Abstract
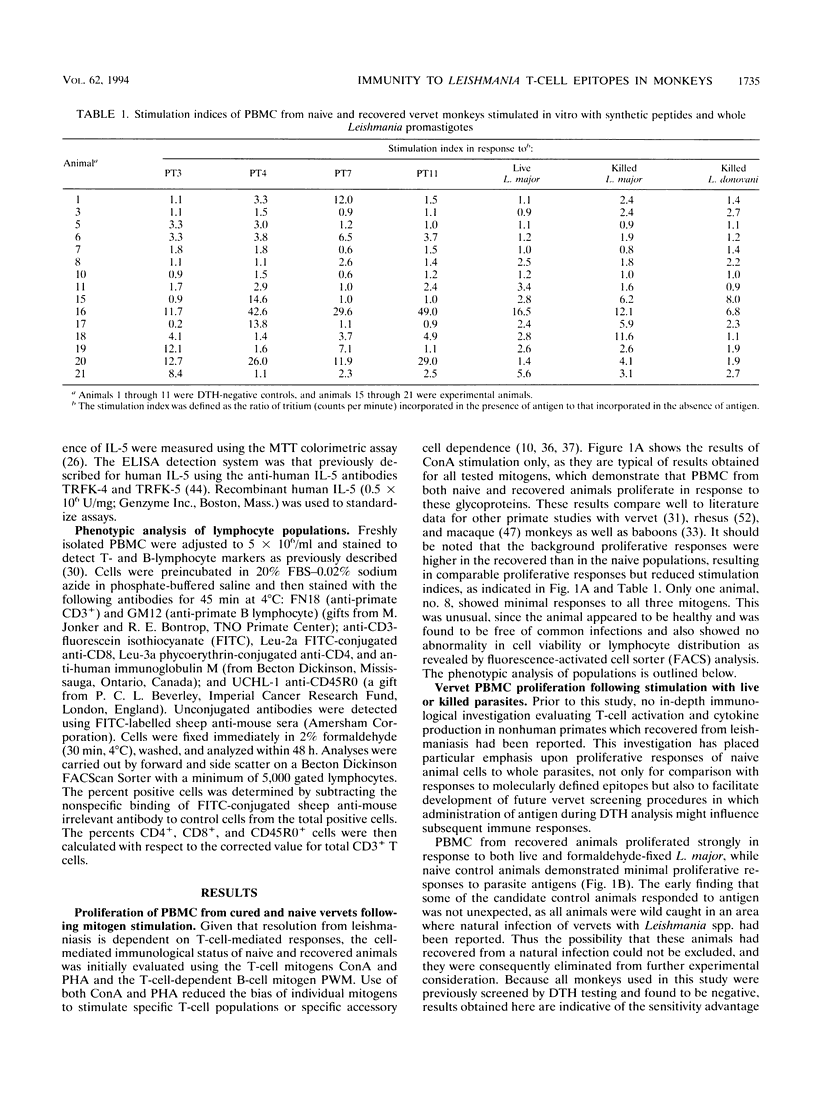
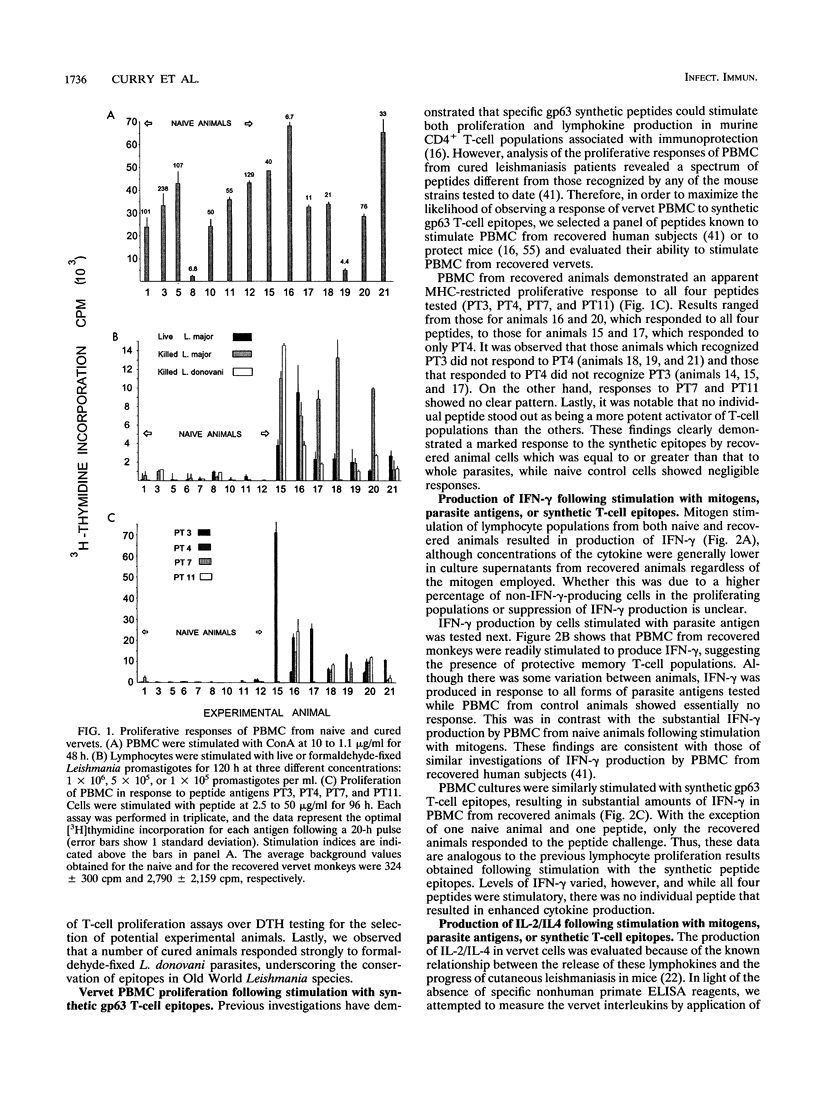
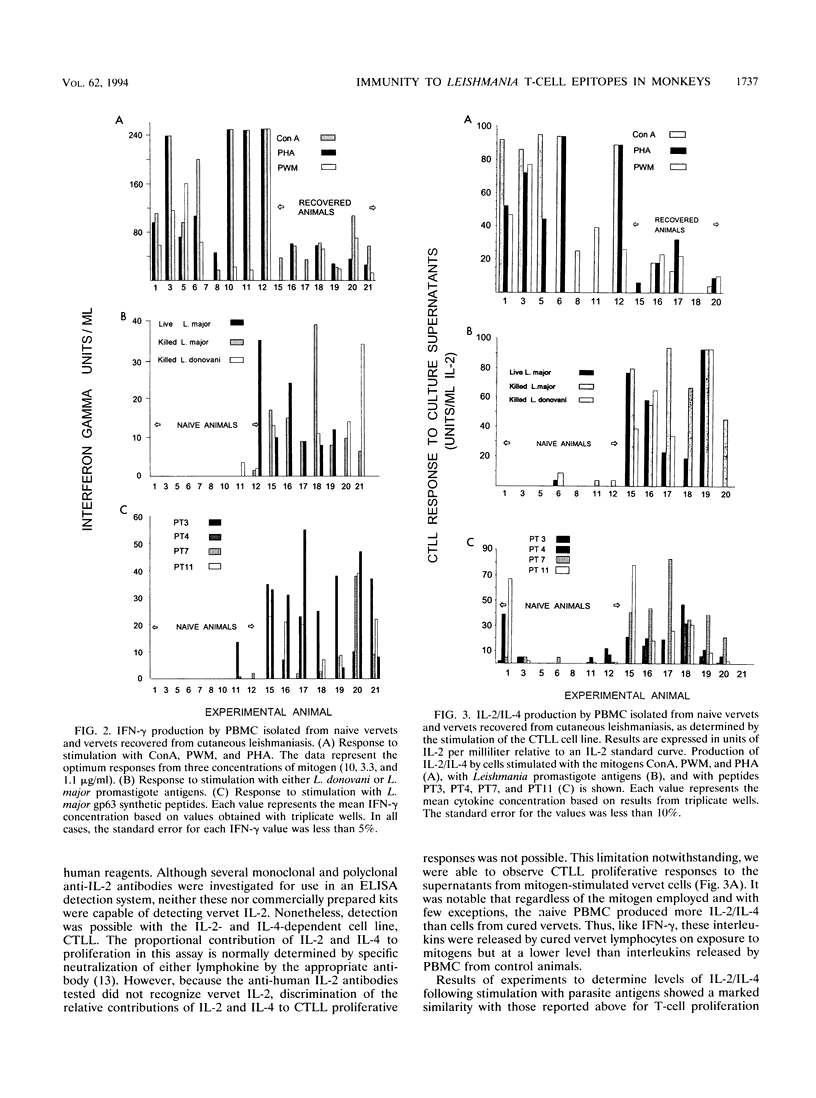
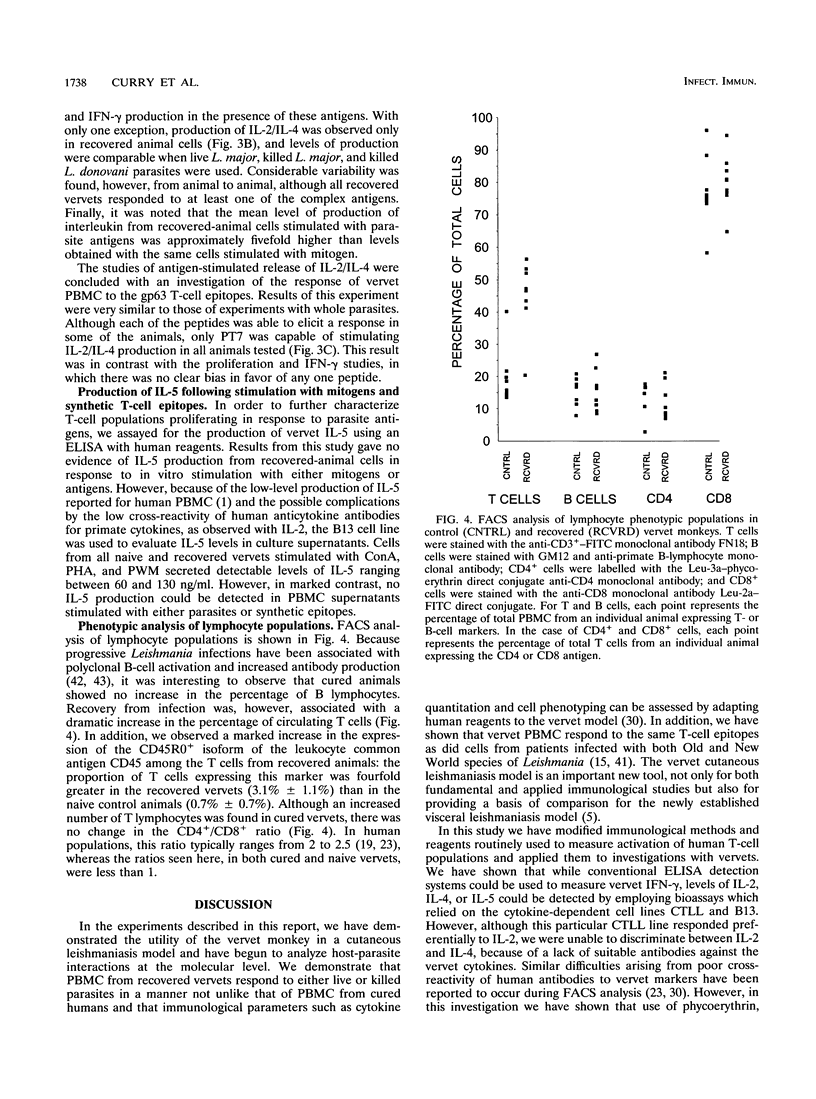
A population of vervet monkeys was immunized with killed parasites and infected with Leishmania major promastigotes either by needle or by infected-fly bite. The responses of recovered monkeys to mitogens, killed parasites, and molecularly defined T-cell epitopes were then compared with those of control animals. Peripheral blood mononuclear cells (PBMC) from both naive and recovered animals proliferated strongly in response to both B- and T-cell mitogens, although the responses of the recovered animals were less strong than those of the naive animals. Cells from recovered vervets, but not those from naive vervets, also proliferated in response to parasite antigens and synthetic T-cell epitopes. Likewise, cells from recovered animals released gamma interferon and either interleukin 2 (IL-2) or IL-4 into culture media in response to both of the above-mentioned antigens, whereas cells from control animals did not. The fact that no IL-5 could be measured following parasite antigen or synthetic T-cell epitope stimulation of PBMC suggested that cells proliferating in response to these molecules belonged to the Th1 subset. Phenotypic analysis of the PBMC showed a marked increase in T-cell but not B-cell populations in recovered animals. Among this population was an increased number of CD45R0+ memory cells. The data from this study are in keeping with the earlier finding that vervet monkeys provide an excellent model system for leishmaniasis. Further, these data support the contention that synthetic T-cell epitopes are prime candidates for molecularly defined Leishmania vaccines.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams J. S., Roncarolo M. G., Yssel H., Andersson U., Gleich G. J., Silver J. E. Strategies of anti-cytokine monoclonal antibody development: immunoassay of IL-10 and IL-5 in clinical samples. Immunol Rev. 1992 Jun;127:5–24. doi: 10.1111/j.1600-065x.1992.