Abstract
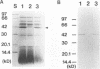
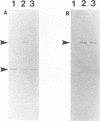
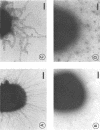
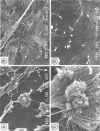
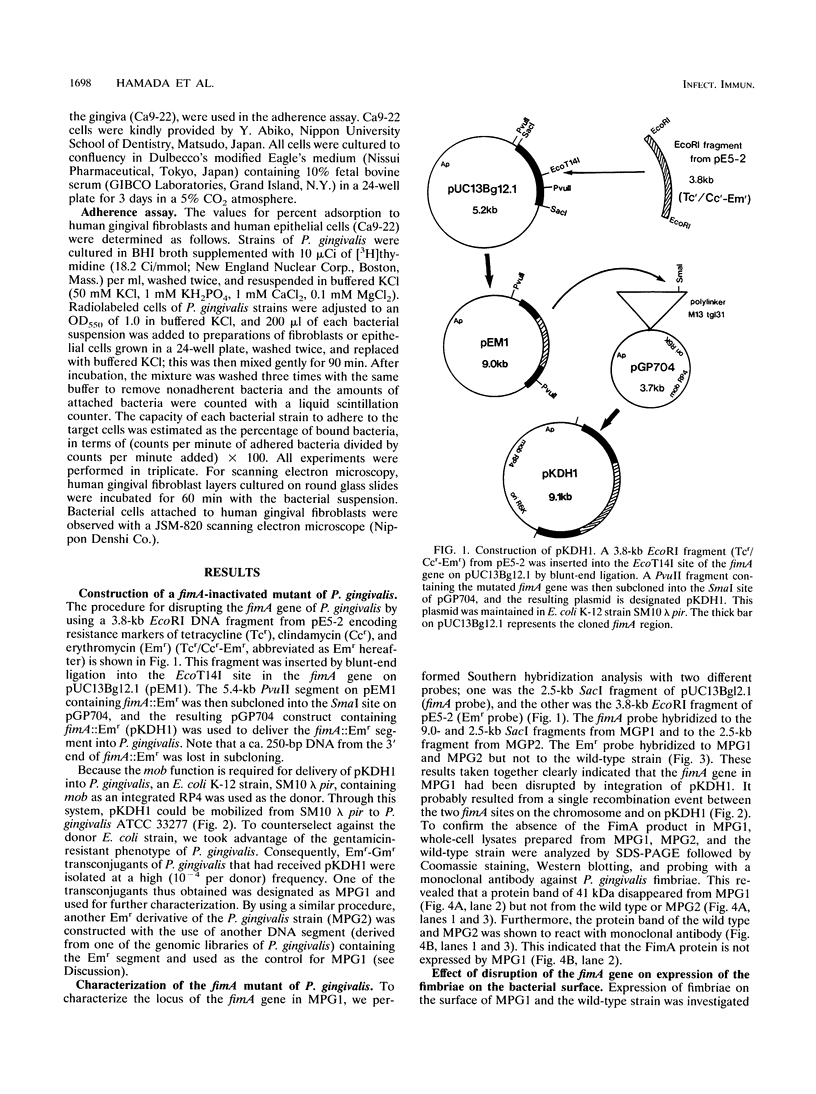
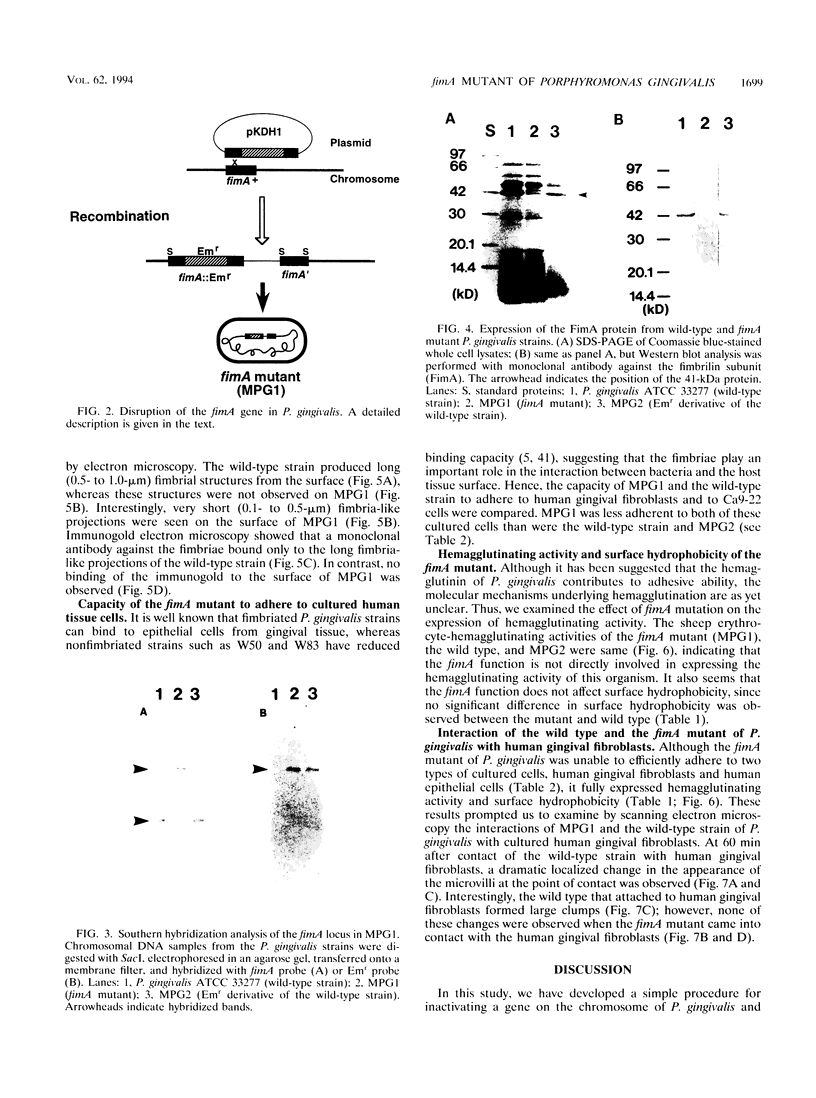
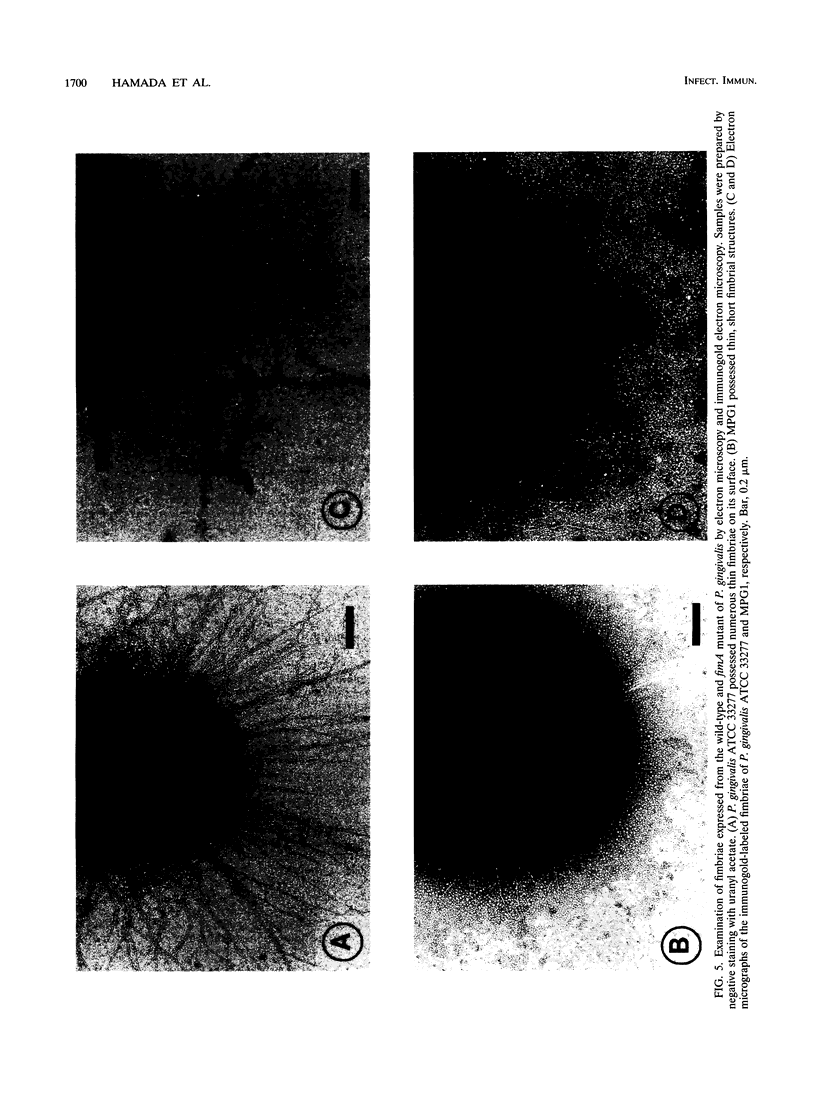
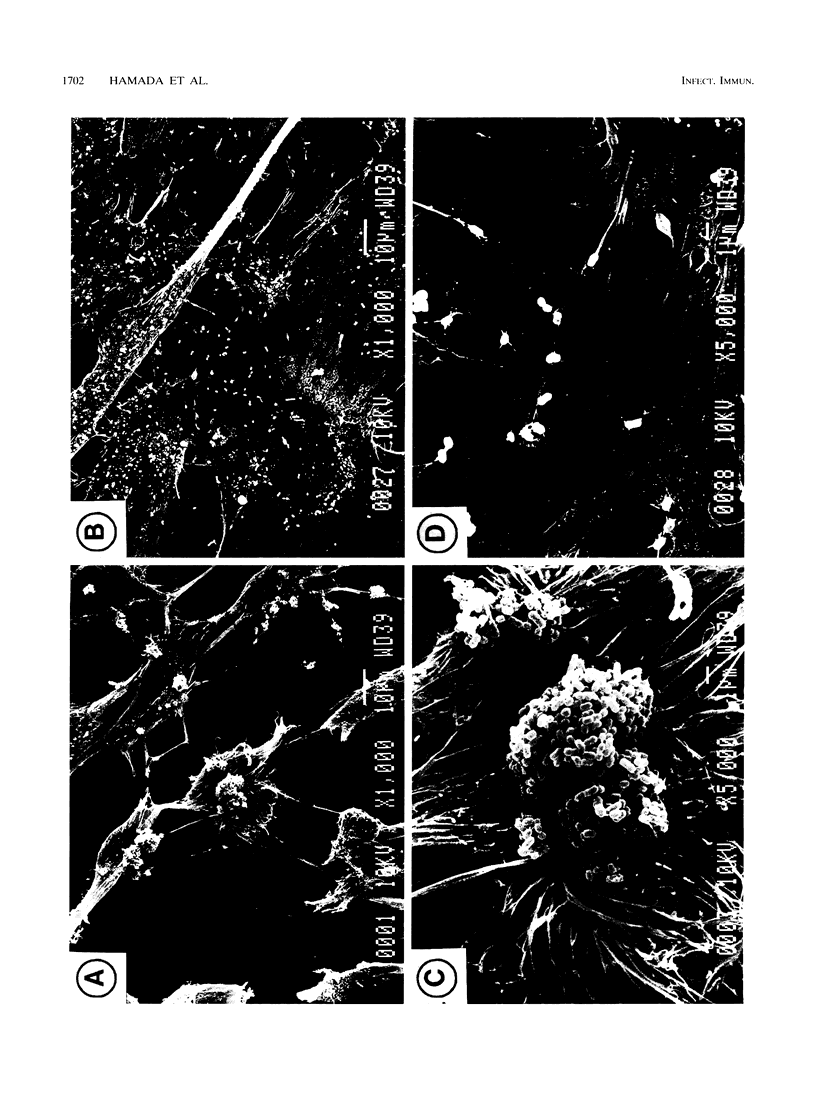
Although fimbriae of Porphyromonas gingivalis have been implicated as playing a major role in adherence to gingival tissue surfaces, no conclusive genetic evidence has yet been obtained. The fimA gene, the determinant for the major fimbrial subunit protein, was cloned and sequenced (D. P. Dickinson, M. A. Kubiniec, F. Yoshimura, and R. J. Genco, J. Bacteriol. 170:1658-1665, 1988). We undertook to inactivate the fimA gene by a homologous recombination technique and examined the fimA mutant for changes in surface properties, including production of fimbriae, adherence to human gingival fibroblasts and epithelial cells, hemagglutinating activity, and surface hydrophobicity. To inactivate the fimA gene, we disrupted a fimA clone by insertion of a DNA segment containing an erythromycin resistance (Emr) gene. This was then delivered into P. gingivalis ATCC 33277 from an Escherichia coli K-12 strain, SM10 lambda pir, by using a mobilizable suicide vector, pGP704; recombination at the fimA locus led to the isolation of a fimA mutant. Disruption of the fimA locus and disappearance of FimA production were confirmed by Southern hybridization with a fimA-specific DNA probe and Western immunoblotting with a monoclonal antibody against the FimA protein, respectively. The fimA mutant constructed failed to express long (0.5- to 1.0-micron) fimbriae from the bacterial surface and had a diminished adhesive capacity to tissue-cultured human gingival fibroblasts and epithelial cells. Observation of the bacteria adhering to human gingival fibroblasts by scanning electron microscopy revealed that the wild-type strain had dramatic local changes in the appearance of the microvilli at the point