Abstract
In the embryonic midgut of Drosophila, Wingless (Wg) signaling elicits threshold-specific transcriptional response, that is, low-signaling levels activate target genes, whereas high-signaling levels repress them. Wg-mediated repression of the HOX gene Ultrabithorax (Ubx) is conferred by a response sequence within the Ubx B midgut enhancer, called WRS-R. It further depends on the Teashirt (Tsh) repressor, which acts through the WRS-R without binding to it. Here, we show that Wg-mediated repression of Ubx B depends on Brinker, which binds to the WRS-R. Furthermore, Brinker blocks transcriptional activation by ubiquitous Wg signaling. Brinker binds to Tsh in vitro, recruits Tsh to the WRS-R, and we find mutual physical interactions between Brinker, Tsh, and the corepressor dCtBP. This suggests that the three proteins may form a ternary repressor complex at the WRS-R to quench the activity of the nearby-bound dTCF/Armadillo transcription complex. Finally, brinker and tsh produce similar mutant phenotypes in the ventral epidermis, and double mutants mimic overactive Wg signaling in this tissue. This suggests that Brinker may have a widespread function in antagonizing Wg signaling.
Keywords: Drosophila, Brinker, transcriptional repression, Teashirt, dCtBP, Wingless
Wingless (Wg) signaling is essential at different stages during Drosophila development. In the early embryo, wg is expressed in segmental stripes in the epidermis, and together with engrailed determines the parasegmental borders (Perrimon 1994). Later, during embryogenesis, wg has a critical function during endoderm induction: wg is expressed in a single parasegment (ps) in the midgut, in which it controls the expression of two HOX genes, Ultrabithorax (Ubx) and labial, as well as the formation of the middle gut constriction, which separates the anterior and the posterior midgut (Bienz 1994). In imaginal discs, Wg signaling controls multiple processes, including the specification of ventral cell fates in the leg disc (Struhl and Basler 1993), the dorso-ventral compartment boundary, the prospective margin in the wing disc (Klein 2001), and the formation of head cuticle in the eye disc (Treisman and Rubin 1995; Royet and Finkelstein 1996).
Armadillo is a key effector of the Wg pathway (Cavallo et al. 1997). It is stabilized by Wg signaling and, consequently, translocates into the nucleus, in which it activates the transcription of Wg target genes by binding to dTCF (Brunner et al. 1997; Riese et al. 1997; van de Wetering et al. 1997). dTCF belongs to the TCF/LEF family of sequence-specific high mobility group (HMG) proteins that are thought to be architectural factors mediating assembly of multiprotein enhancer complexes (Grosschedl et al. 1994). In the absence of Wg signaling, dTCF actively represses Wg target genes by binding to the corepressor Groucho (Cavallo et al. 1998) and the CREB-binding protein (dCBP), which can acetylate the Armadillo-binding domain of dTCF (Waltzer and Bienz 1998). Thus, Wg signaling converts dTCF from a transcriptional repressor to an activator (Bienz 1998).
A number of Wg target genes have been identified whose expression is stimulated directly by dTCF/Armadillo. These include Ubx (Riese et al. 1997), dpp (Yang et al. 2000), and stripe (Piepenburg et al. 2000), each of which contains Wg-responsive enhancers with binding sites for dTCF. Other examples are teashirt (tsh) (Mathies et al. 1994), engrailed (Hooper 1994), Dfrizzled2 (Cadigan et al. 1998), and shavenbaby (Payre et al. 1999), but it has not been shown whether these genes are controlled directly by dTCF. Furthermore, Wg signaling can also repress target genes, for example the HOX genes Ubx and labial in the embryonic midgut (Hoppler and Bienz 1995; Yu et al. 1998). Similarly, Wg represses its own expression in the midgut (Yu et al. 1998) and in the wing imaginal disc along the margin (Rulifson et al. 1996). dpp is antagonized by Wg in multiple embryonic and larval tissues (Ma and Moses 1995; Treisman and Rubin 1995; Brook and Cohen 1996; Jiang and Struhl 1996). Notably, in some tissues, Wg signaling acts at multiple threshold levels to control the expression of its target genes, for example, in the embryonic midgut. In this issue, Ubx and labial are stimulated by low Wg levels and repressed by high Wg levels (Bienz 1997). Wg also acts at multiple thresholds in the wing (Zecca et al. 1996; Neumann and Cohen 1997) and in the leg imaginal discs (Lecuit and Cohen 1997).
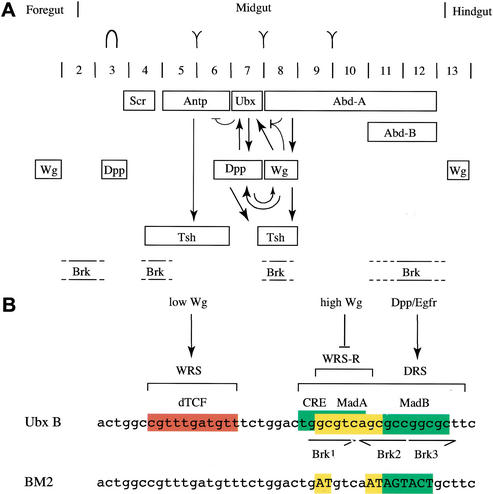
Transcriptional repression mediated by Wg is not well understood. Perhaps the best-studied model is the midgut enhancer of Ubx, called Ubx B (Thüringer et al. 1993), which is repressed by high levels of Wg signaling in the posterior embryonic midgut (Yu et al. 1998; Fig. 1A). This repression is conferred by the WRS-R (Yu et al. 1998), a sequence that is distinct from the WRS, that is, the dTCF-binding site that confers Wg-mediated stimulation of this enhancer (Riese et al. 1997; Fig. 1B). The WRS-R coincides with the DRS, a tandem of binding sites for the Dpp effector Mad that mediates transcriptional stimulation by Dpp signaling (Kim et al. 1997; Szüts et al. 1998). Furthermore, it has been shown that Tsh acts through the WRS-R to repress Ubx B in response to high Wg levels (Waltzer et al. 2001). However, Tsh does not bind to the WRS-R directly (Waltzer et al. 2001), so the DNA-binding protein conferring the Wg-mediated repression remained elusive.
Figure 1.
Gene expression in the midgut and signal-responsive sequences in Ubx B. (A) Expression of HOX proteins (top), Wg and Dpp in the midgut mesoderm (Bienz 1996) in relation to Tsh expression and estimated domains of brk expression (Jazwinska et al. 1999b; E. Saller and M. Bienz, unpubl.). Expression of tsh in ps8 requires wg and dpp (Mathies et al. 1994). Midgut limits, positions of gastric caeca (in ps3) and of the gut constrictions are indicated above parasegments (ps); note that the middle gut constriction bissects the midgut into anterior and posterior. Critical regulatory interactions between the genes in the middle midgut are shown (arrows, stimulatory; barred line, repressive). (B, top) Signal-responsive sequences within the Ubx B midgut enhancer, with the WRS, DRS, and WRS-R indicated. These include binding sites for the Wg effector dTCF, the Dpp effector Mad, and the cAMP response element (CRE)-like sequence. Note that the three Brinker-binding sites coincide with the Mad sites (Saller and Bienz 2001). (Bottom) Sequence of the BM2 enhancer with mutations in the Mad/Brinker-binding sites.
