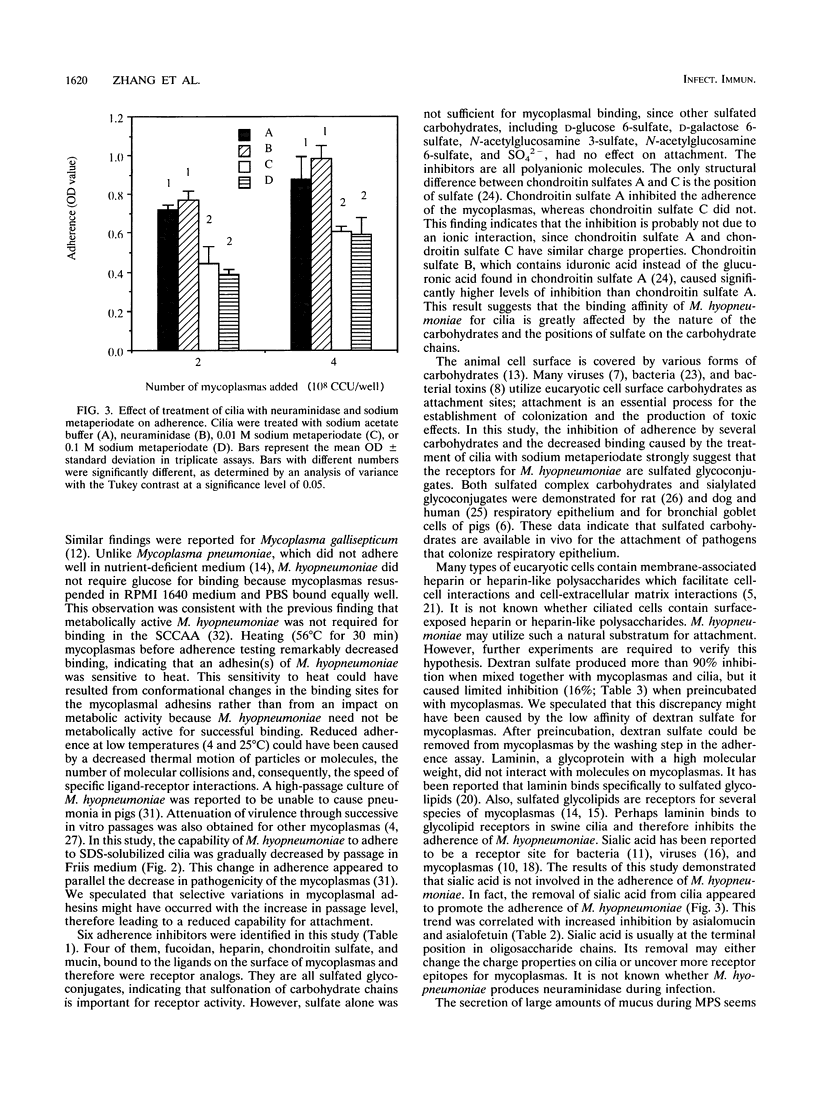
A microtiter plate adherence assay for Mycoplasma hyopneumoniae was established by use of purified swine tracheal cilia which contained receptors for the mycoplasmas. M. hyopneumoniae bound specifically to plates coated with solubilized cilia. The binding was dependent on both the concentration of cilia and the number of mycoplasmas. Dextran sulfate, heparin, chondroitin sulfate, laminin, mucin, and fucoidan significantly inhibited the binding of the mycoplasmas. The six inhibitors also disrupted the adherence of the mycoplasmas to intact ciliated cells. Preincubation with either mycoplasmas or cilia indicated that heparin, mucin, fucoidan, and chondroitin sulfate interacted with the adhesive molecules on the surface of the mycoplasmas, while laminin blocked the receptors in cilia. The basis for the inhibition induced by dextran sulfate was unknown. Treatment of cilia with neuraminidase appeared to promote adherence of the mycoplasmas, whereas treatment of cilia with sodium metaperiodate decreased binding. These results indicate that receptors for M. hyopneumoniae in the ciliated epithelium of the respiratory tract of pigs are glycoconjugate in nature.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson M., Medalia O., Schori L., Mirelman D., Sharon N., Ofek I. Prevention of colonization of the urinary tract of mice with Escherichia coli by blocking of bacterial adherence with methyl alpha-D-mannopyranoside. J Infect Dis. 1979 Mar;139(3):329–332. doi: 10.1093/infdis/139.3.329. [DOI] [PubMed] [Google Scholar]
- Blanchard B., Vena M. M., Cavalier A., Le Lannic J., Gouranton J., Kobisch M. Electron microscopic observation of the respiratory tract of SPF piglets inoculated with Mycoplasma hyopneumoniae. Vet Microbiol. 1992 Mar;30(4):329–341. doi: 10.1016/0378-1135(92)90020-t. [DOI] [PubMed] [Google Scholar]
- Collier A. M., Hu P. C., Clyde W. A., Jr The changing pathogenicity of Mycoplasma pneumoniae with passage in vitro. Correlates of virulence. Diagn Microbiol Infect Dis. 1985 Jul;3(4):321–328. doi: 10.1016/0732-8893(85)90006-9. [DOI] [PubMed] [Google Scholar]
- DeBey M. C., Jacobson C. D., Ross R. F. Histochemical and morphologic changes of porcine airway epithelial cells in response to infection with Mycoplasma hyopneumoniae. Am J Vet Res. 1992 Sep;53(9):1705–1710. [PubMed] [Google Scholar]