of contact with large bacterial clumps, whereas the fimA mutant did not. In contrast, neither the hemagglutinating activity nor the surface hydrophobicity was changed in the fimA mutant. These data thus constitute the first direct genetic evidence demonstrating that the FimA protein of P. gingivalis is essential for the interaction of the organism with human gingival tissue cells through a function(s) encoded by the fimA gene.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Childs W. C., 3rd, Gibbons R. J. Selective modulation of bacterial attachment to oral epithelial cells by enzyme activities associated with poor oral hygiene. J Periodontal Res. 1990 May;25(3):172–178. doi: 10.1111/j.1600-0765.1990.tb01040.x. [DOI] [PubMed] [Google Scholar]
- Dickinson D. P., Kubiniec M. A., Yoshimura F., Genco R. J. Molecular cloning and sequencing of the gene encoding the fimbrial subunit protein of Bacteroides gingivalis. J Bacteriol. 1988 Apr;170(4):1658–1665. doi: 10.1128/jb.170.4.1658-1665.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan M. J., Nakao S., Skobe Z., Xie H. Interactions of Porphyromonas gingivalis with epithelial cells. Infect Immun. 1993 May;61(5):2260–2265. doi: 10.1128/iai.61.5.2260-2265.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Etherden I. Comparative hydrophobicities of oral bacteria and their adherence to salivary pellicles. Infect Immun. 1983 Sep;41(3):1190–1196. doi: 10.1128/iai.41.3.1190-1196.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulbourne P. A., Ellen R. P. Evidence that Porphyromonas (Bacteroides) gingivalis fimbriae function in adhesion to Actinomyces viscosus. J Bacteriol. 1991 Sep;173(17):5266–5274. doi: 10.1128/jb.173.17.5266-5274.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier D., Mayrand D. Functional characterization of extracellular vesicles produced by Bacteroides gingivalis. Infect Immun. 1987 Jan;55(1):111–117. doi: 10.1128/iai.55.1.111-117.