Brinker was initially discovered as an antagonist of Dpp signaling (Campbell and Tomlinson 1999; Jazwinska et al. 1999a; Minami et al. 1999). It is a sequence-specific DNA-binding protein with a distantly related homeodomain (Sivasankaran et al. 2000; Rushlow et al. 2001; Zhang et al. 2001) and functions as a transcriptional repressor (Sivasankaran et al. 2000; Hasson et al. 2001; Kirkpatrick et al. 2001; Rushlow et al. 2001; Zhang et al. 2001). Recently, we have discovered that Brinker binds to the Mad-binding sites within Ubx B to antagonize Dpp-mediated stimulation of this enhancer (Saller and Bienz 2001). Because these overlap the WRS-R (see above), we asked whether Brinker might be involved in Wg-mediated repression. This is the case. Here, we show that brinker is required for repression of Ubx and wg by high levels of Wg signaling in the embryonic midgut. Furthermore, Brinker competes efficiently with Wg signaling, blocking Wg-mediated stimulation of Ubx B. We provide evidence that Brinker can recruit Tsh to the WRS-R to form a repressor complex, and that the two proteins can recruit the corepressor dCtBP. This suggests a mechanism by which Brinker can block dTCF/Armadillo-mediated stimulation of Wg target genes. Finally, we show that Brinker also antagonizes wg in the ventral epidermis of Drosophila embryos.
Results
brinker is required for Wg-mediated repression of Ubx and wg
In wild-type embryos, Ubx B directs β-galactosidase (lacZ) expression in the middle midgut, between the anterior and the posterior midgut constriction (ps6-9) (Fig. 2A). In brinker mutants, Ubx B is derepressed at both ends of the midgut, approximately in ps2 and ps12 (Saller and Bienz 2001; arrowheads in Fig. 2B). If Wg is overexpressed throughout the midgut, lacZ staining is strong anterior to the middle gut constriction (i.e., to the left of the vertical bar in Fig. 2C), but is much reduced posterior to this constriction (Fig. 2C, open triangle; Yu et al. 1998). The stimulation in the anterior midgut is partly due to the overexpressed Wg, and partly to endogenous Dpp, which is ectopically activated throughout this region by constitutive Wg signaling (Yu et al. 1996). The lack of staining posterior to the middle gut constriction reflects repression of Ubx B by Wg signaling, which reaches particularly high levels near the Wg source (in ps8, indicated by asterisks in Fig. 2; Yu et al. 1998). However, if Wg is overexpressed in brinker mutant embryos, lacZ staining is evenly strong throughout the midgut, from ps2 to ps12 (Fig. 2D). Thus, brinker is required in the posterior midgut for the repression of Ubx B in response to high Wg levels.
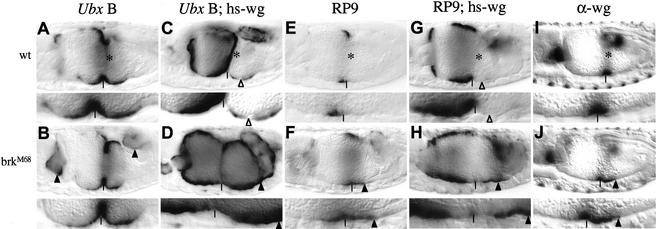
Figure 2.
brinker is required for Wingless-mediated repression of Ubx and wg. Side views of 12-to-14-hour-old embryos, wild-type or brk mutant, bearing Ubx reporter genes and hs-wg as indicated, stained with antibody against lacZ (A–H) or Wg (I,J); (bottom) an enlarged section is shown that spans the relevant gut region (ps6–ps9). (*) Wingless sources in the wild type; (▵) point to transcriptional repression due to high Wg levels, arrowheads to stimulation and derepression in response to ectopic Wg. Note the absence of Wg-mediated repression posterior to the middle gut constrictions in brk mutants. These constrictions are indicated by vertical lines in this and all subsequent figures; anterior to the left, dorsal up.
We asked whether brinker mutation affects Ubx itself. Ubx is expressed in ps7 of the midgut, with a sharp posterior expression limit coinciding with the middle gut constriction (Bienz and Tremml 1988). Although Ubx is derepressed in the posteriormost region of the midgut in brinker mutants, we could not detect any Ubx derepression into ps8 (Saller and Bienz 2001), probably because Ubx is repressed in this region by the HOX gene abdominal-A (presumably independently of Brinker; Bienz and Tremml 1988). We thus used an extended Ubx enhancer, called RP9, which faithfully mimics Ubx expression in the midgut, but which is less efficiently repressed by abdominal-A than Ubx itself (Thüringer and Bienz 1993). Like Ubx itself, RP9 directs lacZ expression in ps7 of the visceral mesoderm (Fig. 2E) in response to Dpp and low levels of Wg signaling, but is repressed posterior to the middle gut constriction in response to high Wg levels (in contrast to Ubx B, which has lost much of its responsiveness to high Wg levels, and thus mediates broader expression; Yu et al. 1998). In brinker mutant embryos, RP9-mediated lacZ staining extends clearly beyond the middle gut constriction and is strong in ps8 (Fig. 2F; Saller and Bienz 2001). Furthermore, in brinker mutants, RP9 responds to ubiquitous Wg in the posterior midgut by conferring lacZ staining in this region (arrowhead in Fig. 2H), although there is no trace of RP9-mediated staining in this region in a wild-type embryo that expresses ubiquitous Wg (open triangle in Fig. 2G). These results indicate that brinker is required for repression of RP9—and by extrapolation of Ubx—posterior to the middle gut constriction, near the Wg source.
Next we asked whether brinker also controls wg, which, at late embryonic stages, autorepresses itself in ps8 (Yu et al. 1998). In wild-type embryos, wg is initially expressed in ps8 (Bienz et al. 1988), but subsequently, when the middle gut constriction forms, shifts slightly toward anterior, so it is expressed at this stage in a band of 6–8 cells wide spanning this constriction (Fig. 2I). However, in brinker mutant embryos, there is a clear posterior expansion of Wg staining that is now detectable in a band of ∼8–12 cells wide (Fig. 2J, arrowhead). Thus, brinker is required for autorepression of wg in this region of the midgut.
Brinker repressor blocks Wg-induced stimulation of target genes
Our results suggest that the Brinker repressor may be able to overcome Wg-mediated target gene activation in the middle midgut. To test this hypothesis, we monitored the activity of Ubx B after simultaneous overexpression of Brinker and Wg in the mesoderm. As mentioned above, Wg overexpression alone leads to strong staining in the anterior part of the midgut (ps3–ps7) and substantial reduction of staining posterior to the middlegut constriction (Figs. 2A,C and 3A,C). However, ectopic Brinker repressed Ubx B virtually throughout the midgut, whether or not Wg was present (Fig. 3B,D). This shows that Brinker can repress a Wg-responsive enhancer even in the presence of strong Wg stimulation. Evidently, the Brinker repressor is dominant over the stimulatory dTCF/Armadillo transcription complex.
