Abstract
The mammalian ATF/CREB family of transcription factors comprises a large group of basic-region leucine zipper (bZIP) proteins whose members mediate diverse transcriptional regulatory functions. Here we report that expression of a specific mouse ATF gene, ATFx, is down-regulated in a variety of cells undergoing apoptosis following growth factor deprivation. When stably expressed in an interleukin 3 (IL-3)-dependent cell line, ATFx suppresses apoptosis resulting from cytokine deprivation. Conversely, a dominant-negative ATFx mutant induces apoptosis of cells cultured in the presence of growth factors. We also show that 24p3, a secreted lipocalin that induces apoptosis when added to hematopoietic cells, represses ATFx expression. However, constitutive expression of ATFx renders cells resistant to 24p3-mediated apoptosis. Collectively, our results indicate that ATFx is an anti-apoptotic factor, a novel role for an ATF protein.
Keywords: Apoptosis, ATFx, IL-3 deprivation, bZIP transcription factor, 24p3
To a great extent, eukaryotic gene regulation is controlled at the transcriptional level by promoter-specific activator proteins (activators). In higher eukaryotes, activators are often present as families of related proteins. One well-studied example is the ATF/CREB family, whose members contain related basic-region leucine zipper (bZIP) DNA-binding domains (Hai and Hartman 2001). ATF proteins (ATFs) bind as homo- or heterodimers to a consensus DNA-binding site, TGAC GTCA (Lee et al. 1987). Outside of their bZIP DNA-binding regions, ATFs diverge considerably, and it is these nonconserved portions that contain the diverse transcriptional regulatory domains. ATF family members are classified into subgroups based on their sequence similarity outside of their bZIP domain (Hai and Hartman 2001).
It has become increasingly clear that transcriptional regulation plays an important role in cell proliferation and death. The balance between proliferation and death is of fundamental importance for normal development and physiological function. During development, cells undergo terminal differentiation and ultimately die by apoptosis (Vaux and Korsmeyer 1999). In contrast, tumor cells either fail to complete the differentiation process or regain the ability to reverse the differentiation program and reenter the cell cycle (Williams 1991; Hartwell and Kastan 1994). Therefore, factors that are differentially expressed during cell cycle progression and have a negative effect on cell death/apoptosis programs might be both critical for normal development and significant for cancer etiology.
In some cells, apoptosis can be induced by deprivation of growth factors. For example, neuronal death occurs following withdrawal of nerve growth factor (Ross 1996). Numerous examples of cell death occur in hematopoietic cells following cytokine deprivation (Georgopoulos 1997; Kuo and Leiden 1999). In an analogous fashion, nonhematopoietic mammalian cells undergo apoptosis when deprived of serum (Collins et al. 1994). In several instances, apoptosis induced by growth factor deprivation has been shown to be transcription-dependent (Ishida et al. 1992) and is associated with marked changes in the gene expression profile (e.g., see Devireddy et al. 2001).
Because of the importance of apoptosis to cell homeostasis, it is of particular interest to identify factors involved in this process. Recently, we have used expression profiling to analyze gene expression in interleukin-3 (IL-3)-dependent murine FL5.12 cells following cytokine deprivation (Devireddy et al. 2001). Among the most dramatic changes was the decreased expression of ATFx (also known in murine cells as ATF7 [Peters et al. 2001] and in human cells as hATF5 [Pati et al. 1999]), a member of the ATF/CREB family of transcription factors, raising the possibility that ATFx has a role in cell survival. Here we test this idea and study the role of ATFx in apoptosis.
Results
Repression of ATFx expression in cells undergoing apoptosis following growth factor deprivation
We have previously found that ATFx is down-regulated in murine pro-B lymphocytic FL5.12 cells following IL-3 deprivation (Devireddy et al. 2001). To determine whether this phenomenon is specific to IL-3-dependent cell lines or is instead a more general event, we analyzed ATFx expression in several other growth factor-dependent cell lines and primary cells.
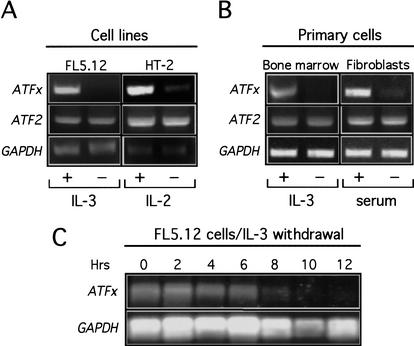
Figure 1A shows, consistent with our previous results, that ATFx expression in FL5.12 cells was markedly reduced after IL-3 deprivation, whereas expression of GAPDH and another ATF gene, ATF2, was unaffected. Similarly, ATFx mRNA levels were reduced following induction of apoptosis by cytokine deprivation in IL-2-dependent HT-2 cells and IL-3-dependent primary mouse bone marrow cells (Fig. 1A,B). ATFx expression was also repressed in primary fetal fibroblasts following induction of apoptosis by serum deprivation (Fig. 1B). In all instances, the levels of ATF2 and GAPDH mRNA were unaffected. Thus, ATFx is selectively down-regulated in diverse cells undergoing apoptosis following growth factor deprivation. A time-course analysis revealed that in FL5.12 cells, ATFx repression first occurred ∼4 h after IL-3 deprivation (Fig. 1C), concomitant with the onset of apoptosis (McCubrey et al. 1989; Boise et al. 1993).
