Abstract
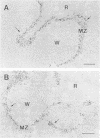
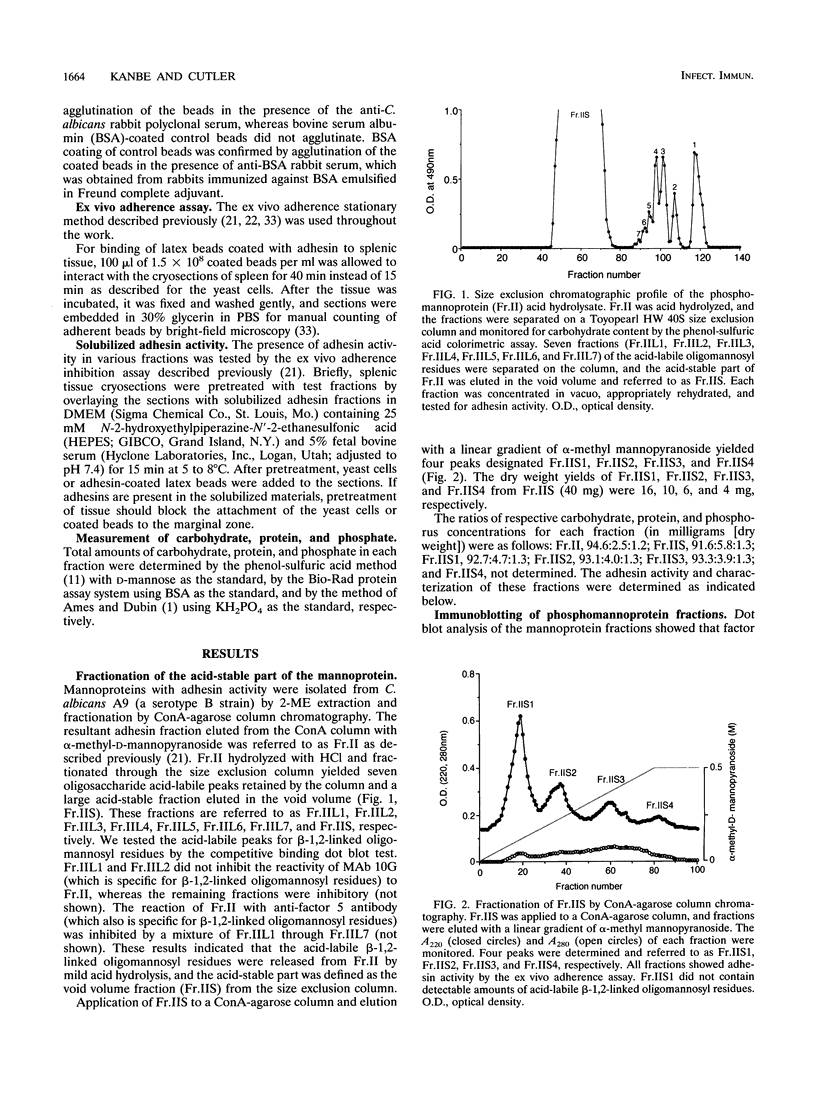
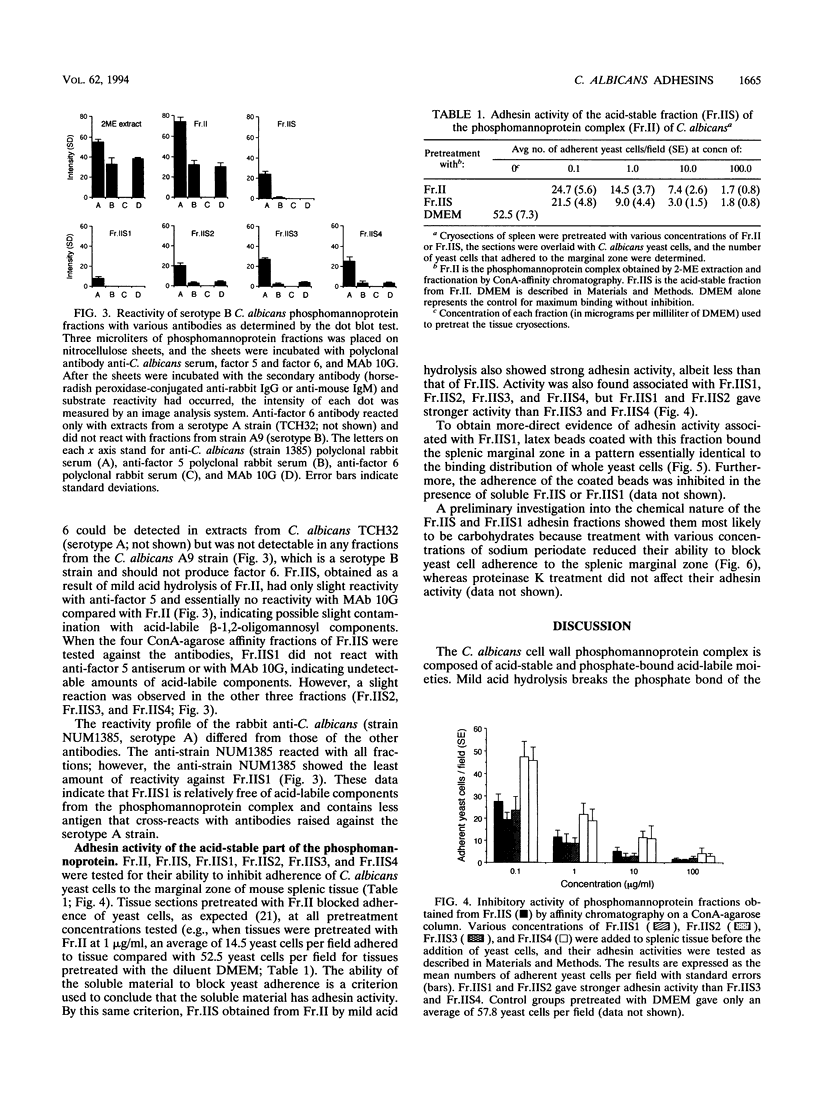
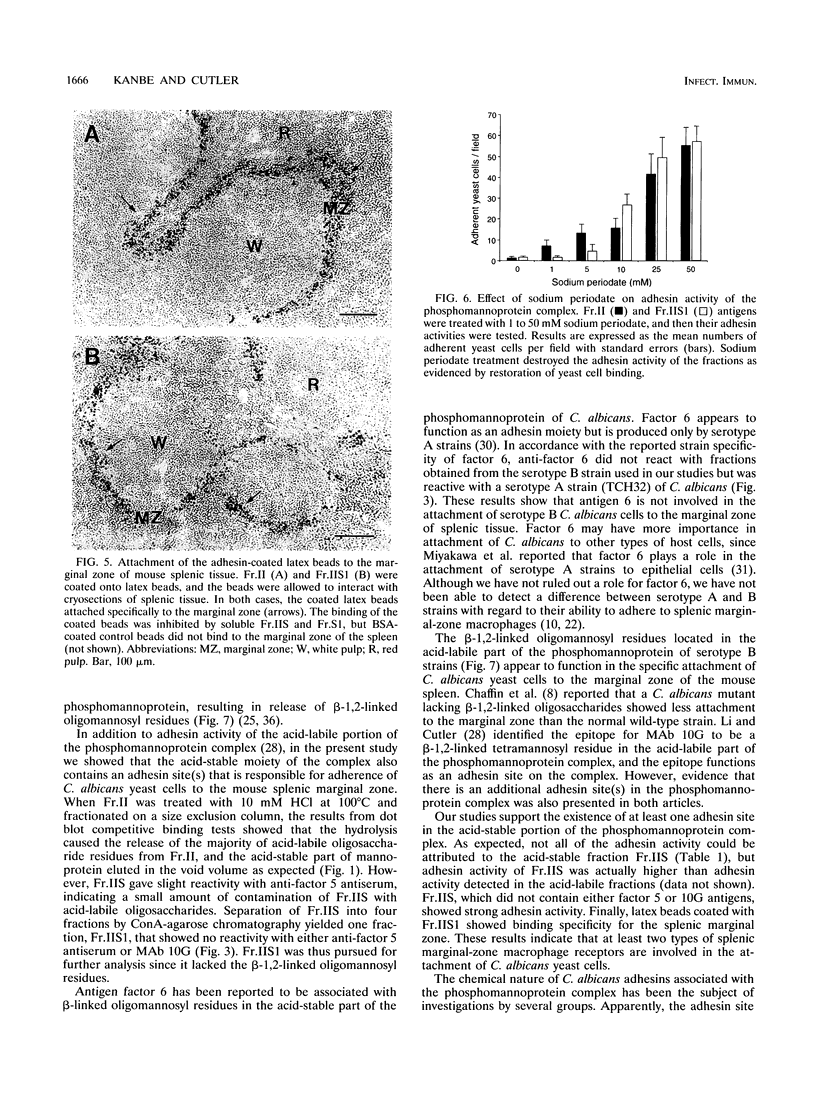
Previously, we showed that Candida albicans hydrophilic yeast cells adhere specifically to mouse splenic marginal-zone macrophages. The adhesins are part of the yeast cell wall phosphomannoprotein complex, and one adhesin site, which reacts with the monoclonal antibody 10G, was identified as a beta-1,2-linked tetramannose in the acid-labile portion of the complex. We report here that the acid-stable part of the complex, which has not been reported previously to have adhesin activity, is in large part responsible for yeast cell binding to the splenic marginal zone. The phosphomannoprotein complex, termed Fr.II, was isolated from C. albicans serotype B yeast cells by beta-mercaptoethanol extraction and concanavalin A-agarose affinity chromatography. Fr.II is devoid of the serotype A-specific antigen factor 6, which functions in yeast cell attachment to epithelial cells. The acid-stable part of Fr.II (i.e., Fr.IIS) was obtained by mild acid hydrolysis and size exclusion fractionation. Fr.IIS was further fractionated into four fractions, Fr.IIS1, Fr.IIS2, Fr.IIS3, and Fr.IIS4, by concanavalin A-agarose column chromatography and elution with a linear gradient of alpha-methyl-D-mannopyranoside. Adhesin activity of these fractions was determined by their ability to block yeast cell binding to the splenic marginal zone. Fr.IIS1 and Fr.IIS2 yielded more material and stronger adhesin activity than either Fr.IIS3 or Fr.IIS4. Only Fr.IIS1 did not react with antibodies (anti-factor 5 and monoclonal antibody 10G) specific for the acid-labile beta-1,2-linked oligosaccharides. Fr.IIS1-coated latex beads attached specifically to the marginal zone in a pattern identical to that of yeast cell binding. Furthermore, Fr.IIS1-latex bead attachment was inhibited by soluble Fr.II or Fr.IIS. Initial chemical analyses indicate that the adhesin site on Fr.IIS1 is a carbohydrate because adhesin activity was destroyed by periodate oxidation but not by proteinase K digestion.