Abstract
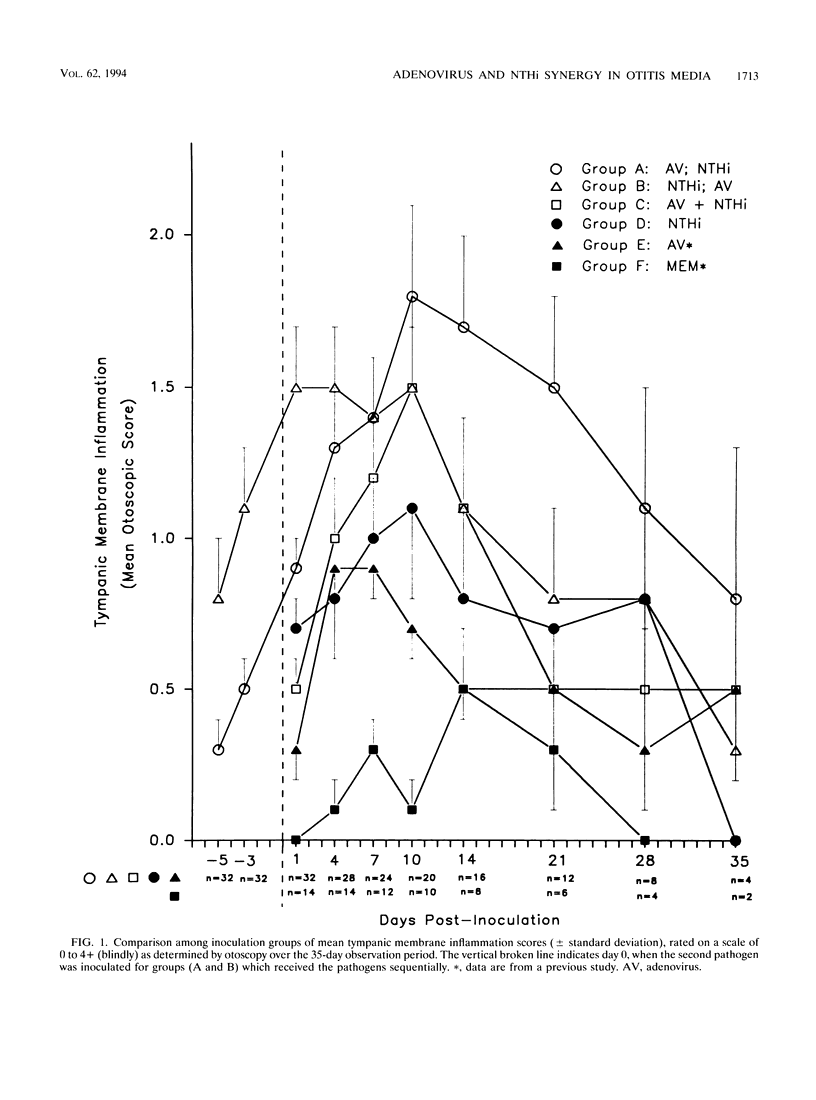
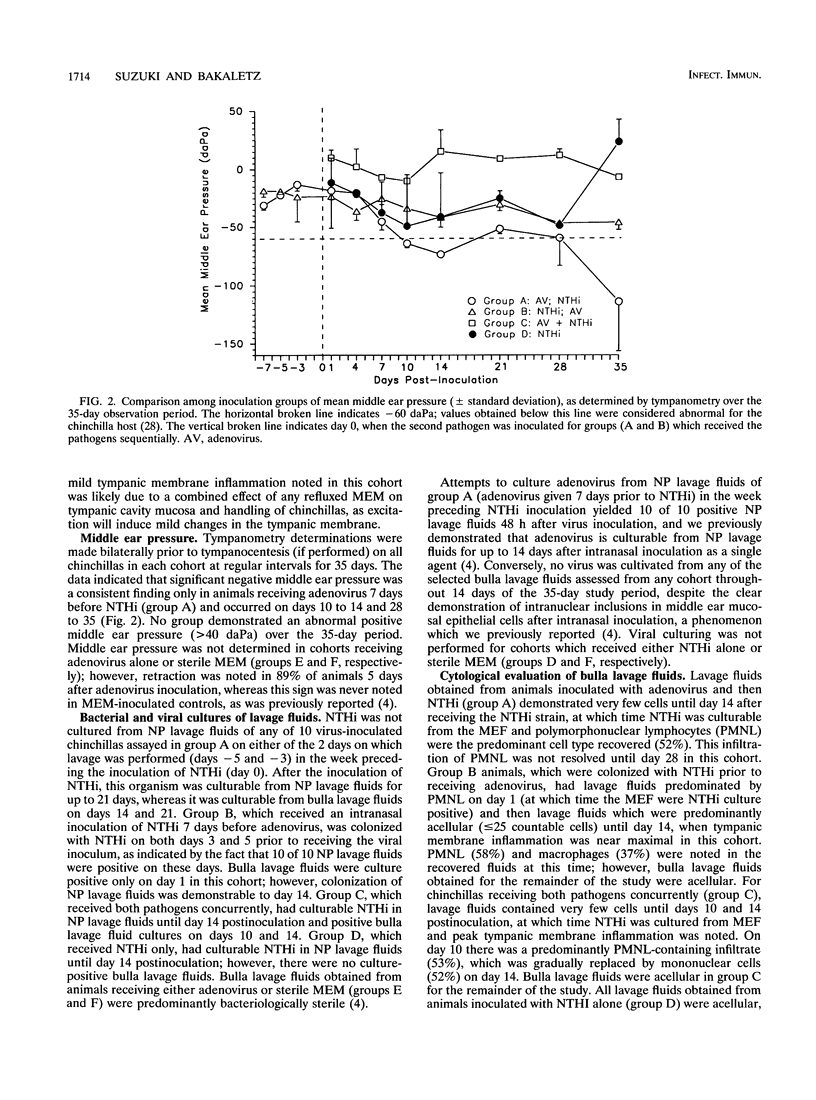
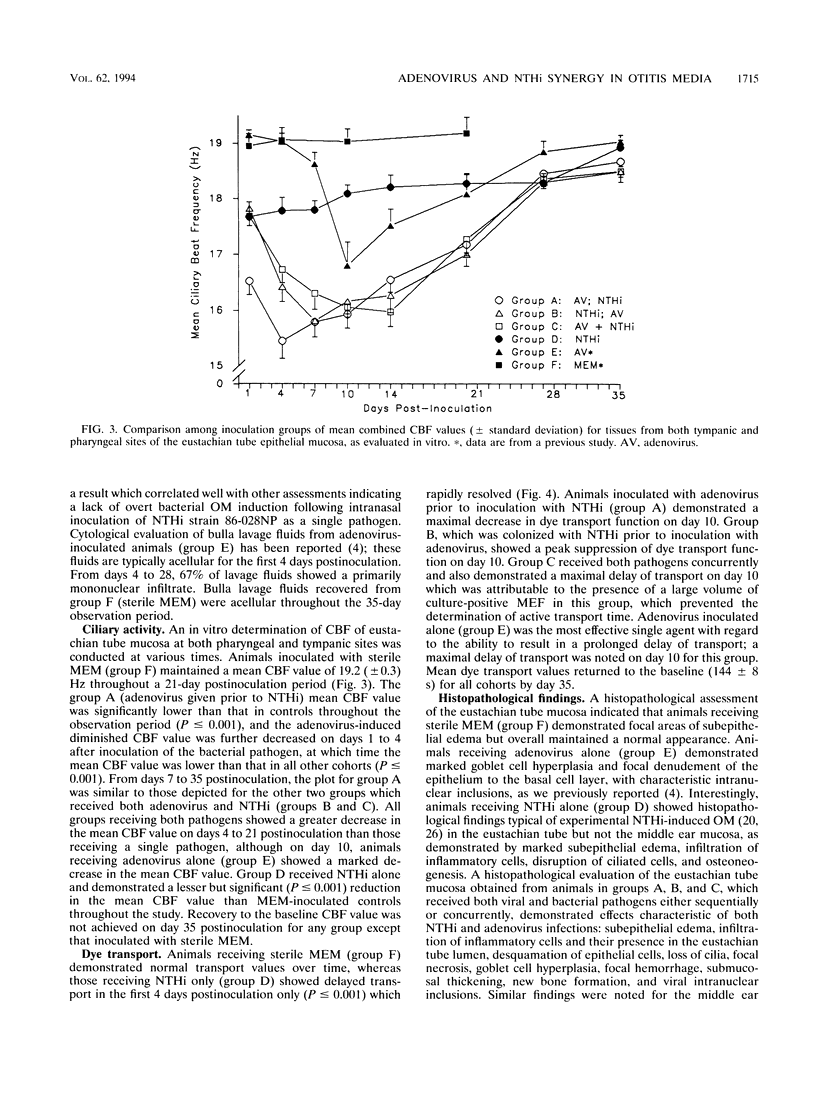
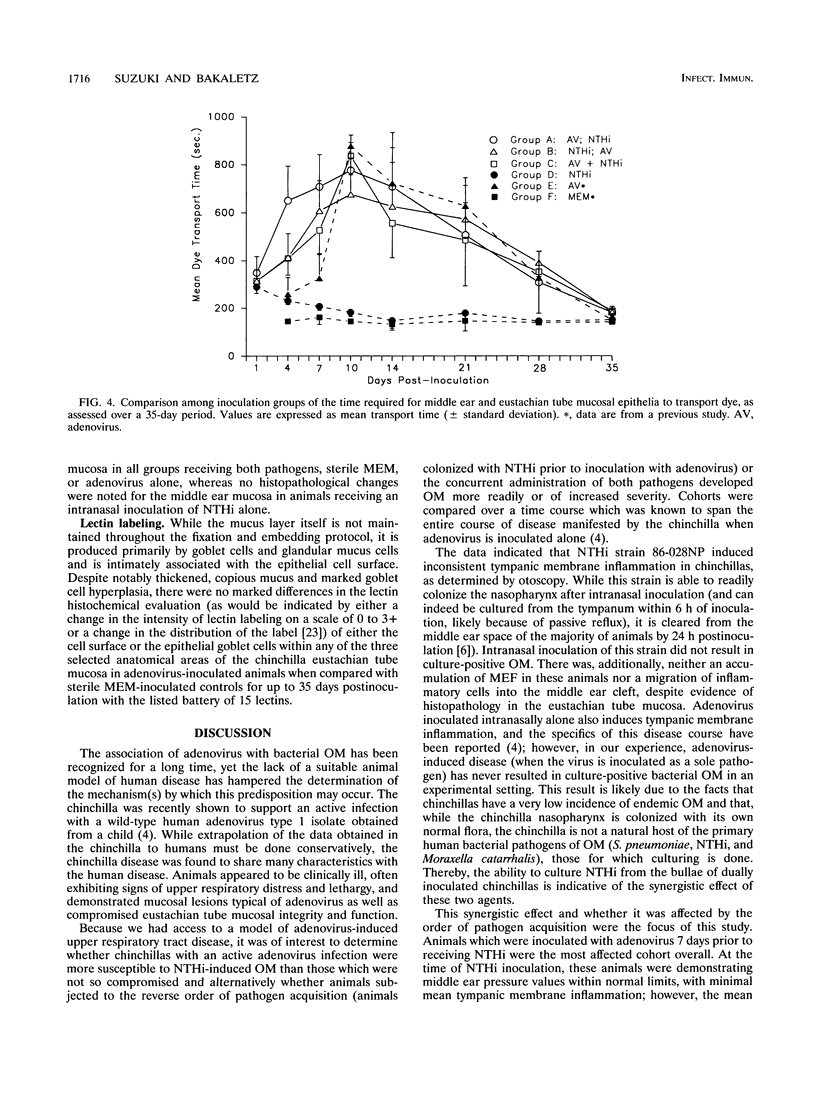
We recently reported the development of a chinchilla model of experimental otitis media (OM) that uses a pediatric clinical isolate of adenovirus type 1 (4) and in which an active infection with the wild-type strain was demonstrated. To expand upon these findings, this study was designed to determine whether we could demonstrate adenovirus infection-induced predisposition to bacterial OM in the chinchilla, as has been shown in human epidemiological studies (D. A. Clements, F. W. Henderson, and E. C. Neebe, p. 27-29, in D. J. Lim, C. D. Bluestone, J. O. Klein, D. J. Nelson, and P. L. Ogra, ed., Proceedings of the Fifth International Symposium on Recent Advances in Otitis Media, 1993; F. W. Henderson, A. M. Collier, M. A. Sanyai, et al., N. Engl. J. Med. 306:1377-1383, 1982). In addition, we were interested in determining whether altering the order of pathogen acquisition would further affect the outcome of disease incidence and severity. Toward this end, cohorts of chinchillas were inoculated intranasally with a strain of nontypeable Haemophilus influenzae (NTHi) (86-028NP) which colonizes the chinchilla nasopharynx but does not consistently induce culture-positive OM when inoculated intranasally (L. O. Bakaletz, T. M. Hoepf, D. J. Lim, and B. Tallan, Abstr. 90th Annu. Meet. Am. Soc. Microbiol. 