Figure 1.
Repression of ATFx expression in cells undergoing apoptosis following growth factor deprivation. (A) Cell lines. RNA was isolated from FL5.12 and HT-2 cells 24 h after removal of IL-3 or IL-2, respectively. RT-PCR analysis was used to monitor expression of ATFx and ATF2, as well as GAPDH as an internal control. (B) Primary cells. ATFx, ATF2, and GAPDH expression was monitored by RT-PCR analysis in mouse bone marrow cells and human fibroblasts 24 h after IL-3 deprivation or serum-withdrawal, respectively. (C) Time-course analysis of ATFx expression following IL-3 deprivation. RNA was isolated from FL5.12 cells at 2-h intervals following IL-3 withdrawal, and ATFx and GAPDH expression was monitored by Northern blot analysis.
ATFx suppresses apoptosis in IL-3-deprived FL5.12 cells
The rapid down-regulation of ATFx in cells undergoing apoptosis raised the possibility that ATFx normally functions to promote cell survival. To test this idea, we asked whether ectopic expression of ATFx would suppress apoptosis of growth factor-deprived cells.
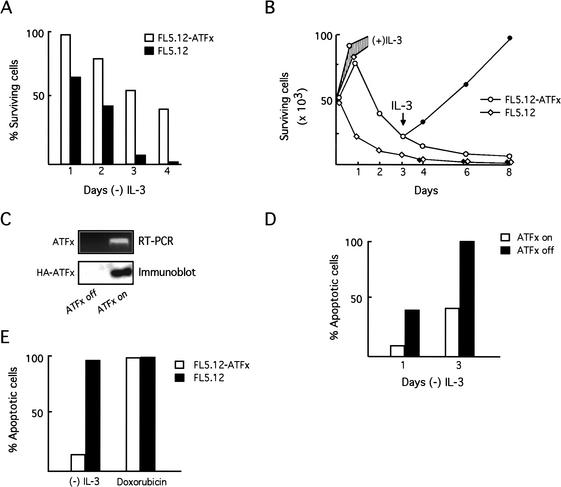
We derived an FL5.12 cell line stably expressing N-terminal hemagglutinin (HA)-tagged ATFx from the constitutive cytomegalovirus (CMV) promoter (FL5.12-ATFx cells). ATFx protein expression was confirmed by staining FL5.12-ATFx cells with an anti-HA polyclonal antibody (see below). Figure 2A shows, as expected, that FL5.12 cells underwent rapid apoptosis when deprived of IL-3, whereas FL5.12-ATFx cells displayed significant resistance to cell death. For example, 4 d after IL-3 deprivation there were no viable FL5.12 cells, whereas ∼50% of the FL5.12-ATFx cells were nonapoptotic. Figure 2B shows that 3 d following readdition of IL-3 to the culture medium, the surviving FL5.12-ATFx cells resumed normal exponential growth, consistent with these cells being nonapoptotic. In contrast, there were no detectable surviving cells when FL5.12 cells were treated analogously.
Figure 2.
ATFx suppresses apoptosis in IL-3-deprived FL5.12 cells. (A) Constitutive ATFx expression. FL5.12 cells stably expressing ATFx from the constitutive CMV promoter (FL5.12-ATFx) were monitored for cell viability following IL-3 removal in parallel with the vector-transfected control (FL5.12). Cell viability was determined using a live/dead cytotoxicity two-color assay: the number of live cells (stained with calcein AM) versus dead cells (stained with ethidium homodimer-1) was quantitatively assessed. (B) FL5.12-ATFx cells are nonapoptotic. FL5.12 and FL5.12-ATFx cells were monitored for growth in the absence of IL-3 (open symbols) and following IL-3 readdition at day 3 (filled symbols). Cell viability was determined by the live/dead assay described in A. Growth curves of FL5.12 and FL5.12-ATFx cells cultured continuously in the presence of IL-3 are also shown (shaded region). (C) An ATFx-inducible cell line. ATFx was placed under the control of a tetracycline-responsive promoter and stably transfected into FL5.12 cells. Induction of ATFx expression was assessed upon removal of the tetracycline-derivative doxycycline by RT-PCR (top) and immunoblot analysis (bottom). (D) Inducible expression of ATFx inhibits apoptosis. The ATFx-inducible cell line was cultured under conditions of IL-3 deprivation in the presence (ATFx off) or absence (ATFx on) of doxycycline. Cells were stained with annexin V to monitor cell viability, and the percentage of apoptotic cells was quantitatively assessed. (E) The anti-apoptotic activity of ATFx is selective. FL5.12 and FL5.12-ATFx cells were treated with doxorubicin, an apoptosis-inducing DNA damaging agent. Cells were stained with annexin V to monitor cell viability, and the percentage of apoptotic cells was quantitatively assessed.