- Dimmock N. J. Review article initial stages in infection with animal viruses. J Gen Virol. 1982 Mar;59(Pt 1):1–22. doi: 10.1099/0022-1317-59-1-1. [DOI] [PubMed] [Google Scholar]
- Eidels L., Proia R. L., Hart D. A. Membrane receptors for bacterial toxins. Microbiol Rev. 1983 Dec;47(4):596–620. doi: 10.1128/mr.47.4.596-620.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis N. F. Some recommendations concerning primary isolation of Mycoplasma suipneumoniae and Mycoplasma flocculare a survey. Nord Vet Med. 1975 Jun;27(6):337–339. [PubMed] [Google Scholar]
- Gesner B., Thomas L. Sialic acid binding sites: role in hemagglutination by Mycoplasma gallisepticum. Science. 1966 Feb 4;151(3710):590–591. doi: 10.1126/science.151.3710.590. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Isayama Y. Evidence for sialyl glycoconjugates as receptors for Bordetella bronchiseptica on swine nasal mucosa. Infect Immun. 1987 Jul;55(7):1607–1609. doi: 10.1128/iai.55.7.1607-1609.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahane I., Banai M., Razin S., Feldner J. Attachment of mycoplasmas to host cell membranes. Rev Infect Dis. 1982 May-Jun;4 (Suppl):S185–S192. doi: 10.1093/clinids/4.supplement_1.s185. [DOI] [PubMed] [Google Scholar]
- Karlsson K. A. Animal glycolipids as attachment sites for microbes. Chem Phys Lipids. 1986 Dec 15;42(1-3):153–172. doi: 10.1016/0009-3084(86)90050-2. [DOI] [PubMed] [Google Scholar]
- Krivan H. C., Olson L. D., Barile M. F., Ginsburg V., Roberts D. D. Adhesion of Mycoplasma pneumoniae to sulfated glycolipids and inhibition by dextran sulfate. J Biol Chem. 1989 Jun 5;264(16):9283–9288. [PubMed] [Google Scholar]
- Lingwood C. A., Quinn P. A., Wilansky S., Nutikka A., Ruhnke H. L., Miller R. B. Common sulfoglycolipid receptor for mycoplasmas involved in animal and human infertility. Biol Reprod. 1990 Oct;43(4):694–697. doi: 10.1095/biolreprod43.4.694. [DOI] [PubMed] [Google Scholar]
- Mebus C. A., Underdahl N. R. Scanning electron microscopy of trachea and bronchi from gnotobiotic pigs inoculated with Mycoplasma hyopneumoniae. Am J Vet Res. 1977 Aug;38(8):1249–1254. [PubMed] [Google Scholar]
- Razin S., Jacobs E. Mycoplasma adhesion. J Gen Microbiol. 1992 Mar;138(3):407–422. doi: 10.1099/00221287-138-3-407. [DOI] [PubMed] [Google Scholar]
- Ro L. H., Ross R. F. Comparison of Mycoplasma hyopneumoniae strains by serologic methods. Am J Vet Res. 1983 Nov;44(11):2087–2094. [PubMed] [Google Scholar]
