Abstract
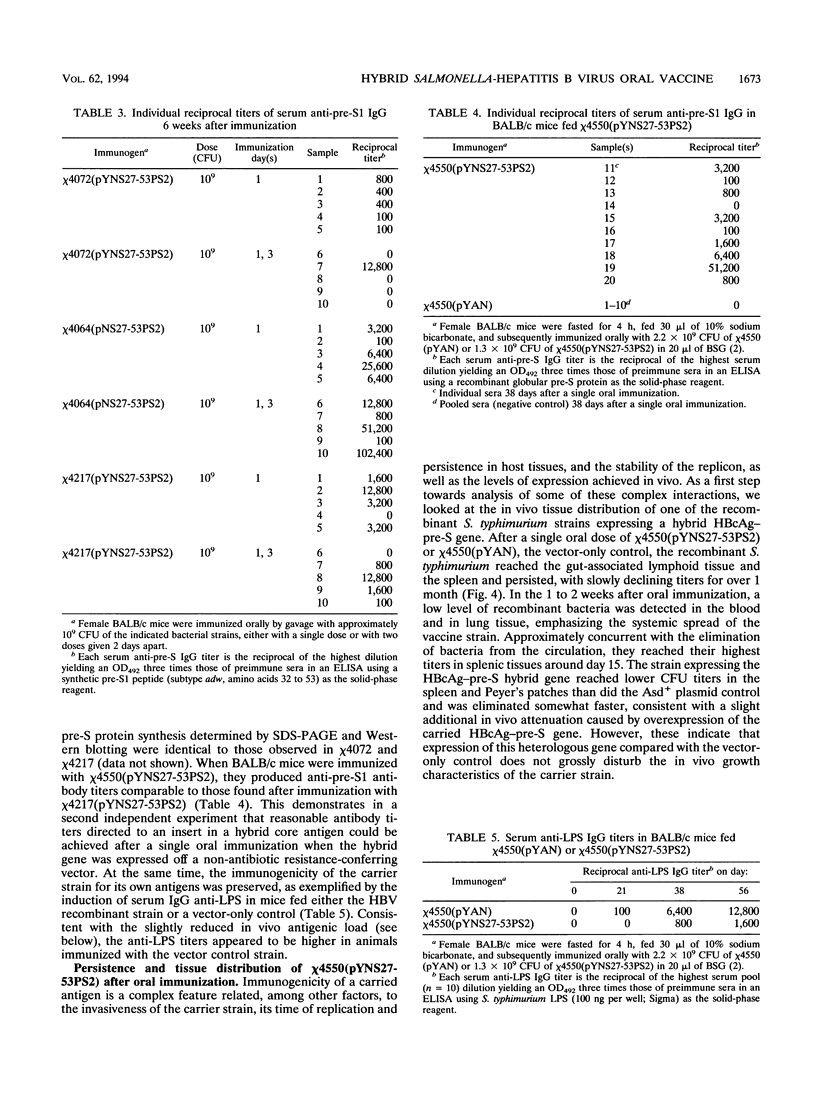
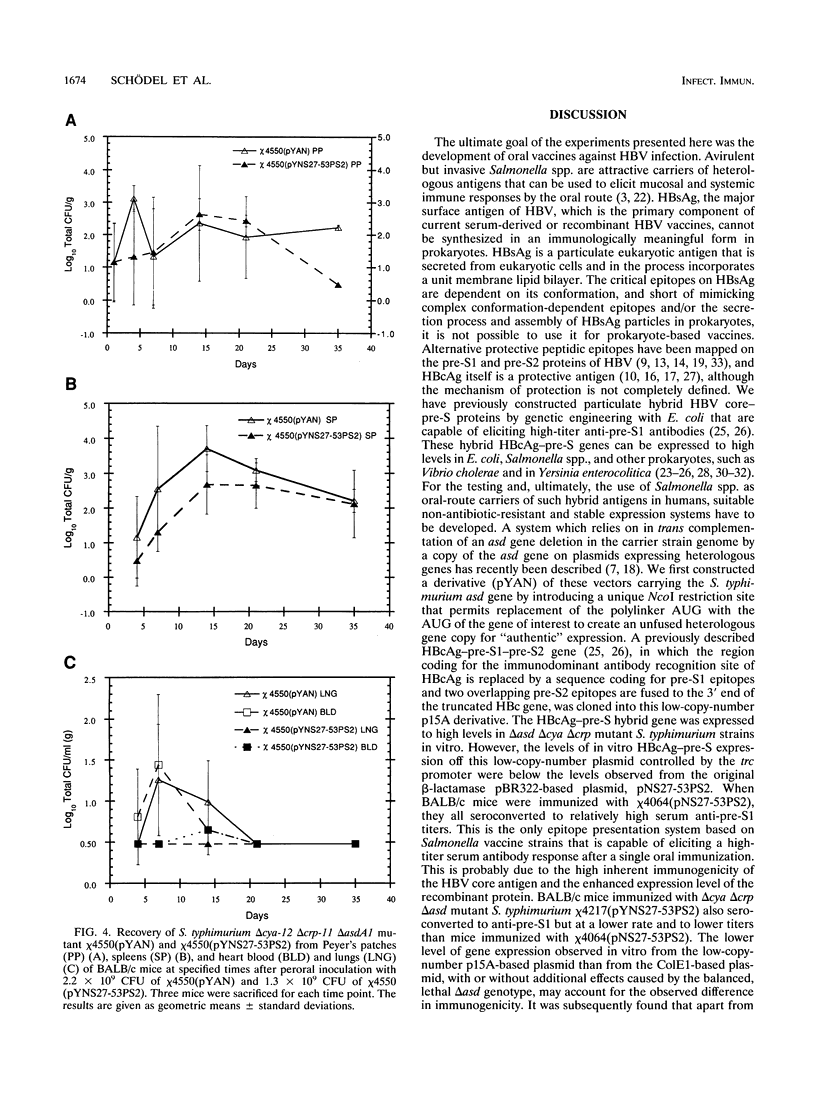
Avirulent salmonellae expressing foreign genes are attractive for use as oral vaccine carriers. To facilitate the stable expression of heterologous genes without conferring antibiotic resistance, a deletion of the asdA1 gene was introduced into Salmonella typhimurium and S. typhi delta cya delta crp mutant vaccine strains. An asd-complementing plasmid expressing hybrid hepatitis B virus nucleocapsid-pre-S (HBcAg-pre-S) particles was constructed. These hybrid HBcAg-pre-S particle genes were stably expressed in S. typhimurium and S. typhi delta cya delta crp mutant vaccine strains in this balanced, lethal host-vector combination. A single oral immunization of BALB/c mice with a recombinant S. typhimurium delta cya delta crp mutant synthesizing hybrid HBcAg-pre-S elicited potentially virus-neutralizing anti-pre-S serum immunoglobulin G antibodies. In addition, serum immunoglobulin G recognizing S. typhimurium lipopolysaccharide was induced. Distribution in tissue after oral immunization was analyzed in one plasmid-strain combination. The recombinant S. typhimurium colonized the gut-associated lymphoid tissue and the spleen and persisted for over 4 weeks, retaining the HBcAg-pre-S expression plasmid. An isogenic virulence plasmid-cured S. typhimurium delta cya delta crp strain expressing the same HBcAg-pre-S gene had reduced immunogenicity for the carried antigen after oral immunization.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bichko V., Schödel F., Nassal M., Gren E., Berzinsh I., Borisova G., Miska S., Peterson D. L., Gren E., Pushko P. Epitopes recognized by antibodies to denatured core protein of hepatitis B virus. Mol Immunol. 1993 Feb;30(3):221–231. doi: 10.1016/0161-5890(93)90051-c. [DOI] [PubMed] [Google Scholar]
- CURTIS S. R., 3rd CHROMOSOMAL ABERRATIONS ASSOCIATED WITH MUTATIONS TO BACTERIOPHAGE RESISTANCE IN ESCHERICHIA COLI. J Bacteriol. 1965 Jan;89:28–40. doi: 10.1128/jb.89.1.28-40.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Kelly S. M. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect Immun. 1987 Dec;55(12):3035–3043. doi: 10.1128/iai.55.12.3035-3043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delos S., Villar M. T., Hu P., Peterson D. L. Cloning, expression, isolation and characterization of the pre-S domains of hepatitis B surface antigen, devoid of the S protein. Biochem J. 1991 Jun 1;276(Pt 2):411–416. doi: 10.1042/bj2760411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienes H. P., Gerlich W. H., Wörsdörfer M., Gerken G., Bianchi L., Hess G., Meyer zum Büschenfelde K. H. Hepatic expression patterns of the large and middle hepatitis B virus surface proteins in viremic and nonviremic chronic hepatitis B. Gastroenterology. 1990 Apr;98(4):1017–1023. doi: 10.1016/0016-5085(90)90028-y. [DOI] [PubMed] [Google Scholar]