1990, abstr. B-66, p. 37, 1990), adenovirus type 1 and then inoculated 7 days later with NTHi, NTHi and then inoculated 7 days later with adenovirus type 1, or both pathogens concurrently. All cohorts were observed over a 35-day period and assessed for incidence and severity of OM by several methodologies. The data collectively indicated that all animals receiving both pathogens developed OM of greater severity than those receiving only a single agent. Adenovirus inoculation followed 7 days later by NTHi inoculation was the order of pathogen acquisition which induced the most prolonged presence of NTHi in both the nasopharynx and the middle ear, the most severe tympanic membrane inflammation overall, and the most significant damage to and altered function of both middle ear and eustachian tube mucosae.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson J. S., Giebink G. S., Quie P. G. Influenza A virus-induced polymorphonuclear leukocyte dysfunction in the pathogenesis of experimental pneumococcal otitis media. Infect Immun. 1982 Apr;36(1):289–296. doi: 10.1128/iai.36.1.289-296.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arola M., Ruuskanen O., Ziegler T., Mertsola J., Näntö-Salonen K., Putto-Laurila A., Viljanen M. K., Halonen P. Clinical role of respiratory virus infection in acute otitis media. Pediatrics. 1990 Dec;86(6):848–855. [PubMed] [Google Scholar]
- Bakaletz L. O., Daniels R. L., Lim D. J. Modeling adenovirus type 1-induced otitis media in the chinchilla: effect on ciliary activity and fluid transport function of eustachian tube mucosal epithelium. J Infect Dis. 1993 Oct;168(4):865–872. doi: 10.1093/infdis/168.4.865. [DOI] [PubMed] [Google Scholar]
- Bakaletz L. O., Griffith S. R., Lim D. J. Effect of prostaglandin E2 and bacterial endotoxin on the rate of dye transport in the eustachian tube of the chinchilla. Ann Otol Rhinol Laryngol. 1989 Apr;98(4 Pt 1):278–282. doi: 10.1177/000348948909800408. [DOI] [PubMed] [Google Scholar]
