Abstract
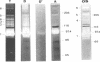
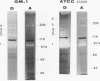
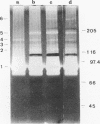
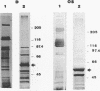
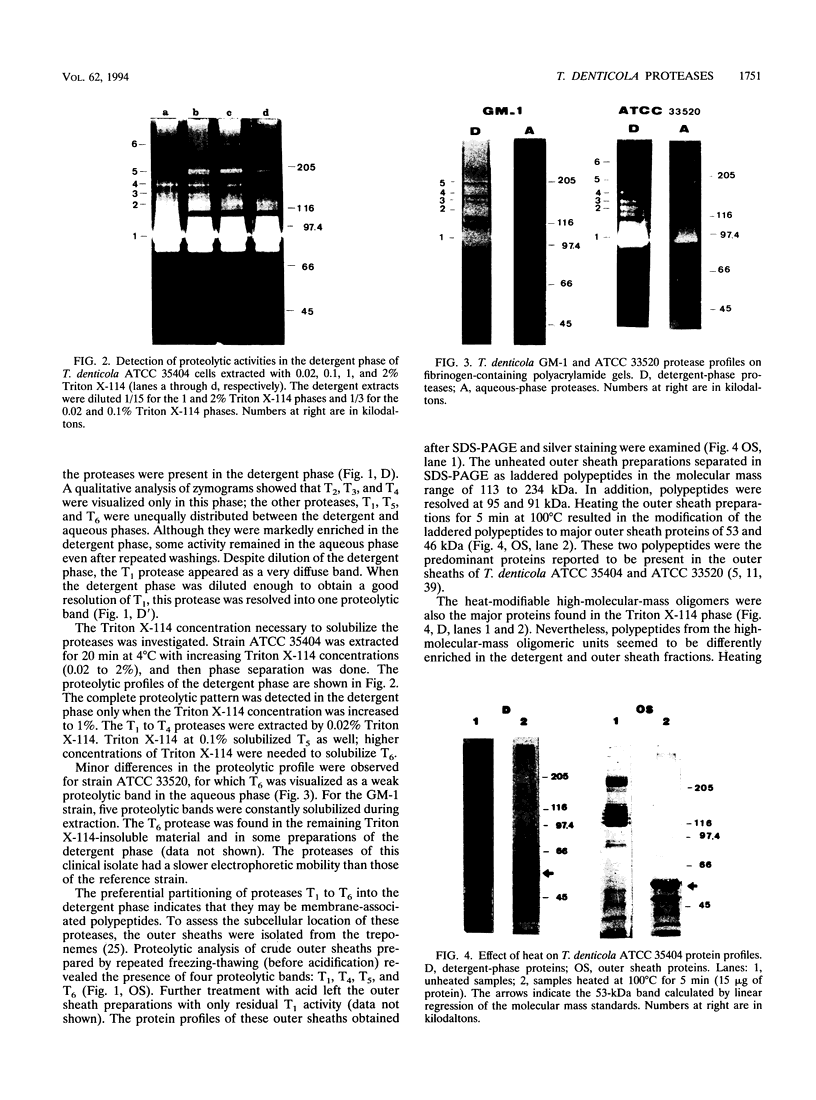
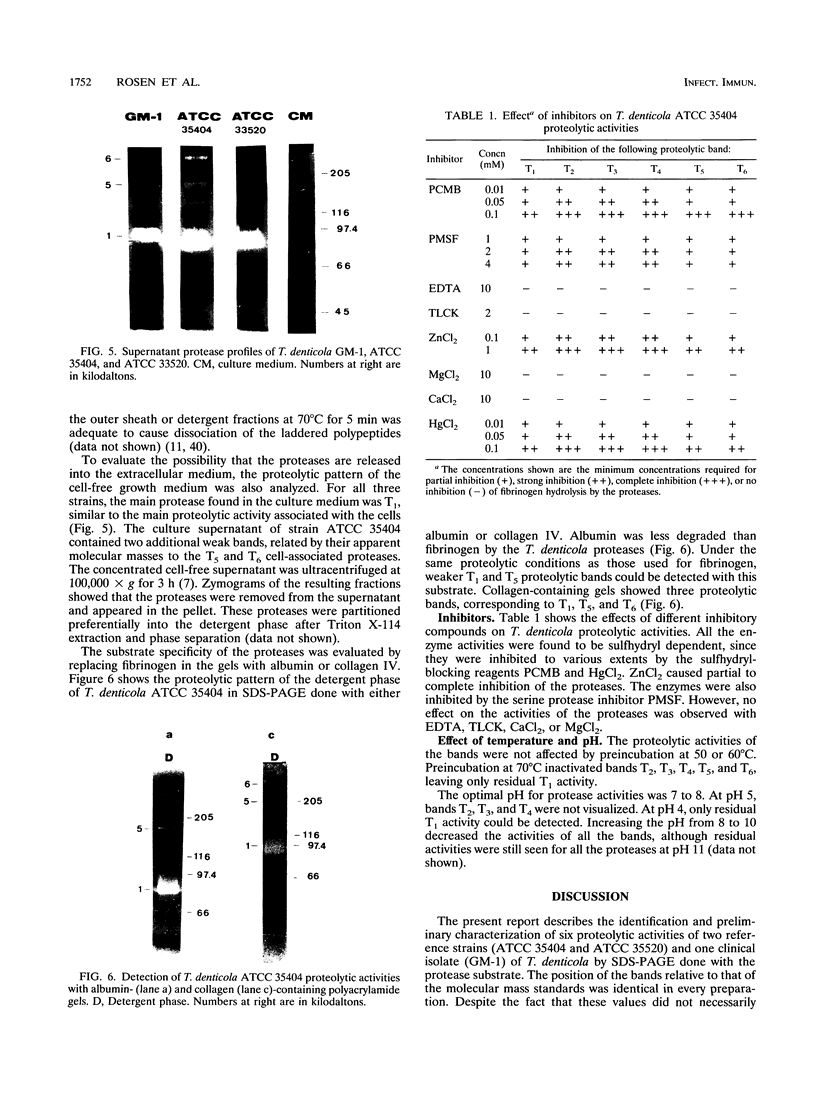
Several fibrinolytic activities of Treponema denticola, an oral spirochete associated with gingivitis and periodontal disease, were identified and characterized following phase partitioning with the nonionic detergent Triton X-114. The apparent molecular masses of the proteases ranged from 91 to 228 kDa when analyzed in sodium dodecyl sulfate-polyacrylamide gels containing fibrinogen as the protease substrate. A qualitative analysis of zymograms showed that the proteases were highly enriched in the detergent phase, although the 91-, 173-, and 228-kDa proteases were also found in the aqueous phase. Zymograms of crude outer sheaths prepared by repeated freezing-thawing revealed that the proteases may be associated with this subcellular compartment. The proteases displayed substrate specificity towards fibrinogen, were susceptible to sulfhydryl group reagents, and had a pH optimum between 7 and 8. The similarities in their sensitivity to inhibitors, temperature stability, pH optimum, and laddered protein profiles suggest that these hydrolytic enzymes may be part of a family of oligomeric proteases that may play an important role in the invasiveness of and tissue damage caused by the spirochete.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blakemore R. P., Canale-Parola E. Arginine catabolism by Treponema denticola. J Bacteriol. 1976 Nov;128(2):616–622. doi: 10.1128/jb.128.2.616-622.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Canale-Parola E. Physiology and evolution of spirochetes. Bacteriol Rev. 1977 Mar;41(1):181–204. doi: 10.1128/br.41.1.181-204.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemetson K. J., Bienz D., Zahno M. L., Lüscher E. F. Distribution of platelet glycoproteins and phosphoproteins in hydrophobic and hydrophilic phases in Triton X-114 phase partition. Biochim Biophys Acta. 1984 Dec 19;778(3):463–469. doi: 10.1016/0005-2736(84)90395-x. [DOI] [PubMed] [Google Scholar]
- Cockayne A., Sanger R., Ivic A., Strugnell R. A., MacDougall J. H., Russell R. R., Penn C. W. Antigenic and structural analysis of Treponema denticola. J Gen Microbiol. 1989 Dec;135(12):3209–3218. doi: 10.1099/00221287-135-12-3209. [DOI] [PubMed] [Google Scholar]