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Asakura K., Iwaguchi S., Homma M., Sukai T., Higashide K., Tanaka K. Electrophoretic karyotypes of clinically isolated yeasts of Candida albicans and C. glabrata. J Gen Microbiol. 1991 Nov;137(11):2531–2538. doi: 10.1099/00221287-137-11-2531. [DOI] [PubMed] [Google Scholar]
- Brawner D. L., Mori M. Adherence of Candida albicans to tissues from mice with drug- or radiation-induced immunodeficiencies. J Infect Dis. 1992 Sep;166(3):587–597. doi: 10.1093/infdis/166.3.587. [DOI] [PubMed] [Google Scholar]
- Calderone R. A., Braun P. C. Adherence and receptor relationships of Candida albicans. Microbiol Rev. 1991 Mar;55(1):1–20. doi: 10.1128/mr.55.1.1-20.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova M., Lopez-Ribot J. L., Monteagudo C., Llombart-Bosch A., Sentandreu R., Martinez J. P. Identification of a 58-kilodalton cell surface fibrinogen-binding mannoprotein from Candida albicans. Infect Immun. 1992 Oct;60(10):4221–4229. doi: 10.1128/iai.60.10.4221-4229.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassone A. Cell wall of Candida albicans: its functions and its impact on the host. Curr Top Med Mycol. 1989;3:248–314. doi: 10.1007/978-1-4612-3624-5_10. [DOI] [PubMed] [Google Scholar]
- Chaffin W. L., Collins B., Marx J. N., Cole G. T., Morrow K. J., Jr Characterization of mutant strains of Candida albicans deficient in expression of a surface determinant. Infect Immun. 1993 Aug;61(8):3449–3458. doi: 10.1128/iai.61.8.3449-3458.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler J. E., Brawner D. L., Hazen K. C., Jutila M. A. Characteristics of Candida albicans adherence to mouse tissues. Infect Immun. 1990 Jun;58(6):1902–1908. doi: 10.1128/iai.58.6.1902-1908.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukayama M., Calderone R. A. Adherence of cell surface mutants of Candida albicans to buccal epithelial cells and analyses of the cell surface proteins of the mutants. Infect Immun. 1991 Apr;59(4):1341–1345. doi: 10.1128/iai.59.4.1341-1345.