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Bell E. B., Sparshott S. M. Interconversion of CD45R subsets of CD4 T cells in vivo. Nature. 1990 Nov 8;348(6297):163–166. doi: 10.1038/348163a0. [DOI] [PubMed] [Google Scholar]
- Belosevic M., Davis C. E., Meltzer M. S., Nacy C. A. Regulation of activated macrophage antimicrobial activities. Identification of lymphokines that cooperate with IFN-gamma for induction of resistance to infection. J Immunol. 1988 Aug 1;141(3):890–896. [PubMed] [Google Scholar]
- Belosevic M., Finbloom D. S., Meltzer M. S., Nacy C. A. IL-2. A cofactor for induction of activated macrophage resistance to infection. J Immunol. 1990 Aug 1;145(3):831–839. [PubMed] [Google Scholar]
- Binhazim A. A., Shin S. S., Chapman W. L., Jr, Olobo J. Comparative susceptibility of African green monkeys (Cercopithecus aethiops) to experimental infection with Leishmania leishmania donovani and Leishmania leishmania infantum. Lab Anim Sci. 1993 Feb;43(1):37–47. [PubMed] [Google Scholar]
- Bouvier J., Etges R. J., Bordier C. Identification and purification of membrane and soluble forms of the major surface protein of Leishmania promastigotes. J Biol Chem. 1985 Dec 15;260(29):15504–15509. [PubMed] [Google Scholar]
- Chouaib S., Fradelizi D. The mechanism of inhibition of human IL 2 production. J Immunol. 1982 Dec;129(6):2463–2468. [PubMed] [Google Scholar]
- Cillari E., Dieli M., Maltese E., Milano S., Salerno A., Liew F. Y. Enhancement of macrophage IL-1 production by Leishmania major infection in vitro and its inhibition by IFN-gamma. J Immunol. 1989 Sep 15;143(6):2001–2005. [PubMed] [Google Scholar]
- Curry R. C., Kiener P. A., Spitalny G. L. A sensitive immunochemical assay for biologically active MuIFN-gamma. J Immunol Methods. 1987 Nov 23;104(1-2):137–142. doi: 10.1016/0022-1759(87)90497-2. [DOI] [PubMed] [Google Scholar]