To verify that ATFx expression inhibits apoptosis, we also derived an ATFx-inducible FL5.12 cell line. ATFx expression was placed under the control of a constitutively active tetracycline transactivator, which can be repressed by doxycycline (Gossen and Bujard 1992). Figure 2C shows that expression of both ATFx mRNA and protein was induced in the absence of doxycycline. Figure 2D shows, as expected, that inducible expression of ATFx also inhibited apoptosis.
Apoptosis can be induced in response to a variety of cellular insults including DNA damage (Hartwell and Kastan 1994; Bunz et al. 1998). To examine whether ATFx is a general inhibitor of apoptosis, we asked whether ATFx expression could suppress apoptosis induced by the DNA-damaging agent doxorubicin. Figure 2E shows that, in contrast to its effect in growth factor-deprived cells, ATFx did not block apoptosis induced by DNA damage. Therefore, the anti-apoptotic activity of ATFx is selective.
Specificity of ATFx anti-apoptotic activity among ATF proteins
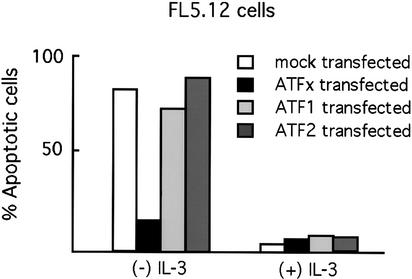
We next asked whether the anti-apoptotic activity was specific to ATFx or was, instead, a general property of ATF proteins. FL5.12 cells were transiently cotransfected with plasmids expressing ATFx, ATF1, or ATF2 together with a plasmid expressing green fluorescence protein (GFP) to mark transfected cells. Cells were cultured in the presence or absence of IL-3, and apoptosis of GFP-positive cells was quantitated. Figure 3 shows, as expected, that growth factor deprivation resulted in apoptosis that was substantially blocked by expression of ATFx. In contrast, expression of ATF2 or ATF1 had no significant effect on apoptosis. Therefore, among ATF proteins the anti-apoptotic activity of ATFx is apparently specific.
Figure 3.
Specificity of the ATFx anti-apoptotic activity among ATF proteins. FL5.12 cells were cotransfected with plasmids expressing green fluorescent protein (GFP) and full-length ATFx, ATF1, ATF2, or vector alone (mock transfected). Transfected cells were then cultured in the presence or absence of growth factor, and the percentage of cells undergoing apoptosis was quantitated after 24 h by analyzing the number of GFP-positive cells that also costained with annexin V.
ATFx is required for cell survival
ATFx is expressed in a wide variety of normal, actively proliferating cells (Pati et al. 1999). To determine whether ATFx provides an essential anti-apoptotic activity under normal conditions, we constructed and analyzed a dominant-negative ATFx mutant. Dominant-negative mutants of bZIP proteins can be derived by deletion of the transcriptional regulatory region, which is located outside of the bZIP DNA-binding domain. Such bZIP protein mutants can still dimerize with the endogenous wild-type ATF protein and bind DNA but cannot carry out their transcriptional regulatory function (Clark and Docherty 1993). Because of the specificity of bZIP dimerization (Vinson et al. 1993), these ATF mutants often selectively block the function of their wild-type counterparts (e.g., see Beier et al. 1999).
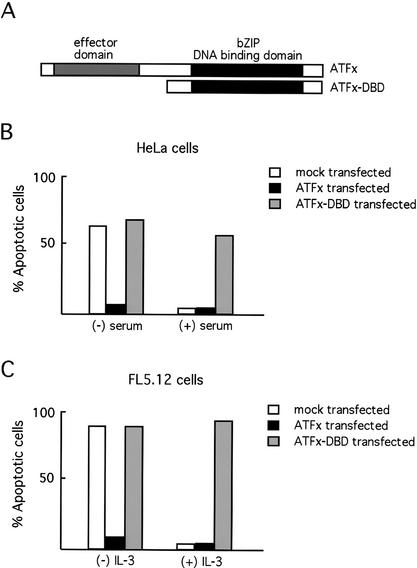
Figure 4A depicts a schematic version of the ATFx dominant-negative mutant (ATFx-DBD) used in these studies. HeLa and FL5.12 cells were transiently cotransfected with plasmids expressing either ATFx or ATFx-DBD together with a GFP expression plasmid to mark transfected cells. The cells were cultured in the presence or absence of the appropriate growth factor (serum or IL-3), and apoptosis of GFP-positive cells was quantitated. As expected, full-length ATFx but not ATFx-DBD suppressed apoptosis following growth factor deprivation in both HeLa and FL5.12 cells (Fig. 4B,C). Conversely, ATFx-DBD but not full-length ATFx induced apoptosis in HeLa and FL5.12 cells grown in the presence of growth factors (Fig. 4B,C). Based on these data as well as that presented above, we interpret these results to indicate that ATFx-DBD induces apoptosis by blocking the anti-apoptotic activity of endogenous ATFx.
Figure 4.