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier D., Mayrand D. Selected characteristics of pathogenic and nonpathogenic strains of Bacteroides gingivalis. J Clin Microbiol. 1987 Apr;25(4):738–740. doi: 10.1128/jcm.25.4.738-740.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanazawa S., Hirose K., Ohmori Y., Amano S., Kitano S. Bacteroides gingivalis fimbriae stimulate production of thymocyte-activating factor by human gingival fibroblasts. Infect Immun. 1988 Jan;56(1):272–274. doi: 10.1128/iai.56.1.272-274.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C., Ebersole J., Felton J., Brunsvold M., Kornman K. S. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science. 1988 Jan 1;239(4835):55–57. doi: 10.1126/science.3336774. [DOI] [PubMed] [Google Scholar]
- Inoshita E., Amano A., Hanioka T., Tamagawa H., Shizukuishi S., Tsunemitsu A. Isolation and some properties of exohemagglutinin from the culture medium of Bacteroides gingivalis 381. Infect Immun. 1986 May;52(2):421–427. doi: 10.1128/iai.52.2.421-427.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogai H., Isogai E., Yoshimura F., Suzuki T., Kagota W., Takano K. Specific inhibition of adherence of an oral strain of Bacteroides gingivalis 381 to epithelial cells by monoclonal antibodies against the bacterial fimbriae. Arch Oral Biol. 1988;33(7):479–485. doi: 10.1016/0003-9969(88)90028-3. [DOI] [PubMed] [Google Scholar]
- Koga T., Nishihara T., Fujiwara T., Nisizawa T., Okahashi N., Noguchi T., Hamada S. Biochemical and immunobiological properties of lipopolysaccharide (LPS) from Bacteroides gingivalis and comparison with LPS from Escherichia coli. Infect Immun. 1985 Mar;47(3):638–647. doi: 10.1128/iai.47.3.638-647.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K. P., Fukushima H., Sagawa H., Walker C. B., Clark W. B. Surface appendages, hemagglutination, and adherence to human epithelial cells of Bacteroides intermedius. Oral Microbiol Immunol. 1989 Dec;4(4):204–210. doi: 10.1111/j.1399-302x.1989.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A. Bacteriology of human experimental gingivitis: effect of plaque and gingivitis score. Infect Immun. 