- Firpo P. P., Axberg I., Scheibel M., Clark E. A. Macaque CD4+ T-cell subsets: influence of activation on infection by simian immunodeficiency viruses (SIV). AIDS Res Hum Retroviruses. 1992 Mar;8(3):357–366. doi: 10.1089/aid.1992.8.357. [DOI] [PubMed] [Google Scholar]
- Frommel T. O., Button L. L., Fujikura Y., McMaster W. R. The major surface glycoprotein (GP63) is present in both life stages of Leishmania. Mol Biochem Parasitol. 1990 Jan 1;38(1):25–32. doi: 10.1016/0166-6851(90)90201-v. [DOI] [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Githure J. I., Reid G. D., Binhazim A. A., Anjili C. O., Shatry A. M., Hendricks L. D. Leishmania major: the suitability of East African nonhuman primates as animal models for cutaneous leishmaniasis. Exp Parasitol. 1987 Dec;64(3):438–447. doi: 10.1016/0014-4894(87)90058-0. [DOI] [PubMed] [Google Scholar]
- Githure J. I., Shatry A. M., Tarara R., Chulay J. D., Suleman M. A., Chunge C. N., Else J. G. The suitability of East African primates as animal models of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1986;80(4):575–576. doi: 10.1016/0035-9203(86)90146-x. [DOI] [PubMed] [Google Scholar]
- Jardim A., Alexander J., Teh H. S., Ou D., Olafson R. W. Immunoprotective Leishmania major synthetic T cell epitopes. J Exp Med. 1990 Aug 1;172(2):645–648. doi: 10.1084/jem.172.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongeneel C. V., Bouvier J., Bairoch A. A unique signature identifies a family of zinc-dependent metallopeptidases. FEBS Lett. 1989 Jan 2;242(2):211–214. doi: 10.1016/0014-5793(89)80471-5. [DOI] [PubMed] [Google Scholar]
- Lawyer P. G., Githure J. I., Anjili C. O., Olobo J. O., Koech D. K., Reid G. D. Experimental transmission of Leishmania major to vervet monkeys (Cercopithecus aethiops) by bites of Phlebotomus duboscqi (Diptera: Psychodidae). Trans R Soc Trop Med Hyg. 1990 Mar-Apr;84(2):229–232. doi: 10.1016/0035-9203(90)90266-h. [DOI] [PubMed] [Google Scholar]