ATFx is required for cell survival. (A) Schematic representation of the full-length ATFx protein and the dominant-negative ATFx mutant (ATFx-DBD). (B) Expression of a dominant-negative ATFx-DBD mutant induces apoptosis in HeLa cells. HeLa cells were cotransfected with plasmids expressing green fluorescent protein (GFP) and full-length ATFx, ATFx-DBD, or vector alone (mock transfected). Transfected cells were then cultured in the presence or absence of serum, and the percentage of cells undergoing apoptosis was quantitated after 24 h by analyzing the number of GFP-positive cells that also costained with annexin V. (C) Expression of a dominant-negative ATFx-DBD mutant induces apoptosis in FL5.12 cells. FL5.12 cells were cotransfected with plasmids expressing GFP and full-length ATFx, ATFx-DBD, or vector alone, and analyzed as in B.
ATFx does not affect cellular proliferation or cell cycle progression
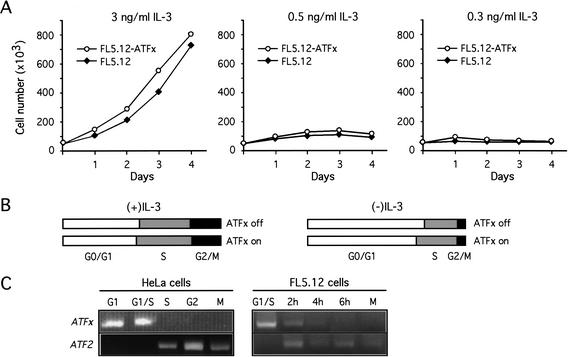
We considered the possibility that ATFx expression might exert its anti-apoptotic activity by affecting the rate of cellular proliferation. We therefore compared the growth kinetics of FL5.12 and FL5.12-ATFx cells. Because the anti-apoptotic activity of ATFx occurs following growth factor deprivation, we also performed the analysis at limiting concentrations of IL-3. Figure 5A shows that the growth curves were similar for FL5.12 and FL5.12-ATFx cells at all concentrations of IL-3 tested. We conclude that ATFx expression does not have a significant effect on the rate of cellular proliferation.
Figure 5.
ATFx does not affect cellular proliferation or cell cycle progression. (A) Cellular proliferation. FL5.12 (closed symbols) and FL5.12-ATFx cells (open symbols) were cultured at the specified concentration of IL-3, and cell growth was monitored at the indicated time points. (B) Cell cycle progression. The ATFx-inducible cell line was cultured in the presence (ATFx off) or absence (ATFx on) of doxycycline. The percentage of cells in each phase of the cell cycle in an asynchronous population were quantitated by flow activated cell sorting (FACS) analysis, and the relative numbers of cells in G0/G1, S, and G2/M phases are shown schematically. (C) ATFx expression is cell-cycle-regulated. HeLa and FL5.12 cells were arrested at G1/S by a double thymidine block or at M phase by nocodazole treatment. ATFx and ATF2 expression was monitored by RT-PCR analysis at the indicated time points following release from the G1/S arrest.
To ask whether ATFx expression might have a more subtle effect on cell growth, we used DNA flow cytometry to compare the cell cycle in asynchronous populations of FL5.12 cells either expressing or not expressing ATFx. Figure 5B shows that at all concentrations of IL-3 tested the number of cells in G1, S, and G2/M was comparable in the presence or absence of overexpressed ATFx, indicating that ATFx does not significantly affect cell cycle progression. Collectively these data show that ATFx overexpression does not directly affect cellular proliferation or progression through the cell cycle. In conjunction with the other data presented in this study, we conclude that the activity of ATFx is purely anti-apoptotic.
To test whether ATFx expression is itself cell-cycle-regulated, we synchronized cells in G1/S and monitored ATFx mRNA levels following release from the arrest. Figure 5C shows that ATFx expression was cell-cycle-regulated in both HeLa and FL5.12 cells, with expression peaking in G1/S and falling to undetectable levels in G2/M. Even following a second round of RT-PCR amplification we failed to detect ATFx transcripts in G2/M cells (data not shown). For comparison, the expression profile of ATF2 was analyzed in parallel. In contrast to the pattern of ATFx expression, ATF2 mRNA was detectable only in S and G2/M. Thus, the G1/S-specific expression pattern of ATFx is not a general characteristic of ATF genes.
Repression of ATFx expression by 24p3
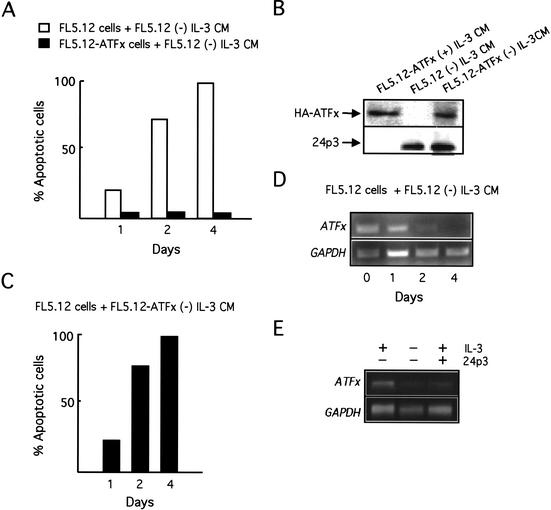
We have recently shown that IL-3 deprivation results in expression and secretion of 24p3, a lipocalin that interacts with a cell surface receptor and induces apoptosis through an autocrine/paracrine pathway (Devireddy et al. 2001). However, IL-3 deprivation does not induce apoptosis in cells constitutively expressing ATFx, which suggests that ectopic expression of ATFx renders the cells resistant to 24p3-mediated apoptosis. To confirm this prediction, we tested the ability of ATFx to suppress apoptosis following 24p3 addition. Conditioned medium (CM) from IL-3-deprived FL5.12 cells contains 24p3 and induces apoptosis upon addition to naive FL5.12 cells (Devireddy et al. 2001). In contrast, FL5.12-ATFx cells, which constitutively express ATFx, were resistant to 24p3-mediated apoptosis (Fig. 6A). Thus, ectopic expression of ATFx can overcome the proapoptotic activity of 24p3.