- Chonmaitree T., Howie V. M., Truant A. L. Presence of respiratory viruses in middle ear fluids and nasal wash specimens from children with acute otitis media. Pediatrics. 1986 May;77(5):698–702. [PubMed] [Google Scholar]
- Chung M. H., Griffith S. R., Park K. H., Lim D. J., DeMaria T. F. Cytological and histological changes in the middle ear after inoculation of influenza A virus. Acta Otolaryngol. 1993 Jan;113(1):81–87. doi: 10.3109/00016489309135771. [DOI] [PubMed] [Google Scholar]
- Doyle W. J. Eustachian tube function in the chinchilla. Arch Otolaryngol. 1985 May;111(5):305–308. doi: 10.1001/archotol.1985.00800070057007. [DOI] [PubMed] [Google Scholar]
- Giebink G. S., Berzins I. K., Marker S. C., Schiffman G. Experimental otitis media after nasal inoculation of Streptococcus pneumoniae and influenza A virus in chinchillas. Infect Immun. 1980 Nov;30(2):445–450. doi: 10.1128/iai.30.2.445-450.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebink G. S., Berzins I. K., Schiffman G., Quie P. G. Experimental otitis media in chinchillas following nasal colonization with type 7F Streptococcus pneumoniae: prevention after vaccination with pneumococcal capsular polysaccharide. J Infect Dis. 1979 Nov;140(5):716–723. doi: 10.1093/infdis/140.5.716. [DOI] [PubMed] [Google Scholar]
- Giebink G. S., Heller K. A., Harford E. R. Tympanometric configurations and middle ear findings in experimental otitis media. Ann Otol Rhinol Laryngol. 1982 Jan-Feb;91(1 Pt 1):20–24. doi: 10.1177/000348948209100106. [DOI] [PubMed] [Google Scholar]
- Giebink G. S., Ripley M. L., Wright P. F. Eustachian tube histopathology during experimental influenza A virus infection in the chinchilla. Ann Otol Rhinol Laryngol. 1987 Mar-Apr;96(2 Pt 1):199–206. doi: 10.1177/000348948709600212. [DOI] [PubMed] [Google Scholar]