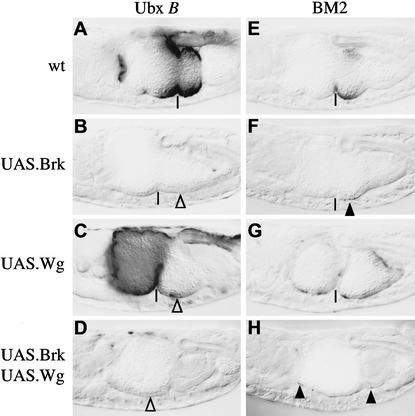
Figure 3.
Brinker blocks Wg-induced transcriptional stimulation by targeting the WRS-R. Side view of ∼14-hour-old embryos bearing Ubx B or BM2 as indicated, stained with antibody against lacZ. (▵) Indicates lack of lacZ staining due to ectopic Wg or Brinker; residual staining is indicated by arrowheads.
Brinker targets the WRS-R to repress Ubx B in response to Wg
A mutant Ubx B enhancer that lacks functional Mad-binding sites (BM2) directs weak lacZ expression posterior to the middle gut constriction (in ps8 and ps9), which reflects its responsiveness to Wg stimulation but not to Dpp (Szüts et al. 1998; Fig. 3E). As expected, BM2 is much less responsive to overexpressed Brinker than the wild-type enhancer (Saller and Bienz 2001), although we noted earlier that there is still a residual Brinker response of BM2 (as indicated by the slight difference in lacZ staining between Fig. 3F and E). This residual response could be due to a fortuitous Brinker-binding site elsewhere in the plasmid (Saller and Bienz 2001).
Ectopic Wg causes an expansion of BM2-mediated lacZ staining in cells scattered throughout the midgut (including the posterior midgut, in which Wg represses Ubx B; Fig. 3G). This confirms that this mutant enhancer can be stimulated, but no longer repressed, by Wg signaling (Yu et al. 1998). Simultaneous overexpression of Brinker and Wg also allows activity of BM2 in individual cells throughout the midgut (Fig. 3H). This lacZ-staining pattern is similar to that due to ectopic Wg alone (Fig. 3G), although, again, there is less lacZ staining in the presence compared with the absence of ubiquitous Brinker, indicating a residual Briner response of BM2 also under these conditions. Nevertheless, these experiments indicate that Brinker depends on the WRS-R sequence to fully antagonize Wg-mediated stimulation of Ubx B.
Brinker recruits Tsh and dCtBP to the WRS-R
We asked whether Brinker might recruit Tsh to the WRS-R. We thus expressed Tsh in bacteria as a GST fusion protein and tested its binding to radioactively labeled in vitro-translated Brinker by pull-down assays. This revealed binding between the two proteins in vitro (Fig. 4A, lanes 1–3). This was confirmed by the converse- binding experiment based on GST–Brinker and radioactively labeled Tsh (Fig. 4A, lanes 4–6).
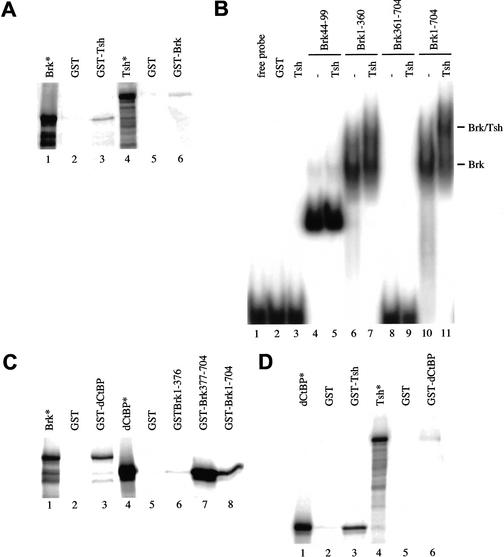
Figure 4.
Direct binding between Brinker, Tsh, and dCtBP. (A,C,D) In vitro pull-down assays with equimolar amounts of GST fusion protein and in vitro-translated radioactively labeled protein as indicated; 10% of the total reaction was loaded in the input lane (*). (B) Gel-shift assays with GST fusion proteins as indicated at top (1 μg per assay for Brinker–GST or fragments thereof; 15 μg per assay for Tsh–GST). The positions of the Brinker/DNA complex and of the ternary Tsh/Brinker/DNA complex are indicated at right.
Next, we performed gel-shift assays to test whether Tsh can bind to Brinker when the latter is bound to the WRS-R. We have shown previously that the amino-terminal homeodomain of Brinker (amino acids 44–99) binds to the Mad-binding sequence within Ubx B, whereas a carboxy-terminal fragment of Brinker lacking this domain does not (Saller and Bienz 2001). We now find that GST–Tsh produces a super-shift if added to full-length Brinker (Fig. 4B, lanes 10 and 11) or to an amino-terminal fragment of Brinker (spanning amino acids 1–360; Fig. 4B, lanes 6 and 7), but fails to produce a super-shift if added to a minimal Brinker fragment spanning the homeodomain (Fig. 4B, lanes 4 and 5). This indicates that Brinker can recruit Tsh to the WRS-R.
Brinker and Tsh both contain a motif (P-DLS-K) known to interact with Drosophila CtBP (dCtBP) (Nibu et al. 1998b). Human CtBP was first identified as a protein that binds to the carboxyl terminus of the adenovirus E1a oncoprotein and functions as a tumor suppressor (Boyd et al. 1993). Furthermore, CtBP proteins are transcriptional corepressors (Turner and Crossley 1998; Criqui-Filipe et al. 1999). dCtBP was isolated in a two-hybrid screen for proteins that bind to the transcriptional repressor Hairy (Poortinga et al. 1998; Zhang and Levine 1999; Phippen et al. 2000). It mediates transcriptional repression by the short-range repressors Knirps, Krüppel, Snail, and Giant in the Drosophila embryo (Nibu et al. 1998a; Nibu and Levine 2001). We thus tested in vitro binding between Brinker and dCtBP, and between Tsh and dCtBP.
In vitro-translated Brinker binds to bacterially expressed GST–dCtBP in pull-down assays (Fig. 4C, lane 3). As expected, an amino-terminal fragment of Brinker spanning amino acids 1–376 fails to bind in vitro-translated dCtBP (Fig. 4C, lane 6). However, a carboxy-terminal fragment of Brinker spanning amino acids 377–704, and to a lesser extent, full-length GST–Brinker, bind to dCtBP (Fig. 4C, lanes 7 and 8). Further pull-down assays show direct binding between dCtBP and Tsh (Fig. 4D). These results suggest that Brinker and Tsh may be able to recruit the corepressor dCtPB, individually or together.