1978 Sep;21(3):830–839. doi: 10.1128/iai.21.3.830-839.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos B. G., Dyer D. W. Restriction fragment length polymorphism analysis of the fimbrillin locus, fimA, of Porphyromonas gingivalis. J Dent Res. 1992 May;71(5):1173–1181. doi: 10.1177/00220345920710050901. [DOI] [PubMed] [Google Scholar]
- Mayrand D., McBride B. C. Exological relationships of bacteria involved in a simple, mixed anaerobic infection. Infect Immun. 1980 Jan;27(1):44–50. doi: 10.1128/iai.27.1.44-50.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Mekalanos J. J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988 Jun;170(6):2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miörner H., Johansson G., Kronvall G. Lipoteichoic acid is the major cell wall component responsible for surface hydrophobicity of group A streptococci. Infect Immun. 1983 Jan;39(1):336–343. doi: 10.1128/iai.39.1.336-343.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y., Gibbons R. J. Attachment of Bacteroides gingivalis to collagenous substrata. J Dent Res. 1988 Aug;67(8):1075–1080. doi: 10.1177/00220345880670080301. [DOI] [PubMed] [Google Scholar]
- Okuda K., Yamamoto A., Naito Y., Takazoe I., Slots J., Genco R. J. Purification and properties of hemagglutinin from culture supernatant of Bacteroides gingivalis. Infect Immun. 1986 Dec;54(3):659–665. doi: 10.1128/iai.54.3.659-665.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peros W. J., Etherden I., Gibbons R. J., Skobe Z. Alteration of fimbriation and cell hydrophobicity by sublethal concentrations of tetracycline. J Periodontal Res. 1985 Jan;20(1):24–30. doi: 10.1111/j.1600-0765.1985.tb00406.x. [DOI] [PubMed] [Google Scholar]