- Cunningham T. M., Walker E. M., Miller J. N., Lovett M. A. Selective release of the Treponema pallidum outer membrane and associated polypeptides with Triton X-114. J Bacteriol. 1988 Dec;170(12):5789–5796. doi: 10.1128/jb.170.12.5789-5796.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoe I. W., Gilchrist J. E. Release of endotoxin in the form of cell wall blebs during in vitro growth of Neisseria meningitidis. J Exp Med. 1973 Nov 1;138(5):1156–1167. doi: 10.1084/jem.138.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etges R. J., Bouvier J., Hoffman R., Bordier C. Evidence that the major surface proteins of three Leishmania species are structurally related. Mol Biochem Parasitol. 1985 Feb;14(2):141–149. doi: 10.1016/0166-6851(85)90033-7. [DOI] [PubMed] [Google Scholar]
- Grenier D., Chao G., McBride B. C. Characterization of sodium dodecyl sulfate-stable Bacteroides gingivalis proteases by polyacrylamide gel electrophoresis. Infect Immun. 1989 Jan;57(1):95–99. doi: 10.1128/iai.57.1.95-99.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier D., Uitto V. J., McBride B. C. Cellular location of a Treponema denticola chymotrypsinlike protease and importance of the protease in migration through the basement membrane. Infect Immun. 1990 Feb;58(2):347–351. doi: 10.1128/iai.58.2.347-351.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapasalo M., Müller K. H., Uitto V. J., Leung W. K., McBride B. C. Characterization, cloning, and binding properties of the major 53-kilodalton Treponema denticola surface antigen. Infect Immun. 1992 May;60(5):2058–2065. doi: 10.1128/iai.60.5.2058-2065.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Holt S. C. Anatomy and chemistry of spirochetes. Microbiol Rev. 1978 Mar;42(1):114–160. doi: 10.1128/mr.42.1.114-160.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick F. H., Gordesky S. E., Marinetti G. V. Differential solubilization of proteins, phospholipids, and cholesterol of erythrocyte membranes by detergents. Biochim Biophys Acta. 1974 Apr 29;345(2):154–161. doi: 10.1016/0005-2736(74)90254-5. [DOI] [PubMed] [Google Scholar]
- LISTGARTEN M. A. ELECTRON MICROSCOPIC OBSERVATIONS ON THE BACTERIAL FLORA OF ACUTE NECROTIZING ULCERATIVE GINGIVITIS. J Periodontol. 1965 Jul-Aug;36:328–339. doi: 10.1902/jop.1965.36.4.328. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liljenberg B., Lindhe J. Juvenile periodontitis. Some microbiological, histopathological and clinical characteristics. J Clin Periodontol. 1980 Feb;7(1):48–61. doi: 10.1111/j.1600-051x.1980.tb01948.x. [DOI] [PubMed] [Google Scholar]
