Abstract
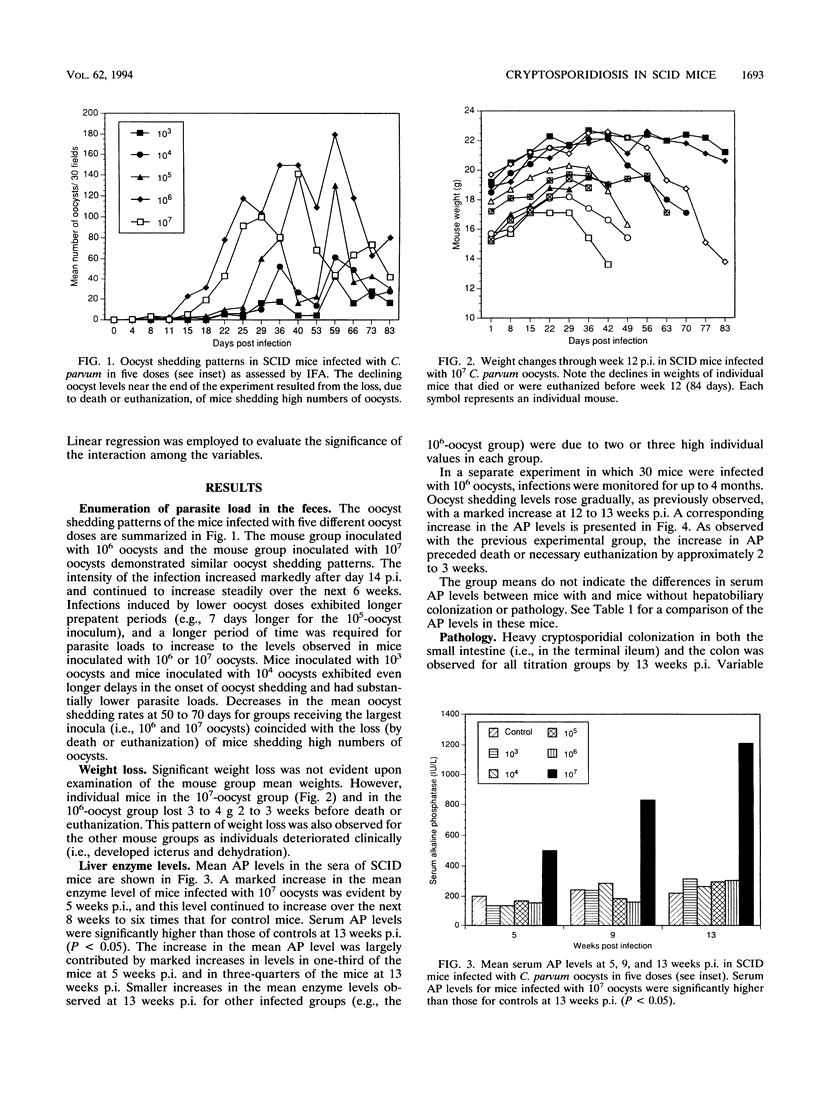
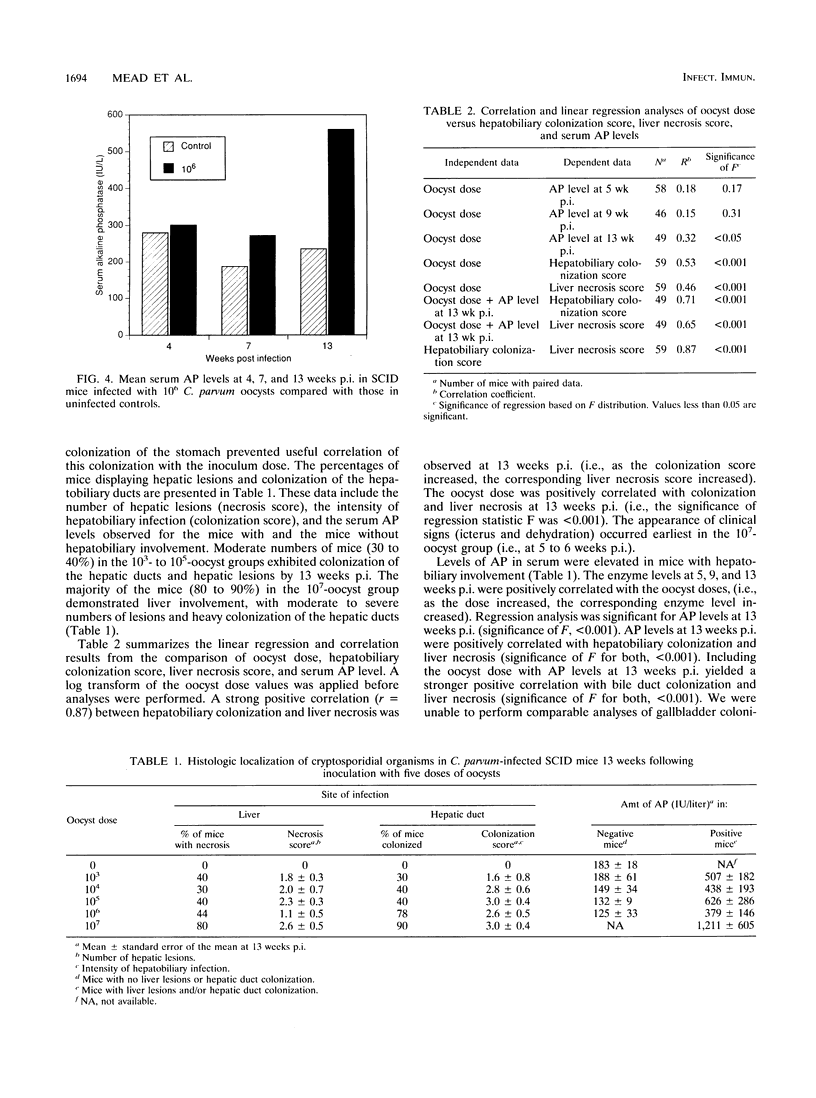
Cryptosporidial infections in severe combined immune deficient (SCID) mice produce a chronic disease state which in the later stages leads to extraintestinal involvement and hepatic dysfunction. To further characterize the infection dynamics in this model and monitor the changes in the hepatic system, a dose titration of the oocyst inoculum was performed and alkaline phosphatase levels in the sera were assayed. Ten SCID mice per dose were inoculated with 10(3), 10(4), 10(5), 10(6), or 10(7) oocysts. Oocyst shedding in the feces was quantified by microscopic enumeration. Mice inoculated with 10(6) oocysts and those inoculated with 10(7) oocysts demonstrated similar oocyst shedding patterns, but the 10(7)-oocyst group exhibited signs of distress (e.g., weight loss and icterus) earlier. The intensity of the infection increased markedly approximately 14 days postinoculation (p.i.) and continued to increase steadily over the next 6 weeks. Inoculation with lower oocyst doses produced a delay in patency (e.g., it occurred 7 days later with the 10(5)-oocyst inoculum and 14 days later with the 10(4)-oocyst inoculum). Mean serum alkaline phosphatase levels in the 10(7)-oocyst group were more than twice control values at 5 weeks p.i. and continued to increase over the next 8 weeks. Oocyst doses and alkaline phosphatase levels were positively correlated with hepatobiliary colonization (r = 0.71) and liver necrosis (r = 0.65) at 13 weeks p.i. A strong positive correlation between hepatobiliary colonization and liver necrosis at 13 weeks p.i. (r = 0.87) was observed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrowood M. J., Sterling C. R. Comparison of conventional staining methods and monoclonal antibody-based methods for Cryptosporidium oocyst detection. J Clin Microbiol. 1989 Jul;27(7):1490–1495. doi: 10.1128/jcm.27.7.1490-1495.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrowood M. J., Sterling C. R. Isolation of Cryptosporidium oocysts and sporozoites using discontinuous sucrose and isopycnic Percoll gradients. J Parasitol. 1987 Apr;73(2):314–319. [PubMed] [Google Scholar]