- Progulske-Fox A., Oberste A., Drummond C., McArthur W. P. Transfer of plasmid pE5-2 from Escherichia coli to Bacteroides gingivalis and B. intermedius. Oral Microbiol Immunol. 1989 Sep;4(3):132–134. doi: 10.1111/j.1399-302x.1989.tb00239.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Perry A., Bayer E. A., Gutnick D. L., Rosenberg E., Ofek I. Adherence of Acinetobacter calcoaceticus RAG-1 to human epithelial cells and to hexadecane. Infect Immun. 1981 Jul;33(1):29–33. doi: 10.1128/iai.33.1.29-33.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Guthrie E. P., Salyers A. A., Gardner J. F. Evidence that the clindamycin-erythromycin resistance gene of Bacteroides plasmid pBF4 is on a transposable element. J Bacteriol. 1985 May;162(2):626–632. doi: 10.1128/jb.162.2.626-632.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Genco R. J. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984 Mar;63(3):412–421. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- Slots J., Gibbons R. J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978 Jan;19(1):254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Listgarten M. A. Bacteroides gingivalis, Bacteroides intermedius and Actinobacillus actinomycetemcomitans in human periodontal diseases. J Clin Periodontol. 1988 Feb;15(2):85–93. doi: 10.1111/j.1600-051x.1988.tb00999.x. [DOI] [PubMed] [Google Scholar]
- Socransky S. S. Relationship of bacteria to the etiology of periodontal disease. J Dent Res. 1970 Mar-Apr;49(2):203–222. doi: 10.1177/00220345700490020401. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Yoshimura F., Kawanami M., Kato H. Detection of fimbrilin gene (fimA) in Porphyromonas (Bacteroides) gingivalis by Southern blot analysis. J Periodontal Res. 1992 Nov;27(6):599–603. doi: 10.1111/j.1600-0765.1992.tb01742.x. [DOI] [PubMed] [Google Scholar]
- Tanner A. C., Haffer C., Bratthall G. T., Visconti R. A., Socransky S. S. A study of the bacteria associated with advancing periodontitis in man. J Clin Periodontol. 1979 Oct;6(5):278–307. doi: 10.1111/j.1600-051x.1979.tb01931.x. [DOI] [PubMed] [Google Scholar]