Figure 6.
Functional relationships between ATFx and 24p3. (A) Constitutive ATFx expression blocks the ability of 24p3 to induce apoptosis. FL5.12 or FL5.12-ATFx cells were incubated with conditioned medium (CM) from FL5.12 cells grown in the absence of IL-3, which contains the apoptosis-inducing factor 24p3. Cell viability was monitored by annexin V staining at the indicated time points following CM addition. (B) ATFx does not interfere with expression or secretion of 24p3. FL5.12 and FL5.12-ATFx cells were cultured in the presence or absence of IL-3, as indicated. The presence of HA-ATFx and 24p3 in the CM was analyzed by immunoblot analysis. (C) ATFx does not block 24p3 secretion. Naive FL5.12 cells were incubated with CM from IL-3-deprived FL5.12-ATFx cells, and cell viability was monitored by annexin V staining at the indicated time points following addition of CM. (D) 24p3 represses ATFx expression. FL5.12 cells were incubated with CM from IL-3-deprived FL5.12 cells, and ATFx and GAPDH expression was monitored by RT-PCR analysis at the indicated time points. (E) FL5.12 cells were incubated in the presence of recombinant 24p3 and IL-3, and ATFx and GAPDH expression was monitored by Northern blot analysis. For comparison, the level of ATFx in the presence and absence of IL-3 is shown as a control.
One possible explanation for the ability of ATFx to suppress apoptosis in IL-3-deprived FL5.12 cells is by affecting the expression or secretion of 24p3. Figure 6B shows that CM from IL-3-deprived FL5.12-ATFx cells contained 24p3 at levels comparable to IL-3-deprived FL5.12 cells. Furthermore, CM from IL-3-deprived FL5.12-ATFx cells induced apoptosis upon addition to FL5.12 cells (Fig. 6C). Thus, constitutive ATFx expression did not block expression or secretion of 24p3.
In the light of these new results, our previous finding that 24p3 induces apoptosis of IL-3-dependent FL5.12 cells even in the presence of cytokine (Devireddy et al. 2001) presents an apparent paradox: in the presence of IL-3, ATFx should be expressed and, based on the results of Figure 6A, cells expressing ATFx should be resistant to 24p3-mediated apoptosis. To resolve this discrepancy we analyzed whether 24p3 affected expression of the endogenous ATFx gene. Figure 6D shows that addition of CM from cytokine-deprived FL5.12 cells to naive FL5.12 cells repressed expression of endogenous ATFx. To confirm that the CM component that repressed ATFx expression was, indeed, 24p3, we performed a separate experiment that monitored the effects of adding 24p3 alone. Figure 6E shows that addition of purified recombinant 24p3 to FL5.12 cells also repressed transcription of ATFx even when IL-3 was present. Thus, at least part of the mechanism by which 24p3 induces apoptosis is to repress transcription of the anti-apoptotic factor ATFx. Ectopic expression of ATFx bypasses this regulation, thereby resulting in cell survival.
Discussion
We have previously shown that ATFx, a member of the ATF/CREB family of transcriptional regulators, is repressed in IL-3-dependent FL5.12 cells following cytokine deprivation, suggesting a role for ATFx in cell survival (Devireddy et al. 2001). In this report, we present several lines of evidence indicating that ATFx has an anti-apoptotic function. First, ATFx expression was inhibited after induction of apoptosis by growth factor deprivation in multiple cell lines and primary cells. Second, ectopic expression of ATFx in an IL-3-dependent cell line suppressed apoptosis following cytokine deprivation. Finally, expression of a dominant-negative ATFx mutant induced apoptosis under normal conditions, implying that ATFx provides an essential anti-apoptotic activity. The role of ATFx in cell survival appears to be purely anti-apoptotic, as overexpression of the gene did not affect the rate of cell growth or cell cycle progression.