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannoum M. A., Burns G. R., Elteen K. A., Radwan S. S. Experimental evidence for the role of lipids in adherence of Candida spp. to human buccal epithelial cells. Infect Immun. 1986 Oct;54(1):189–193. doi: 10.1128/iai.54.1.189-193.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., van Rooijen N., Cutler J. E. Binding of Candida albicans yeast cells to mouse popliteal lymph node tissue is mediated by macrophages. Infect Immun. 1993 Aug;61(8):3244–3249. doi: 10.1128/iai.61.8.3244-3249.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen K. C., Brawner D. L., Riesselman M. H., Jutila M. A., Cutler J. E. Differential adherence of hydrophobic and hydrophilic Candida albicans yeast cells to mouse tissues. Infect Immun. 1991 Mar;59(3):907–912. doi: 10.1128/iai.59.3.907-912.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen K. C., Hazen B. W. Hydrophobic surface protein masking by the opportunistic fungal pathogen Candida albicans. Infect Immun. 1992 Apr;60(4):1499–1508. doi: 10.1128/iai.60.4.1499-1508.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen K. C., Hazen B. W. Surface hydrophobic and hydrophilic protein alterations in Candida albicans. FEMS Microbiol Lett. 1993 Feb 15;107(1):83–87. doi: 10.1111/j.1574-6968.1993.tb06008.x. [DOI] [PubMed] [Google Scholar]
- Ishiguro A., Homma M., Sukai T., Higashide K., Torii S., Tanaka K. Immunoblotting analysis of sera from patients with candidal vaginitis and healthy females. J Med Vet Mycol. 1992;30(4):281–292. doi: 10.1080/02681219280000371. [DOI] [PubMed] [Google Scholar]
- Kanbe T., Han Y., Redgrave B., Riesselman M. H., Cutler J. E. Evidence that mannans of Candida albicans are responsible for adherence of yeast forms to spleen and lymph node tissue. Infect Immun. 1993 Jun;61(6):2578–2584. doi: 10.1128/iai.61.6.2578-2584.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanbe T., Jutila M. A., Cutler J. E. Evidence that Candida albicans binds via a unique adhesion system on phagocytic cells in the marginal zone of the mouse spleen. Infect Immun. 