- Toda K., Otsuka M., Ishikawa Y., Sato M., Yamamoto Y., Nakamura R. Thiol-dependent collagenolytic activity in culture media of Bacteroides gingivalis. J Periodontal Res. 1984 Jul;19(4):372–381. doi: 10.1111/j.1600-0765.1984.tb01010.x. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Yamaji Y., Umemoto T. Correlation between cell-adherent activity and surface structure in Porphyromonas gingivalis. Oral Microbiol Immunol. 1992 Dec;7(6):357–363. doi: 10.1111/j.1399-302x.1992.tb00636.x. [DOI] [PubMed] [Google Scholar]
- White D., Mayrand D. Association of oral Bacteroides with gingivitis and adult periodontitis. J Periodontal Res. 1981 May;16(3):259–265. doi: 10.1111/j.1600-0765.1981.tb00974.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Nishikata M., Suzuki T., Hoover C. I., Newbrun E. Characterization of a trypsin-like protease from the bacterium Bacteroides gingivalis isolated from human dental plaque. Arch Oral Biol. 1984;29(7):559–564. doi: 10.1016/0003-9969(84)90078-5. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Takahashi K., Nodasaka Y., Suzuki T. Purification and characterization of a novel type of fimbriae from the oral anaerobe Bacteroides gingivalis. J Bacteriol. 1984 Dec;160(3):949–957. doi: 10.1128/jb.160.3.949-957.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Takahashi Y., Hibi E., Takasawa T., Kato H., Dickinson D. P. Proteins with molecular masses of 50 and 80 kilodaltons encoded by genes downstream from the fimbrilin gene (fimA) are components associated with fimbriae in the oral anaerobe Porphyromonas gingivalis. Infect Immun. 1993 Dec;61(12):5181–5189. doi: 10.1128/iai.61.12.5181-5189.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambon J. J., Reynolds H. S., Slots J. Black-pigmented Bacteroides spp. in the human oral cavity. Infect Immun. 1981 Apr;32(1):198–203. doi: 10.1128/iai.32.1.198-203.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steenbergen T. J., Kastelein P., Touw J. J., de Graaff J. Virulence of black-pigmented Bacteroides strains from periodontal pockets and other sites in experimentally induced skin lesions in mice. J Periodontal Res. 1982 Jan;17(1):41–49. doi: 10.1111/j.1600-0765.1982.tb01129.x. [DOI] [PubMed] [Google Scholar]