ATFx is a member of a large family of transcription factors originally identified more than a decade ago (Hai et al. 1989). Since then, additional ATFs that mediate diverse cellular activities have been reported. For example, CREB and ATF1 are involved in mediating transcription in response to intracellular cAMP concentrations (De Cesare and Sassone-Corsi 2000). ATF2 (also known as CRE-BP1) and ATF3 have been implicated in transcriptional control of stress-response genes (Hai et al. 1999; Zoumpourlis et al. 2000). ATF6 is involved in regulating genes involved in the ER stress response and serum response (Hai and Hartman 2001). Thus, despite their diverse activities, one common theme is a role for ATF/CREB proteins in intracellular signaling. To our knowledge, ATFx is the only member of the ATF/CREB family shown to have a role in an apoptosis/survival pathway. Although anti-apoptosis represents a new activity for an ATF/CREB protein, there are many precedents for the idea that a transcription factor can participate in an apoptosis/survival pathway. For example, p53, c-myc, and E2F are well-characterized transcription factors that are known to mediate either apoptosis or survival depending on cellular context (Bunz et al. 1998; Juin et al. 1999; Zhang et al. 1999; Muller et al. 2001).
Because ATFx is a member of the ATF/CREB family of transcription factors, it almost certainly functions by modulating the expression of target genes. Specifically, ATFx is a member of the ATF4 subgroup of the ATF/CREB family; ATF4 itself has both activator and repressor activities (Hai and Hartman 2001). Whether ATFx similarly acts like an activator and/or repressor remains to be determined. Candidate genes that may be activated include those that promote cell survival or inhibit apoptosis. Alternatively, ATFx could down-regulate genes encoding proapoptotic proteins. Clearly, an understanding of the molecular basis by which ATFx promotes anti-apoptosis will require identifying its target genes.
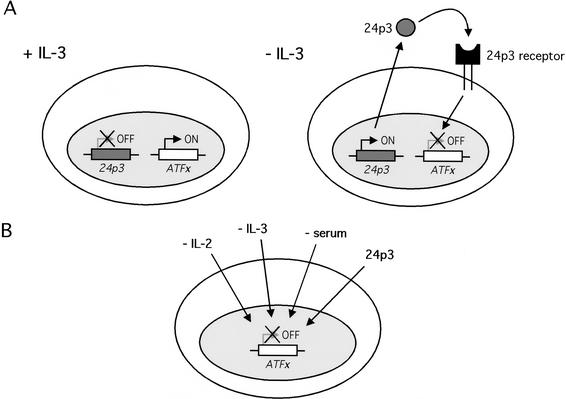
Based on the findings presented in this report and our previous studies, IL-3 deprivation has at least two effects on gene expression: induction of the proapoptotic factor 24p3 and repression of the anti-apoptotic factor ATFx. The results presented here reveal important functional interrelationships between these two proteins (Fig. 7A). Most importantly, addition of 24p3 to FL5.12 cells repressed ATFx expression, indicating that the ATFx gene is a downstream target of the signal transduction cascade initiated by 24p3. The fact that ATFx expression counteracts the proapoptotic effect of 24p3 further suggests that ATFx might be the major (and perhaps sole) gene target of 24p3. Thus, our results indicate that at least part of the mechanism by which 24p3 induces apoptosis is by repressing expression of the anti-apoptotic factor ATFx.
Figure 7.
Role of ATFx in transcriptional regulation of apoptosis. (A) Model for the transcriptional regulation of apoptosis in IL-3-deprived cells. IL-3 deprivation induces expression and secretion of 24p3. The secreted lipocalin then binds to its cell surface receptor, leading to repression of ATFx through an as-of-yet uncharacterized pathway. (B) Transcriptional regulation of ATFx by diverse signals. In addition to the 24p3-mediated pathway, ATFx expression can be down-regulated in appropriate cell types by deprivation of growth factors such as IL-2, IL-3, and serum.
It is clear, however, that the 24p3 pathway is not the only one by which ATFx expression is down-regulated (summarized in Fig. 7B). For example, it is likely that IL-3 deprivation down-regulates ATFx expression through a pathway independent of 24p3: ATFx expression is repressed significantly more rapidly by IL-3 deprivation than by 24p3 addition (cf. Figs. 1C and 6D). Moreover, ATFx expression is down-regulated in appropriate cell types by deprivation of other cytokines, such as IL-2 (Fig. 1A), or serum (Fig. 1B), conditions under which 24p3 is not expressed (Devireddy et al. 2001). Elucidating the various pathways by which ATFx expression is regulated should lead to a better understanding of how apoptosis can be controlled at the transcriptional level.
Materials and methods
ATFx cloning and plasmid construction
The murine ATFx cDNA containing the entire protein-coding region was cloned by reverse transcription-polymerase chain reaction (RT-PCR) using RNA isolated from FL5.12 cells. ATFx expression plasmids were constructed by subcloning the full-length ATFx cDNA containing a hemagglutinin (HA) tag at the N terminus into the vectors pcDNA3.1 (Invitrogen) and pUHD10-3 (Clonetech) to generate CMV–HA-ATFx and TA–HA-ATFx, which expressed ATFx from the constitutive CMV promoter and the tetracycline-inducible promoter, respectively. HA-ATFx was subcloned using the primers 5′-GGGGAT GAATTCCACCATGGAGTATCCATATGATGTTCCAGATT ATGCTATGGCATCCCTACTCAAGAAGGAG-3′ and 5′-GC AGCTACGGGTCCTCTGGCT-3′ directed toward the 5′ and 3′ ends of the gene, respectively. The CMV–HA-ATFx-DBD vector was generated by subcloning the ATFx DNA-binding domain into pcDNA3.1 using the primers 5′-GGGGATGAATTC CACCATGGAGTATCCATATGATGTTCCAGATTATGCT ACCACCCGAGGGGACCGCAAG-3′ and 5′-GCAGCTACGG GTCCTCTGGCT-3′ directed toward the 5′ and 3′ ends of the gene, respectively. Details of vector construction are available from the authors upon request.