- Roberts D. D., Rao C. N., Magnani J. L., Spitalnik S. L., Liotta L. A., Ginsburg V. Laminin binds specifically to sulfated glycolipids. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1306–1310. doi: 10.1073/pnas.82.5.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins B. J., Culp L. A. Glycosaminoglycans in the substrate adhesion sites of normal and virus-transformed murine cells. Biochemistry. 1979 Jan 9;18(1):141–148. doi: 10.1021/bi00568a022. [DOI] [PubMed] [Google Scholar]
- Sharon N., Eshdat Y., Silverblatt F. J., Ofek I. Bacterial adherence to cell surface sugars. Ciba Found Symp. 1981;80:119–141. doi: 10.1002/9780470720639.ch9. [DOI] [PubMed] [Google Scholar]
- Spicer S. S., Chakrin L. W., Wardell J. R., Jr, Kendrick W. Histochemistry of mucosubstances in the canine and human respiratory tract. Lab Invest. 1971 Dec;25(6):483–490. [PubMed] [Google Scholar]
- Spicer S. S., Mochizuki I., Setser M. E., Martinez J. R. Complex carbohydrates of rat tracheobronchial surface epithelium visualized ultrastructurally. Am J Anat. 1980 May;158(1):93–109. doi: 10.1002/aja.1001580109. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson D., Furr P. M., Davies H. A., Manchee R. J., Mouches C., Bove J. M. Mycoplasmal adherence with particular reference to the pathogenicity of Mycoplasma pulmonis. Isr J Med Sci. 1981 Jul;17(7):599–603. [PubMed] [Google Scholar]
- Tuomanen E., Towbin H., Rosenfelder G., Braun D., Larson G., Hansson G. C., Hill R. Receptor analogs and monoclonal antibodies that inhibit adherence of Bordetella pertussis to human ciliated respiratory epithelial cells. J Exp Med. 1988 Jul 1;168(1):267–277. doi: 10.1084/jem.168.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young T. F., Erickson B. Z., Ross R. F., Wannemuehler Y. Hemagglutination and hemagglutination inhibition of turkey red blood cells with Mycoplasma hyopneumoniae. Am J Vet Res. 1989 Jul;50(7):1052–1055. [PubMed] [Google Scholar]
- Zielinski G. C., Ross R. F. Adherence of Mycoplasma hyopneumoniae to porcine ciliated respiratory tract cells. Am J Vet Res. 1993 Aug;54(8):1262–1269. [PubMed] [Google Scholar]
- Zielinski G. C., Ross R. F. Effect of growth in cell cultures and strain on virulence of Mycoplasma hyopneumoniae for swine. Am J Vet Res. 1990 Mar;51(3):344–348. [PubMed] [Google Scholar]
- Zielinski G. C., Young T., Ross R. F., Rosenbusch R. F. Adherence of Mycoplasma hyopneumoniae to cell monolayers. Am J Vet Res. 1990 Mar;51(3):339–343. [PubMed] [Google Scholar]