1992 May;60(5):1972–1978. doi: 10.1128/iai.60.5.1972-1978.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz S. A. Fungal adherence to the vascular compartment: a critical step in the pathogenesis of disseminated candidiasis. Clin Infect Dis. 1992 Jan;14(1):340–347. doi: 10.1093/clinids/14.1.340. [DOI] [PubMed] [Google Scholar]
- Klotz S. A., Smith R. L. A fibronectin receptor on Candida albicans mediates adherence of the fungus to extracellular matrix. J Infect Dis. 1991 Mar;163(3):604–610. doi: 10.1093/infdis/163.3.604. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Shibata N., Nakada M., Chaki S., Mizugami K., Ohkubo Y., Suzuki S. Structural study of cell wall phosphomannan of Candida albicans NIH B-792 (serotype B) strain, with special reference to 1H and 13C NMR analyses of acid-labile oligomannosyl residues. Arch Biochem Biophys. 1990 Apr;278(1):195–204. doi: 10.1016/0003-9861(90)90248-w. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Shibata N., Suzuki S. Evidence for oligomannosyl residues containing both beta-1,2 and alpha-1,2 linkages as a serotype A-specific epitope(s) in mannans of Candida albicans. Infect Immun. 1992 May;60(5):2106–2109. doi: 10.1128/iai.60.5.2106-2109.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer N., Segal E., Lis H., Gov Y. Effect of Candida albicans cell wall components on the adhesion of the fungus to human and murine vaginal mucosa. Mycopathologia. 1988 May;102(2):115–121. doi: 10.1007/BF00437448. [DOI] [PubMed] [Google Scholar]
- Li R. K., Cutler J. E. Chemical definition of an epitope/adhesin molecule on Candida albicans. J Biol Chem. 1993 Aug 25;268(24):18293–18299. [PubMed] [Google Scholar]
- McCourtie J., Douglas L. J. Extracellular polymer of Candida albicans: isolation, analysis and role in adhesion. J Gen Microbiol. 1985 Mar;131(3):495–503. doi: 10.1099/00221287-131-3-495. [DOI] [PubMed] [Google Scholar]
- Miyakawa Y., Kagaya K., Kuribayashi T., Suzuki M., Fukazawa Y. Isolation and chemical and biological characterization of antigenic mutants of Candida albicans serotype A. Yeast. 1989 Apr;5(Spec No):S225–S229. [PubMed] [Google Scholar]