- Garcia L. S., Brewer T. C., Bruckner D. A. Fluorescence detection of Cryptosporidium oocysts in human fecal specimens by using monoclonal antibodies. J Clin Microbiol. 1987 Jan;25(1):119–121. doi: 10.1128/jcm.25.1.119-121.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellin B. G., Soave R. Coccidian infections in AIDS. Toxoplasmosis, cryptosporidiosis, and isosporiasis. Med Clin North Am. 1992 Jan;76(1):205–234. doi: 10.1016/s0025-7125(16)30377-7. [DOI] [PubMed] [Google Scholar]
- Gross T. L., Wheat J., Bartlett M., O'Connor K. W. AIDS and multiple system involvement with cryptosporidium. Am J Gastroenterol. 1986 Jun;81(6):456–458. [PubMed] [Google Scholar]
- Hinnant K., Schwartz A., Rotterdam H., Rudski C. Cytomegaloviral and cryptosporidial cholecystitis in two patients with AIDS. Am J Surg Pathol. 1989 Jan;13(1):57–60. doi: 10.1097/00000478-198901000-00008. [DOI] [PubMed] [Google Scholar]
- Kahn D. G., Garfinkle J. M., Klonoff D. C., Pembrook L. J., Morrow D. J. Cryptosporidial and cytomegaloviral hepatitis and cholecystitis. Arch Pathol Lab Med. 1987 Sep;111(9):879–881. [PubMed] [Google Scholar]
- Kuhls T. L., Greenfield R. A., Mosier D. A., Crawford D. L., Joyce W. A. Cryptosporidiosis in adult and neonatal mice with severe combined immunodeficiency. J Comp Pathol. 1992 May;106(4):399–410. doi: 10.1016/0021-9975(92)90024-o. [DOI] [PubMed] [Google Scholar]
- McGowan I., Hawkins A. S., Weller I. V. The natural history of cryptosporidial diarrhoea in HIV-infected patients. AIDS. 1993 Mar;7(3):349–354. doi: 10.1097/00002030-199303000-00007. [DOI] [PubMed] [Google Scholar]
- Mead J. R., Arrowood M. J., Sidwell R. W., Healey M. C. Chronic Cryptosporidium parvum infections in congenitally immunodeficient SCID and nude mice. J Infect Dis. 1991 Jun;163(6):1297–1304. doi: 10.1093/infdis/163.6.1297. [DOI] [PubMed] [Google Scholar]
- Perryman L. E., Kegerris K. A., Mason P. H. Effect of orally administered monoclonal antibody on persistent Cryptosporidium parvum infection in scid mice. Infect Immun. 1993 Nov;61(11):4906–4908. doi: 10.1128/iai.61.11.4906-4908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman D. J., Cello J. P., Laing F. C. Papillary stenosis and sclerosing cholangitis in the acquired immunodeficiency syndrome. Ann Intern Med. 1987 Apr;106(4):546–549. doi: 10.7326/0003-4819-106-4-546. [DOI] [PubMed] [Google Scholar]
- Solari A., Venegas J., Gonzalez E., Vasquez C. Detection and classification of Trypanosoma cruzi by DNA hybridization with nonradioactive probes. J Protozool. 1991 Nov-Dec;38(6):559–565. doi: 10.1111/j.1550-7408.1991.tb06080.x. [DOI] [PubMed] [Google Scholar]