- Galán J. E., Nakayama K., Curtiss R., 3rd Cloning and characterization of the asd gene of Salmonella typhimurium: use in stable maintenance of recombinant plasmids in Salmonella vaccine strains. Gene. 1990 Sep 28;94(1):29–35. doi: 10.1016/0378-1119(90)90464-3. [DOI] [PubMed] [Google Scholar]
- Gulig P. A., Curtiss R., 3rd Plasmid-associated virulence of Salmonella typhimurium. Infect Immun. 1987 Dec;55(12):2891–2901. doi: 10.1128/iai.55.12.2891-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y., Takai E., Ohnuma H., Kitajima K., Tsuda F., Machida A., Mishiro S., Nakamura T., Miyakawa Y., Mayumi M. A synthetic peptide vaccine involving the product of the pre-S(2) region of hepatitis B virus DNA: protective efficacy in chimpanzees. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9174–9178. doi: 10.1073/pnas.83.23.9174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwarson S., Tabor E., Thomas H. C., Snoy P., Gerety R. J. Protection against hepatitis B virus infection by immunization with hepatitis B core antigen. Gastroenterology. 1985 Mar;88(3):763–767. doi: 10.1016/0016-5085(85)90148-9. [DOI] [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981 Feb;145(2):1110–1111. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Chisari F. V., Nakamura T., Thornton G. B. Two distinct but overlapping antibody binding sites in the pre-S(2) region of HBsAg localized within 11 continuous residues. J Immunol. 1986 Oct 15;137(8):2703–2710. [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Thornton G. B., Hughes J. L. Antibody production to the nucleocapsid and envelope of the hepatitis B virus primed by a single synthetic T cell site. Nature. 1987 Oct 8;329(6139):547–549. doi: 10.1038/329547a0. [DOI] [PubMed] [Google Scholar]
- Milich D. R. T- and B-cell recognition of hepatitis B viral antigens. Immunol Today. 1988 Dec;9(12):380–386. doi: 10.1016/0167-5699(88)91239-X. [DOI] [PubMed] [Google Scholar]
- Murray K., Bruce S. A., Hinnen A., Wingfield P., van Erd P. M., de Reus A., Schellekens H. Hepatitis B virus antigens made in microbial cells immunise against viral infection. EMBO J. 1984 Mar;3(3):645–650. doi: 10.1002/j.1460-2075.1984.tb01861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K., Bruce S. A., Wingfield P., van Eerd P., de Reus A., Schellekens H. Protective immunisation against hepatitis B with an internal antigen of the virus. J Med Virol. 1987 Oct;23(2):101–107. doi: 10.1002/jmv.1890230202. [DOI] [PubMed] [Google Scholar]