Cell lines and transfections
IL-2-dependent murine HT-2 cells, HeLa cells, primary murine bone marrow cells, and human fibroblasts were all obtained from American Type Culture Collection. The FL5.12 cell line has been previously described (Boise et al. 1993). HT-2 cells were maintained in RPMI 1640 medium (GIBCO BRL) supplemented with 10% fetal bovine serum (FBS) and 10 U/mL recombinant IL-2 (Pharmingen); FL5.12 cells were maintained in RPMI medium supplemented with 10% FBS and 3 ng/mL recombinant IL-3. HeLa cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS; mouse bone marrow cells were maintained in DMEM supplemented with 15% FBS, 10 ng/mL human IL-6, 6 ng/mL murine IL-3, and 100 ng/mL murine steel factor; human fibroblasts were maintained in DMEM supplemented with 10% FBS.
Transient and stable transfections were performed using FuGENE 6 (Roche Applied Science) according to the manufacturer's instructions. FL5.12 cells were stably transfected with the CMV–HA-ATFx construct to generate FL5.12-ATFx cells. The ATFx-inducible cell line was generated by stably transfecting FL5.12 cells with the TA–HA-ATFx construct; the method for generating doxycycline-inducible cell lines has been previously described (Gossen and Bujard 1992). ATFx expression was repressed by the addition of 1 μg/mL doxycycline (Sigma).
RNA and protein analysis
RT-PCR and Northern analysis were performed according to standard protocols (Ausubel et al. 2001), except that total RNA was prepared by the RNeasy extraction kit (QIAGEN). Sequences for ATFx, ATF2, and GAPDH primer sets used for RT-PCR analysis are as follows: ATFx, 5′-ATGGCATCCCTACT CAAGAAGGAG-3′ and 5′-AGCGACTTATTCTGGTCTCTC TTC-3′; ATF2, 5′-CCCGTGGCCTTTAAAGTGCGATTCA-3′ and 5′-GCGACAGTATCACCATTGGTAAC; GAPDH, 5′-AC CACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGT TGCTGTA-3′ (all directed toward the 5′ and 3′ ends of the gene, respectively). Immunoblot assays were performed using rabbit polyclonal antibodies that specifically recognized either 24p3 (Chu et al. 1996) or the HA tag (Santa Cruz Biotechnology). Recombinant 24p3 was synthesized as previously described (Devireddy et al. 2001).
Apoptotic and survival assays
Apoptosis was induced by either growth factor withdrawal or addition of 1 μg/mL doxorubicin (Sigma). After 24 h, cells were stained with either annexin V (Oncogene Research Products) or a combination of calcein AM and ethidium homodimer-1 dyes (Molecular Probes) according to the manufacturers' instructions. For cotransfection experiments, cells were transfected with a plasmid expressing green fluorescent protein (GFP; Clonetech) in combination with the ATFx and ATFx-DBD expression vectors. All experiments were performed at least three times with comparable results.
Cell cycle analysis
HeLa and FL5.12 cells were synchronized with either a double-thymidine block or nocodazole as described (Darzynkiewicz 1993), which arrest cells in the G1/S and G2/M phases, respectively. Cells were collected at various time points following release from cell cycle arrest and fixed with ethanol; cellular DNA was stained with propidium iodide. DNA content and cell number were analyzed by FACS analysis, and cell cycle profiles were analyzed using the ModFit program (Verify Software).
Acknowledgments
We thank S. Evans for editorial assistance. This work was supported by a Leukemia and Lymphoma Society grant to L.R.D. and a grant from the National Institutes of Health to M.R.G. M.R.G. is an investigator of the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL michael.green@umassmed.edu; FAX (508) 856-5473.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.992202.