- Letvin N. L., King N. W., Reinherz E. L., Hunt R. D., Lane H., Schlossman S. F. T lymphocyte surface antigens in primates. Eur J Immunol. 1983 Apr;13(4):345–347. doi: 10.1002/eji.1830130414. [DOI] [PubMed] [Google Scholar]
- Liew F. Y. Functional heterogeneity of CD4+ T cells in leishmaniasis. Immunol Today. 1989 Feb;10(2):40–45. doi: 10.1016/0167-5699(89)90302-2. [DOI] [PubMed] [Google Scholar]
- Liew F. Y. Regulation of cell-mediated immunity in leishmaniasis. Curr Top Microbiol Immunol. 1990;155:53–64. doi: 10.1007/978-3-642-74983-4_4. [DOI] [PubMed] [Google Scholar]
- Locksley R. M., Heinzel F. P., Sadick M. D., Holaday B. J., Gardner K. D., Jr Murine cutaneous leishmaniasis: susceptibility correlates with differential expansion of helper T-cell subsets. Ann Inst Pasteur Immunol. 1987 Sep-Oct;138(5):744–749. doi: 10.1016/s0769-2625(87)80030-2. [DOI] [PubMed] [Google Scholar]
- Martin L. N., Gormus B. J., Bozelka B. E. Functional analysis of monkey lymphocyte subsets defined by OKT4 and OKT8 Monoclonal Antibodies. Cell Immunol. 1983 Apr 15;77(2):338–347. doi: 10.1016/0008-8749(83)90034-5. [DOI] [PubMed] [Google Scholar]
- Monaco J. J. A molecular model of MHC class-I-restricted antigen processing. Immunol Today. 1992 May;13(5):173–179. doi: 10.1016/0167-5699(92)90122-N. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Murray H. W. Interferon-gamma, the activated macrophage, and host defense against microbial challenge. Ann Intern Med. 1988 Apr;108(4):595–608. doi: 10.7326/0003-4819-108-4-595. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Stern J. J., Welte K., Rubin B. Y., Carriero S. M., Nathan C. F. Experimental visceral leishmaniasis: production of interleukin 2 and interferon-gamma, tissue immune reaction, and response to treatment with interleukin 2 and interferon-gamma. J Immunol. 1987 Apr 1;138(7):2290–2297. [PubMed] [Google Scholar]