- Neurath A. R., Kent S. B., Parker K., Prince A. M., Strick N., Brotman B., Sproul P. Antibodies to a synthetic peptide from the preS 120-145 region of the hepatitis B virus envelope are virus neutralizing. Vaccine. 1986 Mar;4(1):35–37. doi: 10.1016/s0264-410x(86)80001-9. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Imai M., Usuda S., Tanaka E., Tachibana K., Mishiro S., Machida A., Nakamura T., Miyakawa Y., Mayumi M. Hemagglutination assay of polypeptide coded by the pre-S region of hepatitis B virus DNA with monoclonal antibody: correlation of pre-S polypeptide with the receptor for polymerized human serum albumin in serums containing hepatitis B antigens. J Immunol. 1985 Feb;134(2):1212–1216. [PubMed] [Google Scholar]
- Pardon P., Popoff M. Y., Coynault C., Marly J., Miras I. Virulence-associated plasmids of Salmonella serotype Typhimurium in experimental murine infection. Ann Inst Pasteur Microbiol. 1986 Jul-Aug;137B(1):47–60. doi: 10.1016/s0769-2609(86)80093-x. [DOI] [PubMed] [Google Scholar]
- Schödel F., Milich D. R., Will H. Hepatitis B virus nucleocapsid/pre-S2 fusion proteins expressed in attenuated Salmonella for oral vaccination. J Immunol. 1990 Dec 15;145(12):4317–4321. [PubMed] [Google Scholar]
- Schödel F., Moriarty A. M., Peterson D. L., Zheng J. A., Hughes J. L., Will H., Leturcq D. J., McGee J. S., Milich D. R. The position of heterologous epitopes inserted in hepatitis B virus core particles determines their immunogenicity. J Virol. 1992 Jan;66(1):106–114. doi: 10.1128/jvi.66.1.106-114.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schödel F., Neckermann G., Peterson D., Fuchs K., Fuller S., Will H., Roggendorf M. Immunization with recombinant woodchuck hepatitis virus nucleocapsid antigen or hepatitis B virus nucleocapsid antigen protects woodchucks from woodchuck hepatitis virus infection. Vaccine. 1993;11(6):624–628. doi: 10.1016/0264-410x(93)90307-j. [DOI] [PubMed] [Google Scholar]
- Schödel F. Prospects for oral vaccination using recombinant bacteria expressing viral epitopes. Adv Virus Res. 1992;41:409–446. doi: 10.1016/s0065-3527(08)60041-x. [DOI] [PubMed] [Google Scholar]
- Schödel F., Will H. Construction of a plasmid for expression of foreign epitopes as fusion proteins with subunit B of Escherichia coli heat-labile enterotoxin. Infect Immun. 1989 Apr;57(4):1347–1350. doi: 10.1128/iai.57.4.1347-1350.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]