- Giebink G. S., Wright P. F. Different virulence of influenza A virus strains and susceptibility to pneumococcal otitis media in chinchillas. Infect Immun. 1983 Sep;41(3):913–920. doi: 10.1128/iai.41.3.913-920.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson F. W., Collier A. M., Sanyal M. A., Watkins J. M., Fairclough D. L., Clyde W. A., Jr, Denny F. W. A longitudinal study of respiratory viruses and bacteria in the etiology of acute otitis media with effusion. N Engl J Med. 1982 Jun 10;306(23):1377–1383. doi: 10.1056/NEJM198206103062301. [DOI] [PubMed] [Google Scholar]
- Klein J. O., Teele D. W. Isolation of viruses and mycoplasmas from middle ear effusions: a review. Ann Otol Rhinol Laryngol. 1976 Mar-Apr;85(2 Suppl 25 Pt 2):140–144. doi: 10.1177/00034894760850S226. [DOI] [PubMed] [Google Scholar]
- Korppi M., Katila M. L., Jäskeläinen J., Leinonen M. Role of non-capsulated Haemophilus influenzae as a respiratory pathogen in children. Acta Paediatr. 1992 Dec;81(12):989–992. doi: 10.1111/j.1651-2227.1992.tb12160.x. [DOI] [PubMed] [Google Scholar]
- Koziol J. A., Maxwell D. A., Fukushima M., Colmerauer M. E., Pilch Y. H. A distribution-free test for tumor-growth curve analyses with application to an animal tumor immunotherapy experiment. Biometrics. 1981 Jun;37(2):383–390. [PubMed] [Google Scholar]
- Lewis D. M., Schram J. L., Meadema S. J., Lim D. J. Experimental otitis media in chinchillas. Ann Otol Rhinol Laryngol Suppl. 1980 May-Jun;89(3 Pt 2):344–350. doi: 10.1177/00034894800890s381. [DOI] [PubMed] [Google Scholar]