The repressor activity of Tsh in the midgut depends on brinker
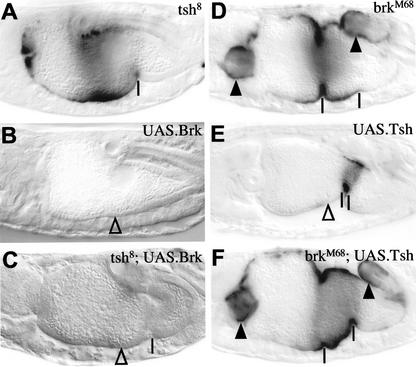
We tested whether Brinker and Tsh interdepend on each other in the transcriptional repression of Ubx B. tsh mutant embryos are easily recognized by their lack of the anterior and middle gut constrictions (Mathies et al. 1994), but the staining pattern of Ubx B in tsh mutant embryos is the same as the pattern seen in wild-type embryos (Fig. 5A). Ectopic Brinker repressed Ubx B throughout the midgut, regardless of whether Tsh was present or not (Fig. 5B,C). Thus, Brinker can repress the Ubx B enhancer in the absence of Tsh.
Figure 5.
Tsh-mediated repression of Ubx B depends on brinker. Side view of ∼14-hour-old mutant embryos as indicated, bearing Ubx B and stained with antibody against lacZ. (▵) Indicates lack of lacZ staining due to ectopic Brinker or Teashirt; derepression of staining is indicated by arrowheads. Note the absence of the anterior and middle gut constrictions in tsh mutants (Mathies et al. 1994) and the absence of the anterior constriction in UAS.Tsh embryos (Waltzer et al. 2001).
We then asked whether Tsh can repress transcription in the absence of Brinker. Overexpression of Tsh throughout the mesoderm causes repression of Ubx B anterior to the middle gut constriction (in ps6 and ps7), but not in ps8, in which the levels of endogenous Wg and Tsh are high (Waltzer et al. 2001; Fig. 5E). However, Tsh cannot repress Ubx B in brinker mutant embryos; the lacZ staining pattern is the same in these mutants, whether or not they also overexpress Tsh (Fig. 5, cf. F with D). Thus, Tsh depends on brinker to repress Ubx B in the midgut.
Brinker and Tsh antagonize Wg signaling in the ventral embryonic epidermis
brinker was identified originally in a screen for mutants that affect Dpp signaling in the ventral epidermis (Jazwinska et al. 1999a). These authors described the brinker mutant phenotype of the larval cuticle, and interpreted this to reflect an expansion of the dorsal epidermis at the expense of the ventral epidermis—consistent with an antagonistic effect of brinker on dorsal Dpp signaling.
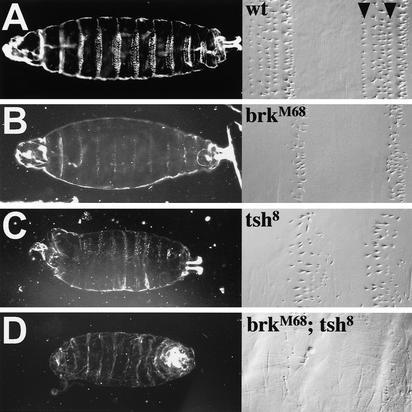
However, when we re-examined the cuticles of brkM68 mutants (the allele described by Jazwinska et al. 1999a), we found that these primarily show a segment polarity phenotype in the ventral epidermis. Wild-type cuticles show segmental stretches of naked cuticle alternating with denticle belts (Fig. 6A, left). In the abdomen, the latter consist of six characteristic rows of denticles, small denticles with hooked ends and flat bases in rows 1–4, large tapered denticles that are less hooked in row 5, and tiny tapered denticles in row 6. The denticles of each row have a characteristic polarity with denticles in rows 1 and 4 pointing toward the anterior, whereas the others point toward the posterior (Fig. 6A, right). brinker mutant larvae show narrowed denticle belts (Fig. 6B, left) of about three disordered rows of tapered denticles, all of which point toward the posterior, usually one row of large denticles followed by two rows of tiny denticles (Fig. 6B, right). Another brinker allele (brkF124; Lammel et al. 2000), which is less strong than brkM68 shows essentially the same mutant phenotype as brkM68, except that the residual denticle belts are slightly broader (data not shown). Therefore, brinker loss clearly causes a major change in the structure and organization of each individual segment in the larval abdomen. Interestingly, this phenotype is also caused by ectopic Wg signaling, which antagonizes epidermal growth factor receptor (EGFR) signaling in the embryonic epidermis (O'Keefe et al. 1997; Szüts et al. 1997).
Figure 6.
brk and tsh antagonize Wg signaling in the ventral embryonic epidermis. Ventral views of larval cuticles, wild type or mutant as indicated, viewed by dark field illumination (left) or by Nomarski optics at high magnification (right; abdominal denticle belts shown). Arrowheads point to denticle rows 1 and 4.
Notably, tsh mutant embryos also retain residual denticle belts with only tapered denticles of the row 5 and 6 type (Gallet et al. 2000; Fig. 6C). The phenotypic similarity between brinker and tsh mutant embryos in the ventral epidermis suggests that both proteins may be involved in the same pathway. To test this hypothesis, we constructed a double-mutant brinker tsh strain and examined their ventral cuticles. These clearly look more severe than either single mutant; they do not show any residual denticle belts (Fig. 6D, left), and only single tiny denticles can be observed occasionally scattered in the posterior abdomen (Fig. 6D, right). In other words, these embryos essentially show the naked cuticle phenotype that results from ubiquitous Wg expression in the embryonic epidermis (Perrimon 1994). This indicates that Brinker and Tsh synergize to promote the formation of ventral denticle belts. In support of this, both brinker and tsh single mutants show loss of segmental rhomboid expression in the trunk of the embryonic epidermis (data not shown), implying that EGFR signaling is not activated in these mutants (O'Keefe et al. 1997; Szüts et al. 1997). As a consequence, shavenbaby fails to be activated (data not shown). Because the latter is a Rhomboid target gene that cell autonomously directs denticle formation (Payre et al. 1999), this explains why the denticle belts do not develop normally in brinker and tsh mutants.
The cuticle phenotype of the brinker tsh double mutants suggests that Brinker and Tsh act together to antagonize Wg signaling in the ventral epidermis. Therefore, the function of Brinker in antagonizing Wg does not appear to be limited to the embryonic midgut.
Discussion
Previous analyses have suggested that Brinker may be dedicated to antagonizing Dpp signaling (Campbell and Tomlinson 1999; Jazwinska et al. 1999a; Minami et al. 1999; Ashe et al. 2000; Sivasankaran et al. 2000; Hasson et al. 2001). This appeared particularly likely, given that the Brinker repressor was found to bind to the same sites as the Dpp effector Mad, and thus to compete with activated Mad for binding to Dpp target genes (Kirkpatrick et al. 2001; Rushlow et al. 2001; Saller and Bienz 2001; Zhang et al. 2001).
We have now discovered that Brinker also antagonizes Wg signaling in two embryonic tissues. However, the underlying repressive mechanism appears to be distinct, as the Brinker repressor binds to a site distinct from that occupied by the Wg effector, the dTCF/Armadillo activator complex. Brinker thus acts at short range to block the activity of this complex.