- Olivier M., Brownsey R. W., Reiner N. E. Defective stimulus-response coupling in human monocytes infected with Leishmania donovani is associated with altered activation and translocation of protein kinase C. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7481–7485. doi: 10.1073/pnas.89.16.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olobo J. O. Reactivity of some monoclonal antibodies specific for human lymphocytes with vervet monkey peripheral blood mononuclear cells. Scand J Immunol Suppl. 1992;11:199–201. doi: 10.1111/j.1365-3083.1992.tb01652.x. [DOI] [PubMed] [Google Scholar]
- Olobo J. O., Reid G. D., Githure J. I., Anjili C. O. IFN-gamma and delayed-type hypersensitivity are associated with cutaneous leishmaniasis in vervet monkeys following secondary rechallenge with Leishmania major. Scand J Immunol Suppl. 1992;11:48–52. doi: 10.1111/j.1365-3083.1992.tb01618.x. [DOI] [PubMed] [Google Scholar]
- Rappocciolo G., Allan J. S., Eichberg J. W., Chanh T. C. A comparative study of natural killer cell activity, lymphoproliferation, and cell phenotypes in nonhuman primates. Vet Pathol. 1992 Jan;29(1):53–59. doi: 10.1177/030098589202900107. [DOI] [PubMed] [Google Scholar]
- Reiner N. E., Finke J. H. Interleukin 2 deficiency in murine Leishmaniasis donovani and its relationship to depressed spleen cell responses to phytohemagglutinin. J Immunol. 1983 Sep;131(3):1487–1491. [PubMed] [Google Scholar]
- Rolink A. G., Melchers F., Palacios R. Monoclonal antibodies reactive with the mouse interleukin 5 receptor. J Exp Med. 1989 May 1;169(5):1693–1701. doi: 10.1084/jem.169.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosnek E. E., Brouwer M. C., Aarden L. A. T cell triggering by lectins. I. Requirements for interleukin 2 production; lectin concentration determines the accessory cell dependency. Eur J Immunol. 1985 Jul;15(7):652–656. doi: 10.1002/eji.1830150703. [DOI] [PubMed] [Google Scholar]