References
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. New York: John Wiley & Sons; 2001. [Google Scholar]
- Beier F, Lee RJ, Taylor AC, Pestell RG, LuValle P. Identification of the cyclin D1 gene as a target of activating transcription factor 2 in chondrocytes. Proc Natl Acad Sci. 1999;96:1433–1438. doi: 10.1073/pnas.96.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nunez G, Thompson CB. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- Chu ST, Huang HL, Chen JM, Chen YH. Demonstration of a glycoprotein derived from the 24p3 gene in mouse uterine luminal fluid. Biochem J. 1996;316:545–550. doi: 10.1042/bj3160545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AR, Docherty K. Negative regulation of transcription in eukaryotes. Biochem J. 1993;296:521–541. doi: 10.1042/bj2960521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MK, Perkins GR, Rodriguez-Tarduchy G, Nieto MA, Lopez-Rivas A. Growth factors as survival factors: Regulation of apoptosis. Bioessays. 1994;16:133–138. doi: 10.1002/bies.950160210. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z. Mammalian cell-cycle analysis. In: Fantes P, Brooks R, editors. The cell cycle. A practical approach. New York: Oxford University Press; 1993. pp. 45–68. [Google Scholar]
- De Cesare D, Sassone-Corsi P. Transcriptional regulation by cyclic AMP-responsive factors. Prog Nucleic Acid Res Mol Biol. 2000;64:343–369. doi: 10.1016/s0079-6603(00)64009-6. [DOI] [PubMed] [Google Scholar]
- Devireddy LR, Teodoro JG, Richard FA, Green MR. Induction of apoptosis by a secreted lipocalin that is transcriptionally regulated by IL-3 deprivation. Science. 2001;293:829–834. doi: 10.1126/science.1061075. [DOI] [PubMed] [Google Scholar]
- Georgopoulos K. Transcription factors required for lymphoid lineage commitment. Curr Opin Immunol. 1997;9:222–227. doi: 10.1016/s0952-7915(97)80139-2. [DOI] [PubMed] [Google Scholar]
- Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai T, Hartman MG. The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors: Activating transcription factor proteins and homeostasis. Gene. 2001;273:1–11. doi: 10.1016/s0378-1119(01)00551-0. [DOI] [PubMed] [Google Scholar]
- Hai TW, Liu F, Coukos WJ, Green MR. Transcription factor ATF cDNA clones: An extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes & Dev. 1989;3:2083–2090. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- Hai T, Wolgang CD, Marsee DK, Allen AE, Sivaprasad U. ATF3 and stress responses. Gene Expr. 1999;7:321–335. [PMC free article] [PubMed] [Google Scholar]
- Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266:1821–1827. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juin P, Hueber AO, Littlewood T, Evan G. c-Myc-induced sensitization to apoptosis is mediated through cytochrome c release. Genes & Dev. 1999;13:1367–1381. doi: 10.1101/gad.13.11.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CT, Leiden JM. Transcriptional regulation of T lymphocyte development and function. Annu Rev Immunol. 1999;17:140–187. doi: 10.1146/annurev.immunol.17.1.149. [DOI] [PubMed] [Google Scholar]
- Lee KA, Hai TY, SivaRaman L, Thimmappaya B, Hurst HC, Jones NC, Green MR. A cellular protein, activating transcription factor, activates transcription of multiple E1A-inducible adenovirus early promoters. Proc Natl Acad Sci. 1987;84:8355–8359. doi: 10.1073/pnas.84.23.8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey J, Holland G, McKearn J, Risser R. Abrogation of factor-dependence in two IL-3-dependent cell lines can occur by two distinct mechanisms. Oncogene Res. 1989;4:97–109. [PubMed] [Google Scholar]
- Muller H, Bracken AP, Vernell R, Moroni MC, Christians F, Grassilli E, Prosperini E, Vigo E, Oliner JD, Helin K. E2Fs regulate the expression of genes involved in differentiation, development, proliferation, and apoptosis. Genes & Dev. 2001;15:267–285. doi: 10.1101/gad.864201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pati D, Meistrich ML, Plon SE. Human Cdc34 and Rad6B ubiquitin-conjugated enzymes target repressors of cyclic AMP-induced transcription for proteolysis. Mol Cell Biol. 1999;19:5001–5013. doi: 10.1128/mcb.19.7.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters CS, Liang X, Li S, Kannan S, Peng Y, Taub R, Diamond RH. ATF-7, a novel bZIP protein, interacts with the PRL-1 protein-tyrosine phosphatase. J Biol Chem. 2001;276:13718–13726. doi: 10.1074/jbc.M011562200. [DOI] [PubMed] [Google Scholar]
- Ross ME. Cell division and the nervous system: Regulating the cycle from neuronal differentiation to death. Trends Neurosci. 1996;19:62–68. doi: 10.1016/0166-2236(96)89622-6. [DOI] [PubMed] [Google Scholar]
- Vaux DL, Korsmeyer SJ. Cell death in development. Cell. 1999;96:245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Vinson CR, Hai T, Boyd SM. Dimerization specificity of the leucine zipper-containing bZIP motif on DNA binding: Prediction and rational design. Genes & Dev. 1993;7:1047–1058. doi: 10.1101/gad.7.6.1047. [DOI] [PubMed] [Google Scholar]
- Williams GT. Programmed cell death: Apoptosis and oncogenesis. Cell. 1991;65:1097–1098. doi: 10.1016/0092-8674(91)90002-g. [DOI] [PubMed] [Google Scholar]
- Zhang HS, Postigo AA, Dean DC. Active transcriptional repression by the Rb-E2F complex mediates G1 arrest triggered by p16INK4a, TGFβ, and contact inhibition. Cell. 1999;97:53–61. doi: 10.1016/s0092-8674(00)80714-x. [DOI] [PubMed] [Google Scholar]
- Zoumpourlis V, Papassava P, Linardopoulos S, Gillespie D, Balmain A, Pintzas A. High levels of phosphorylated c-Jun, Fra-1, Fra-2 and ATF-2 proteins correlate with malignant phenotypes in the multistage mouse skin carcinogenesis model. Oncogene. 2000;19:4011–4021. doi: 10.1038/sj.onc.1203732. [DOI] [PubMed] [Google Scholar]