The Brinker/Tsh repressor quenches the activity of dTCF/Armadillo
Most likely, Brinker uses a mechanism called quenching to block dTCF/Armadillo (Fig. 7). Quenching involves interaction of repressors (and the corepressors they recruit) with activators bound to nearby sites (Gray and Levine 1996). Brinker is known to be able to quench target genes by recruiting the corepressor Groucho (Hasson et al. 2001; Zhang et al. 2001), which is involved in multiple quenching processes (Zhang and Levine 1999). groucho antagonizes wg (Cavallo et al. 1998), and TCF factors can bind to Groucho proteins directly (Levanon et al. 1998; Roose et al. 1998), so dTCF may thus be able to recruit Groucho unassisted. However, these findings do not rule out the possibility that dTCF relies on cooperation with Brinker to achieve Groucho recruitment.
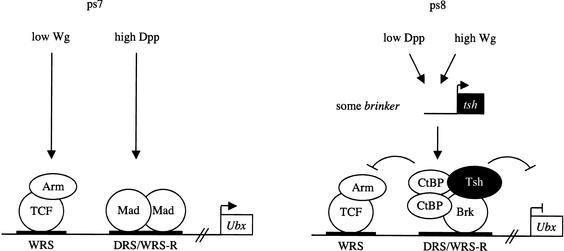
Figure 7.
A morphogenetic switch in the Ubx midgut enhancer: a model. In ps7, low Wg levels synergize with high Dpp levels to stimulate Ubx B; the WRS is occupied by dTCF/Armadillo, the WRS-R by activated Mad. In ps8, the Dpp-signaling levels are low, so Brinker (which is expressed in ps8; Fig. 1A) displaces Mad from the WRS-R. In addition, high Wingless levels (and low Dpp) induce local expression of Tsh in this region. Brinker thus recruits Tsh to the WRS-R, and both proteins together may recruit the quenching factor dCtBP to block the stimulatory activity of the nearby-bound dTCF/Armadillo transcription complex. Note that, although on overexpression, Brinker is sufficient to repress Ubx B in the absence of tsh (Fig. 5) at normal expression levels, Brinker critically depends on tsh to repress Ubx B (Fig. 2). Thus, Tsh is the pivotal factor in this process. Tsh is up-regulated by high Wg levels and, on recruitment by Brinker, confers repression by quenching Wg-mediated activation.
Brinker can bind to the corepressor dCtBP (Fig. 4), so Brinker may recruit dCtBP instead of, or in addition to, Groucho. Recall that Tsh plays a critical role in the Wg-mediated repression in the midgut (Waltzer et al. 2001), just like Brinker itself (Fig. 2). Moreover, Tsh can bind to Brinker as well as to dCtBP, so it seems plausible that Tsh plays a pivotal role in assisting Brinker in the recruitment of dCtBP. Like Groucho, dCtBP is a corepressor with quenching activity (Nibu et al. 1998b). In addition, Tsh may itself be involved in the quenching process. It has been suggested that quenching may be based on obstruction of the interaction between the activation domain of a transcriptional activator and the general transcription machinery (Courey and Jia 2001)—intriguingly, hypophosphorylated Tsh binds to the carboxy-terminal activation domain of Armadillo to modulate Wg signaling (Gallet et al. 1999).
Interpretation of distinct Wg-signaling thresholds: a morphogenetic switch at the transcriptional level
The Drosophila midgut has provided a model system in which Wg signaling regulates gene transcription in a concentration-dependent manner; low signaling levels activate Wg target genes, whereas high levels repress the same genes. The discovery that Brinker confers transcriptional repression by Wg completes our picture of the DNA-binding proteins that interpret these different signaling thresholds. dTCF confers Wg-induced stimulation of target genes (Riese et al. 1997), but its activity can be blocked by Brinker, which confers Wg-mediated repression of the same genes (Fig. 7). dTCF depends on Armadillo for its activity, whereas Brinker depends on Tsh to block the activity of the dTCF/Armadillo complex. In turn, the availability of Armadillo depends directly on Wg signaling, which promotes its stabilization and nuclear translocation (Cavallo et al. 1997), whereas the availability of Tsh depends on transcription of its gene (which itself depends on wg; Mathies et al. 1994). In other words, high Wg signaling induces locally the expression of the Tsh corepressor, which then cooperates with Brinker to repress Wg target genes in the same cells (Fig. 7). One of these targets is wg itself, so Brinker and Tsh take part in the negative feedback loop of Wg signaling in the middle midgut.
The link to Dpp signaling
Ubx B is not only a Wg-responsive enhancer, but it is also stimulated by Dpp signaling (Thüringer and Bienz 1993). Furthermore, Dpp signaling antagonizes Wg-mediated repression (Yu et al. 1998). This can be explained in two ways. First, high levels of Dpp-activated Mad are expected to compete with Brinker for binding to the WRS-R. Second, the brinker gene itself may be down-regulated by Dpp signaling, as this is the case in other tissues (Campbell and Tomlinson 1999; Jazwinska et al. 1999a; Minami et al. 1999; Marty et al. 2000; Sivasankaran et al. 2000), so Brinker may only be present at very low levels in cells within the Dpp-signaling domain. We cannot detect brinker expression in this domain, whereas low levels of expression are detectable in the neighboring Wg-signaling domain (in ps8; Fig. 1A). In contrast, in the latter domain, in which the levels of activated Mad are expected to be low, Brinker successfully competes with Mad for binding to the WRS-R and, together with Tsh, which is present at high levels in this domain, blocks the activity of dTCF/Armadillo (Fig. 7). Note that Dpp signaling promotes this repression indirectly, by contributing to the stimulation of Tsh expression in ps8 (Mathies et al. 1994).
Dpp and Wg signaling cooperate in multiple developmental contexts. In some contexts they synergize (e.g., Campbell et al. 1993; Cohen et al. 1993; Thüringer and Bienz 1993), whereas in other contexts, they antagonize each other (e.g., Ma and Moses 1995; Treisman and Rubin 1995; Theisen et al. 1996). Given that most, if not all, Dpp target genes, and multiple Wg target genes, are repressible by Brinker, this suggests that Brinker may have a universal key role in this decision between synergy and antagonism; absence of Brinker allows synergy between Dpp and Wg, whereas presence of Brinker (and Tsh) mediates antagonism.
Materials and methods
Fly stains
The loss-of-function alleles brkF124 (Lammel et al. 2000), brkM68 (Jazwinska et al. 1999a), and tsh8 (Fasano 1991) were used for analysis. The following lacZ reporter constructs were used: RP9, Ubx B (Thüringer et al. 1993), and BM2 (Szüts et al. 1998). Wg was ubiquitously overexpressed using hs-wg (Noordermeer et al. 1992). The following Gal4 driver and producer lines were used: 24B.Gal4 (for mesodermal expression) (Brand and Perrimon 1993), Arm.Gal4 (for ubiquitous expression) (Sanson et al. 1996), UAS.Wg (Lawrence et al. 1996), UAS.Tsh (Gallet et al. 1998), and UAS.Brk (Lammel et al. 2000).
Heat-shock regimes and phenotypic analyses
Standard crosses were set up, and embryos were collected at 25°C. Mutant embryos and embryos overexpressing various proteins were identified by blue balancers and by their gut phenotypes. To generate the brk; tsh double-mutant strain, GFP balancer chromosomes were used that allow unambigous identification of double-mutant larval cuticles.