- Roosnek E. E., Brouwer M. C., Aarden L. A. T cell triggering by lectins. II. Stimuli for induction of interleukin 2 responsiveness and interleukin 2 production differ only in quantitative aspects. Eur J Immunol. 1985 Jul;15(7):657–661. doi: 10.1002/eji.1830150704. [DOI] [PubMed] [Google Scholar]
- Rothbard J. B., Taylor W. R. A sequence pattern common to T cell epitopes. EMBO J. 1988 Jan;7(1):93–100. doi: 10.1002/j.1460-2075.1988.tb02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbard J. Synthetic peptides as vaccines. Nature. 1987 Nov 12;330(6144):106–107. doi: 10.1038/330106b0. [DOI] [PubMed] [Google Scholar]
- Russell D. G., Alexander J. Effective immunization against cutaneous leishmaniasis with defined membrane antigens reconstituted into liposomes. J Immunol. 1988 Feb 15;140(4):1274–1279. [PubMed] [Google Scholar]
- Russo D. M., Jardim A., Carvalho E. M., Sleath P. R., Armitage R. J., Olafson R. W., Reed S. G. Mapping human T cell epitopes in leishmania gp63. Identification of cross-reactive and species-specific epitopes. J Immunol. 1993 Feb 1;150(3):932–939. [PubMed] [Google Scholar]
- Sacks D. L. B cell dependent T lymphocyte responses in leishmaniasis. Mem Inst Oswaldo Cruz. 1988 Nov;83 (Suppl 1):506–513. doi: 10.1590/s0074-02761988000500060. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Scott P. A., Asofsky R., Sher F. A. Cutaneous leishmaniasis in anti-IgM-treated mice: enhanced resistance due to functional depletion of a B cell-dependent T cell involved in the suppressor pathway. J Immunol. 1984 Apr;132(4):2072–2077. [PubMed] [Google Scholar]
- Schumacher J. H., O'Garra A., Shrader B., van Kimmenade A., Bond M. W., Mosmann T. R., Coffman R. L. The characterization of four monoclonal antibodies specific for mouse IL-5 and development of mouse and human IL-5 enzyme-linked immunosorbent. J Immunol. 1988 Sep 1;141(5):1576–1581. [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P. The role of TH1 and TH2 cells in experimental cutaneous leishmaniasis. Exp Parasitol. 1989 Apr;68(3):369–372. doi: 10.1016/0014-4894(89)90120-3. [DOI] [PubMed] [Google Scholar]