- Lim D. J., Lewis D. M., Schram J. L., Birck H. G. Otitis media with effusion. Cytological and microbiological correlates. Arch Otolaryngol. 1979 Jul;105(7):404–412. doi: 10.1001/archotol.1979.00790190030006. [DOI] [PubMed] [Google Scholar]
- Linder T. E., Lim D. J., DeMaria T. F. Changes in the structure of the cell surface carbohydrates of the chinchilla tubotympanum following Streptococcus pneumoniae-induced otitis media. Microb Pathog. 1992 Oct;13(4):293–303. doi: 10.1016/0882-4010(92)90039-q. [DOI] [PubMed] [Google Scholar]
- Murphy T. F., Bernstein J. M., Dryja D. M., Campagnari A. A., Apicella M. A. Outer membrane protein and lipooligosaccharide analysis of paired nasopharyngeal and middle ear isolates in otitis media due to nontypable Haemophilus influenzae: pathogenetic and epidemiological observations. J Infect Dis. 1987 Nov;156(5):723–731. doi: 10.1093/infdis/156.5.723. [DOI] [PubMed] [Google Scholar]
- Okazaki N., DeMaria T. F., Briggs B. R., Lim D. J. Experimental otitis media with effusion induced by nonviable Hemophilus influenzae: cytologic and histologic study. Am J Otolaryngol. 1984 Mar-Apr;5(2):80–92. doi: 10.1016/s0196-0709(84)80026-5. [DOI] [PubMed] [Google Scholar]
- Park K., Bakaletz L. O., Coticchia J. M., Lim D. J. Effect of influenza A virus on ciliary activity and dye transport function in the chinchilla eustachian tube. Ann Otol Rhinol Laryngol. 1993 Jul;102(7):551–558. doi: 10.1177/000348949310200711. [DOI] [PubMed] [Google Scholar]
- Rosenfeld R. M., Doyle W. J., Swarts J. D., Seroky J., Pinero B. P. Third-generation cephalosporins in the treatment of acute pneumococcal otitis media. An animal study. Arch Otolaryngol Head Neck Surg. 1992 Jan;118(1):49–52. doi: 10.1001/archotol.1992.01880010053015. [DOI] [PubMed] [Google Scholar]