- Miyakawa Y., Kuribayashi T., Kagaya K., Suzuki M., Nakase T., Fukazawa Y. Role of specific determinants in mannan of Candida albicans serotype A in adherence to human buccal epithelial cells. Infect Immun. 1992 Jun;60(6):2493–2499. doi: 10.1128/iai.60.6.2493-2499.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray T. L., Payne C. D., Morrow B. J. Candida albicans acid proteinase: characterization and role in candidiasis. Adv Exp Med Biol. 1991;306:173–183. doi: 10.1007/978-1-4684-6012-4_22. [DOI] [PubMed] [Google Scholar]
- Riesselman M. H., Kanbe T., Cutler J. E. Improvements and important considerations of an ex vivo assay to study Candida albicans-splenic tissue interactions. J Immunol Methods. 1991 Dec 15;145(1-2):153–160. doi: 10.1016/0022-1759(91)90321-6. [DOI] [PubMed] [Google Scholar]
- Sandin R. L., Rogers A. L., Patterson R. J., Beneke E. S. Evidence for mannose-mediated adherence of Candida albicans to human buccal cells in vitro. Infect Immun. 1982 Jan;35(1):79–85. doi: 10.1128/iai.35.1.79-85.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer R. T., Horst M. N., Garner R. E., Hudson J., Jenkins P. R., Richardson A. L. Altered hepatic clearance and killing of Candida albicans in the isolated perfused mouse liver model. Infect Immun. 1990 Sep;58(9):2869–2874. doi: 10.1128/iai.58.9.2869-2874.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata N., Arai M., Haga E., Kikuchi T., Najima M., Satoh T., Kobayashi H., Suzuki S. Structural identification of an epitope of antigenic factor 5 in mannans of Candida albicans NIH B-792 (serotype B) and J-1012 (serotype A) as beta-1,2-linked oligomannosyl residues. Infect Immun. 1992 Oct;60(10):4100–4110. doi: 10.1128/iai.60.10.4100-4110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosh F. D., Douglas L. J. Characterization of a fucoside-binding adhesin of Candida albicans. Infect Immun. 1992 Nov;60(11):4734–4739. doi: 10.1128/iai.60.11.4734-4739.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]