- Tatsumi M., Imamura Y. Accessory cells distinct from macrophages in lymphocyte proliferation of cynomolgus monkeys. Immunology. 1984 May;52(1):25–30. [PMC free article] [PubMed] [Google Scholar]
- Titus R. G., Milon G., Marchal G., Vassalli P., Cerottini J. C., Louis J. A. Involvement of specific Lyt-2+ T cells in the immunological control of experimentally induced murine cutaneous leishmaniasis. Eur J Immunol. 1987 Oct;17(10):1429–1433. doi: 10.1002/eji.1830171007. [DOI] [PubMed] [Google Scholar]
- Titus R. G., Sherry B., Cerami A. Tumor necrosis factor plays a protective role in experimental murine cutaneous leishmaniasis. J Exp Med. 1989 Dec 1;170(6):2097–2104. doi: 10.1084/jem.170.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga E. A., Snoek M., Jansen H. M., Bos J. D., van Lier R. A., Kapsenberg M. L. Human atopen-specific types 1 and 2 T helper cell clones. J Immunol. 1991 Nov 1;147(9):2942–2949. [PubMed] [Google Scholar]
- Wilson M. E., Hardin K. K., Donelson J. E. Expression of the major surface glycoprotein of Leishmania donovani chagasi in virulent and attenuated promastigotes. J Immunol. 1989 Jul 15;143(2):678–684. [PubMed] [Google Scholar]
- Wilson M. E., Hardin K. K. The major Leishmania donovani chagasi surface glycoprotein in tunicamycin-resistant promastigotes. J Immunol. 1990 Jun 15;144(12):4825–4834. [PubMed] [Google Scholar]
- Yang D. M., Rogers M. V., Liew F. Y. Identification and characterization of host-protective T-cell epitopes of a major surface glycoprotein (gp63) from Leishmania major. Immunology. 1991 Jan;72(1):3–9. [PMC free article] [PubMed] [Google Scholar]
- de Vries J. E., Caviles A. P., Jr, Bont W. S., Mendelsohn J. The role of monocytes in human lymphocyte activation by mitogens. J Immunol. 1979 Mar;122(3):1099–1107. [PubMed] [Google Scholar]