The following conditions were used to express Wg ubiquitously from the hs-wg transposon: embryos were collected on apple plates for16 h at 18°C, then immersed in a 37°C waterbath for 30 min. Subsequently, the embryos were aged for 2 h at 25°C prior to the next heat shock or fixation. Embryos were subjected to three heat shocks. Staining of embryos was performed as described (Yu et al. 1996). The following antibodies were used: anti-lacZ (Promega), anti-Wg (Brook and Cohen 1996).
Cuticle preparations were done as described (Szüts et al. 1997).
GST fusion proteins, pull-down, and gel-shift assays
Full-length dCtBP, full-length Brinker, and Brinker fragments were subcloned into pGEX-2TK (Pharmacia) or pT7βlink using standard PCR-cloning procedures. Full-length Tsh subcloned into pGEX-2T or pT7βlink was a gift from Lucas Waltzer (Centre de Biologie du Dévelppemont Université Paul Sabatier, Toulouse, France). GST fusion proteins were produced in Escherichia coli BL21, purified by affinity chromatography on GST-agarose beads, and eluted for gel-shift assays as described (Saller and Bienz 2001). Gel-shift experiments with the WRS-R probe were performed as described (Saller and Bienz 2001). For super-shift assays, purified GST–Tsh fusion protein was added to the DNA/GST-Brinker incubation mix for 10 min prior to loading on the gel. Pull-down assays were performed as described (Waltzer and Bienz 1998).
Acknowledgments
We thank Lucas Waltzer, Susan Parkhurst, Uwe Lammel, and Stephen Kerridge for plasmids and fly strains, Jan de Boer and Barry Thomson for advice and discussion. E.S. is supported by a long-term fellowship from the Swiss National Science Foundation.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL mb2@mrc-lmb.cam.ac.uk; FAX 44-1-223-412142.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.230002.
References
- Ashe HL, Mannervik M, Levine M. Dpp signaling thresholds in the dorsal ectoderm of the Drosophila embryo. Development. 2000;127:3305–3312. doi: 10.1242/dev.127.15.3305. [DOI] [PubMed] [Google Scholar]
- Bienz M. Homeotic genes and positional signaling in the Drosophila viscera. Trends Genet. 1994;10:22–26. doi: 10.1016/0168-9525(94)90015-9. [DOI] [PubMed] [Google Scholar]
- ————— Induction of the endoderm in Drosophila. Sem Cell Dev Biol. 1996;7:113–119. [Google Scholar]
- ————— Endoderm induction in Drosophila: The nuclear targets of the inducing signals. Curr Opin Genet Dev. 1997;7:683–688. doi: 10.1016/s0959-437x(97)80017-2. [DOI] [PubMed] [Google Scholar]
- ————— TCF: Transcriptional activator or repressor? Curr Opin Cell Biol. 1998;10:366–372. doi: 10.1016/s0955-0674(98)80013-6. [DOI] [PubMed] [Google Scholar]
- Bienz M, Tremml G. Domain of Ultrabithorax expression in Drosophila visceral mesoderm from autoregulation and exclusion. Nature. 1988;33:576–578. doi: 10.1038/333576a0. [DOI] [PubMed] [Google Scholar]
- Bienz M, Saari G, Tremml G, Müller J, Züst B, Lawrence PA. Differential regulation of Ultrabithorax in two germ layers of Drosophila. Cell. 1988;53:567–576. doi: 10.1016/0092-8674(88)90573-9. [DOI] [PubMed] [Google Scholar]
- Boyd JM, Subramanian T, Schaeper U, La Regina M, Bayley S, Chinnadurai G. A region in the C-terminus of adenovirus 2/5 E1a protein is required for association with a cellular phosphoprotein and important for the negative modulation of T24-ras mediated transformation, tumorigenesis and metastasis. EMBO J. 1993;12:469–478. doi: 10.1002/j.1460-2075.1993.tb05679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brook WJ, Cohen SM. Antagonistic interactions between wingless and decapentaplegic responsible for dorsal-ventral pattern in the Drosophila leg. Science. 1996;273:1373–1377. doi: 10.1126/science.273.5280.1373. [DOI] [PubMed] [Google Scholar]
- Brunner E, Peter O, Schweizer L, Basler K. pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature. 1997;385:829–833. doi: 10.1038/385829a0. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Fish MP, Rulifson EJ, Nusse R. Wingless repression of Drosophila frizzled 2 expression shapes the Wingless morphogen gradient in the wing. Cell. 1998;93:767–777. doi: 10.1016/s0092-8674(00)81438-5. [DOI] [PubMed] [Google Scholar]
- Campbell G, Tomlinson A. Transducing the Dpp morphogen gradient in the wing of Drosophila:Regulation of Dpp targets by brinker. Cell. 1999;96:553–562. doi: 10.1016/s0092-8674(00)80659-5. [DOI] [PubMed] [Google Scholar]
- Campbell G, Weaver T, Tomlinson A. Axis specification in the developing Drosophila appendage: The role of wingless, decapentaplegic, and the homeobox gene aristaless. Cell. 1993;74:1113–1123. doi: 10.1016/0092-8674(93)90732-6. [DOI] [PubMed] [Google Scholar]
- Cavallo R, Rubenstein D, Peifer M. Armadillo and dTCF: A marriage made in the nucleus. Curr Opin Genet Dev. 1997;7:459–466. doi: 10.1016/s0959-437x(97)80071-8. [DOI] [PubMed] [Google Scholar]
- Cavallo RA, Cox RT, Moline MM, Roose J, Polevoy GA, Clevers H, Peifer M, Bejsovec A. Drosophila Tcf and Groucho interact to repress Wingless signaling activity. Nature. 1998;395:604–608. doi: 10.1038/26982. [DOI] [PubMed] [Google Scholar]
- Cohen B, Simcox AA, Cohen SM. Allocation of the thoracic imaginal primordia in the Drosophila embryo. Development. 1993;117:597–608. doi: 10.1242/dev.117.2.597. [DOI] [PubMed] [Google Scholar]
- Courey AJ, Jia S. Transcriptional repression: The long and the short of it. Genes & Dev. 2001;15:2786–2796. doi: 10.1101/gad.939601. [DOI] [PubMed] [Google Scholar]
- Criqui-Filipe P, Ducret C, Maira SM, Wasylyk B. Net, a negative Ras-switchable TCF, contains a second inhibition domain, the CID, that mediates repression through interactions with CtBP and de-acetylation. EMBO J. 1999;18:3392–3403. doi: 10.1093/emboj/18.12.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano L, Röder L, Coré N, Alexandre E, Vola C, Jacq B, Kerridge S. The gene teashirt is required for the development of Drosophila embryonic trunk segments and encodes a protein with widely spaced zinc finger motifs. Cell. 1991;64:63–79. doi: 10.1016/0092-8674(91)90209-h. [DOI] [PubMed] [Google Scholar]
- Gallet A, Erkner A, Charroux B, Fasano L, Kerridge S. Trunk-specific modulation of wingless signaling in Drosophila by teashirt binding to armadillo. Curr Biol. 1998;8:893–902. doi: 10.1016/s0960-9822(07)00369-7. [DOI] [PubMed] [Google Scholar]
- Gallet A, Angelats C, Erkner A, Charroux B, Fasano L, Kerridge S. The C-terminal domain of armadillo binds to hypophosphorylated teashirt to modulate wingless signaling in Drosophila. EMBO J. 1999;18:2208–2217. doi: 10.1093/emboj/18.8.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallet A, Angelats C, Kerridge S, Therond PP. Cubitus interruptus-independent transduction of the Hedgehog signal in Drosophila. Development. 2000;127:5509–5522. doi: 10.1242/dev.127.24.5509. [DOI] [PubMed] [Google Scholar]
- Gray S, Levine M. Short-range transcriptional repressors mediate both quenching and direct repression within complex loci in Drosophila. Genes & Dev. 1996;10:700–710. doi: 10.1101/gad.10.6.700. [DOI] [PubMed] [Google Scholar]
- Grosschedl R, Giese K, Pagel J. HMG domain proteins: Architectural elements in the assembly of nucleoprotein structures. Trends Genet. 1994;10:94–100. doi: 10.1016/0168-9525(94)90232-1. [DOI] [PubMed] [Google Scholar]
- Hasson P, Muller B, Basler K, Paroush Z. Brinker requires two corepressors for maximal and versatile repression in Dpp signaling. EMBO J. 2001;20:5725–5736. doi: 10.1093/emboj/20.20.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper JE. Distinct pathways for autocrine and paracrine Wingless signaling in Drosophila embryos. Nature. 1994;372:461–464. doi: 10.1038/372461a0. [DOI] [PubMed] [Google Scholar]
- Hoppler S, Bienz M. Two different thresholds of wingless signaling with distinct developmental consequences in the Drosophila midgut. EMBO J. 1995;14:5016–5026. doi: 10.1002/j.1460-2075.1995.tb00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinska A, Kirov N, Wieschaus E, Roth S, Rushlow C. The Drosophila gene brinker reveals a novel mechanism of Dpp target gene regulation. Cell. 1999a;96:563–573. doi: 10.1016/s0092-8674(00)80660-1. [DOI] [PubMed] [Google Scholar]
- Jazwinska A, Rushlow C, Roth S. The role of brinker in mediating the graded response to Dpp in early Drosophila embryos. Development. 1999b;126:3323–3334. doi: 10.1242/dev.126.15.3323. [DOI] [PubMed] [Google Scholar]
- Jiang J, Struhl G. Complementary and mutually exclusive activities of decapentaplegic and wingless organize axial patterning during Drosophila leg development. Cell. 1996;86:401–409. doi: 10.1016/s0092-8674(00)80113-0. [DOI] [PubMed] [Google Scholar]
- Kim J, Johnson K, Chen HJ, Carroll S, Laughon A. Drosophila Mad binds to DNA and directly mediates activation of vestigial by Decapentaplegic. Nature. 1997;388:304–308. doi: 10.1038/40906. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick H, Johnson K, Laughon A. Repression of dpp targets by binding of brinker to mad sites. J Biol Chem. 2001;276:18216–18222. doi: 10.1074/jbc.M101365200. [DOI] [PubMed] [Google Scholar]
- Klein T. Wing disc development in the fly: The early stages. Curr Opin Genet Dev. 2001;11:470–475. doi: 10.1016/s0959-437x(00)00219-7. [DOI] [PubMed] [Google Scholar]
- Lammel U, Meadows L, Saumweber H. Analysis of Drosophila salivary gland, epidermis and CNS development suggests an additional function of brinker in anterior-posterior cell fate specification. Mech Dev. 2000;92:179–191. doi: 10.1016/s0925-4773(99)00337-8. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Sanson B, Vincent JP. Compartments, wingless and engrailed: Patterning the ventral epidermis of Drosophila embryos. Development. 1996;122:4095–4103. doi: 10.1242/dev.122.12.4095. [DOI] [PubMed] [Google Scholar]
- Lecuit T, Cohen SM. Proximal-distal axis formation in the Drosophila leg. Nature. 1997;388:139–145. doi: 10.1038/40563. [DOI] [PubMed] [Google Scholar]
- Levanon D, Goldstein RE, Bernstein Y, Tang H, Goldenberg D, Stifani S, Paroush Z, Groner Y. Transcriptional repression by AML1 and LEF-1 is mediated by the TLE/Groucho corepressors. Proc Natl Acad Sci. 1998;95:11590–11595. doi: 10.1073/pnas.95.20.11590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Moses K. Wingless and patched are negative regulators of the morphogenetic furrow and can affect tissue polarity in the developing Drosophila compound eye. Development. 1995;121:2279–2289. doi: 10.1242/dev.121.8.2279. [DOI] [PubMed] [Google Scholar]
- Marty T, Muller B, Basler K, Affolter M. Schnurri mediates dpp-dependent repression of brinker transcription. Nat Cell Biol. 2000;2:745–749. doi: 10.1038/35036383. [DOI] [PubMed] [Google Scholar]
- Mathies LD, Kerridge S, Scott MP. Role of the teashirt gene in Drosophila midgut morphogenesis: Secreted proteins mediate the action of homeotic genes. Development. 1994;120:2799–2809. doi: 10.1242/dev.120.10.2799. [DOI] [PubMed] [Google Scholar]
- Minami M, Kinoshita N, Kamoshida Y, Tanimoto H, Tabata T. brinker is a target of Dpp in Drosophila that negatively regulates Dpp-dependent genes. Nature. 1999;398:242–246. doi: 10.1038/18451. [DOI] [PubMed] [Google Scholar]
- Neumann CJ, Cohen SM. Long-range action of Wingless organizes the dorsal-ventral axis of the Drosophila wing. Development. 1997;124:871–880. doi: 10.1242/dev.124.4.871. [DOI] [PubMed] [Google Scholar]
- Nibu Y, Levine MS. CtBP-dependent activities of the short-range Giant repressor in the Drosophila embryo. Proc Natl Acad Sci. 2001;98:6204–6208. doi: 10.1073/pnas.111158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibu Y, Zhang H, Bajor E, Barolo S, Small S, Levine M. dCtBP mediates transcriptional repression by Knirps, Krüppel and Snail in the Drosophila embryo. EMBO J. 1998a;17:7009–7020. doi: 10.1093/emboj/17.23.7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibu Y, Zhang H, Levine M. Interaction of short-range repressors with Drosophila CtBP in the embryo. Science. 1998b;280:101–104. doi: 10.1126/science.280.5360.101. [DOI] [PubMed] [Google Scholar]
- Noordermeer J, Johnston P, Rijsewijk F, Nusse R, Lawrence PA. The consequences of ubiquitous expression of the wingless gene in the Drosophila embryo. Development. 1992;116:711–719. doi: 10.1242/dev.116.3.711. [DOI] [PubMed] [Google Scholar]
- O'Keefe L, Dougan ST, Gabay L, Raz E, Shilo BZ, DiNardo S. Spitz and Wingless, emanating from distinct borders, cooperate to establish cell fate across the Engrailed domain in the Drosophila epidermis. Development. 1997;124:4837–4845. doi: 10.1242/dev.124.23.4837. [DOI] [PubMed] [Google Scholar]
- Payre F, Vincent A, Carreno S. ovo/svb integrates Wingless and DER pathways to control epidermis differentiation. Nature. 1999;400:271–275. doi: 10.1038/22330. [DOI] [PubMed] [Google Scholar]
- Perrimon N. The genetic basis of patterned baldness in Drosophila. Cell. 1994;76:781–784. doi: 10.1016/0092-8674(94)90351-4. [DOI] [PubMed] [Google Scholar]
- Phippen TM, Sweigart AL, Moniwa M, Krumm A, Davie JR, Parkhurst SM. Drosophila C-terminal binding protein functions as a context-dependent transcriptional co-factor and interferes with both mad and groucho transcriptional repression. J Biol Chem. 2000;275:37628–37637. doi: 10.1074/jbc.M004234200. [DOI] [PubMed] [Google Scholar]
- Piepenburg O, Vorbruggen G, Jäckle H. Drosophila segment borders result from unilateral repression of hedgehog activity by wingless signaling. Mol Cell. 2000;6:203–209. [PubMed] [Google Scholar]
- Poortinga G, Watanabe M, Parkhurst SM. Drosophila CtBP: A Hairy-interacting protein required for embryonic segmentation and hairy-mediated transcriptional repression. EMBO J. 1998;17:2067–2078. doi: 10.1093/emboj/17.7.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riese J, Yu X, Munnerlyn A, Eresh S, Hsu SC, Grosschedl R, Bienz M. LEF-1, a nuclear factor coordinating signaling inputs from wingless and decapentaplegic. Cell. 1997;88:777–787. doi: 10.1016/s0092-8674(00)81924-8. [DOI] [PubMed] [Google Scholar]
- Roose J, Molenaar M, Peterson J, Hurenkamp J, Brantjes H, Moerer P, van de Wetering M, Destree O, Clevers H. The Xenopus Wnt effector XTcf-3 interacts with Groucho-related transcriptional repressors. Nature. 1998;395:608–612. doi: 10.1038/26989. [DOI] [PubMed] [Google Scholar]
- Royet J, Finkelstein R. hedgehog, wingless and orthodenticle specify adult head development in Drosophila. Development. 1996;122:1849–1858. doi: 10.1242/dev.122.6.1849. [DOI] [PubMed] [Google Scholar]
- Rulifson EJ, Micchelli CA, Axelrod JD, Perrimon N, Blair SS. wingless refines its own expression domain on the Drosophila wing margin. Nature. 1996;384:72–74. doi: 10.1038/384072a0. [DOI] [PubMed] [Google Scholar]
- Rushlow C, Colosimo PF, Lin MC, Xu M, Kirov N. Transcriptional regulation of the Drosophila gene zen by competing Smad and Brinker inputs. Genes & Dev. 2001;15:340–351. doi: 10.1101/gad.861401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saller E, Bienz M. Direct competition between Brinker and Drosophila Mad in Dpp target gene transcription. EMBO Rep. 2001;2:298–305. doi: 10.1093/embo-reports/kve068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanson B, White P, Vincent JP. Uncoupling cadherin-based adhesion from wingless signaling in Drosophila. Nature. 1996;383:627–630. doi: 10.1038/383627a0. [DOI] [PubMed] [Google Scholar]
- Sivasankaran R, Vigano MA, Muller B, Affolter M, Basler K. Direct transcriptional control of the Dpp target omb by the DNA binding protein Brinker. EMBO J. 2000;19:6162–6172. doi: 10.1093/emboj/19.22.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G, Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993;72:527–540. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- Szüts D, Freeman M, Bienz M. Antagonism between EGFR and Wingless signaling in the larval cuticle of Drosophila. Development. 1997;124:3209–3219. doi: 10.1242/dev.124.16.3209. [DOI] [PubMed] [Google Scholar]
- Szüts D, Eresh S, Bienz M. Functional intertwining of Dpp and EGFR signaling during Drosophila endoderm induction. Genes & Dev. 1998;12:2022–2035. doi: 10.1101/gad.12.13.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen H, Haerry TE, O'Connor MB, Marsh JL. Developmental territories created by mutual antagonism between Wingless and Decapentaplegic. Development. 1996;122:3939–3948. doi: 10.1242/dev.122.12.3939. [DOI] [PubMed] [Google Scholar]
- Thüringer F, Bienz M. Indirect autoregulation of a homeotic Drosophila gene mediated by extracellular signaling. Proc Nat Acad Sci. 1993;90:3899–3903. doi: 10.1073/pnas.90.9.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thüringer F, Cohen SM, Bienz M. Dissection of an indirect autoregulatory response of a homeotic Drosophila gene. EMBO J. 1993;12:2419–30. doi: 10.1002/j.1460-2075.1993.tb05896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman JE, Rubin GM. wingless inhibits morphogenetic furrow movement in the Drosophila eye disc. Development. 1995;121:3519–3527. doi: 10.1242/dev.121.11.3519. [DOI] [PubMed] [Google Scholar]
- Turner J, Crossley M. Cloning and characterization of mCtBP2, a co-repressor that associates with basic Krüppel-like factor and other mammalian transcriptional regulators. EMBO J. 1998;17:5129–5140. doi: 10.1093/emboj/17.17.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- Waltzer L, Bienz M. Drosophila CBP represses the transcription factor TCF to antagonize Wingless signaling. Nature. 1998;395:521–525. doi: 10.1038/26785. [DOI] [PubMed] [Google Scholar]
- Waltzer L, Vandel L, Bienz M. Teashirt is required for transcriptional repression mediated by high Wingless levels. EMBO J. 2001;20:137–145. doi: 10.1093/emboj/20.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, van Beest M, Clevers H, Jones T, Hursh DA, Mortin MA. decapentaplegic is a direct target of dTcf repression in the Drosophila visceral mesoderm. Development. 2000;127:3695–3702. doi: 10.1242/dev.127.17.3695. [DOI] [PubMed] [Google Scholar]
- Yu X, Hoppler S, Eresh S, Bienz M. decapentaplegic, a target gene of the wingless signaling pathway in the Drosophila midgut. Development. 1996;122:849–858. doi: 10.1242/dev.122.3.849. [DOI] [PubMed] [Google Scholar]
- Yu X, Riese J, Eresh S, Bienz M. Transcriptional repression due to high levels of Wingless signaling. EMBO J. 1998;17:7021–7032. doi: 10.1093/emboj/17.23.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca M, Basler K, Struhl G. Direct and long-range action of a wingless morphogen gradient. Cell. 1996;87:833–844. doi: 10.1016/s0092-8674(00)81991-1. [DOI] [PubMed] [Google Scholar]
- Zhang H, Levine M. Groucho and dCtBP mediate separate pathways of transcriptional repression in the Drosophila embryo. Proc Natl Acad Sci. 1999;96:535–540. doi: 10.1073/pnas.96.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Levine M, Ashe HL. Brinker is a sequence-specific transcriptional repressor in the Drosophila embryo. Genes & Dev. 2001;15:261–266. doi: 10.1101/gad.861201. [DOI] [PMC free article] [PubMed] [Google Scholar]