- Listgarten M. A. Structure of the microbial flora associated with periodontal health and disease in man. A light and electron microscopic study. J Periodontol. 1976 Jan;47(1):1–18. doi: 10.1902/jop.1976.47.1.1. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A., Laughon B. E., Stoll J. The bacteriology of acute necrotizing ulcerative gingivitis. J Periodontol. 1982 Apr;53(4):223–230. doi: 10.1902/jop.1982.53.4.223. [DOI] [PubMed] [Google Scholar]
- Maher P. A., Singer S. J. Anomalous interaction of the acetylcholine receptor protein with the nonionic detergent Triton X-114. Proc Natl Acad Sci U S A. 1985 Feb;82(4):958–962. doi: 10.1073/pnas.82.4.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda K., Kawata T. Isolation, properties, and reassembly of outer sheath carrying a polygonal array from an oral treponeme. J Bacteriol. 1982 Jun;150(3):1405–1413. doi: 10.1128/jb.150.3.1405-1413.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikx F. H., Maltha J. C., van Campen G. J. Spirochetes in early lesions of necrotizing ulcerative gingivitis experimentally induced in beagles. Oral Microbiol Immunol. 1990 Apr;5(2):86–89. doi: 10.1111/j.1399-302x.1990.tb00233.x. [DOI] [PubMed] [Google Scholar]
- Norris S. J. Polypeptides of Treponema pallidum: progress toward understanding their structural, functional, and immunologic roles. Treponema Pallidum Polypeptide Research Group. Microbiol Rev. 1993 Sep;57(3):750–779. doi: 10.1128/mr.57.3.750-779.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K., Makinen K. K., Loesche W. J. Purification and characterization of an enzyme produced by Treponema denticola capable of hydrolyzing synthetic trypsin substrates. Infect Immun. 1986 Jul;53(1):213–220. doi: 10.1128/iai.53.1.213-220.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K., Naito Y., Ohta K., Fukumoto Y., Kimura Y., Ishikawa I., Kinoshita S., Takazoe I. Bacteriological study of periodontal lesions in two sisters with juvenile periodontitis and their mother. Infect Immun. 1984 Jul;45(1):118–121. doi: 10.1128/iai.45.1.118-121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf J. D., Chamberlain N. R., Clausell A., Norgard M. V. Identification and localization of integral membrane proteins of virulent Treponema pallidum subsp. pallidum by phase partitioning with the nonionic detergent triton X-114. Infect Immun. 1988 Feb;56(2):490–498. doi: 10.1128/iai.56.2.490-498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglie R., Newman M. G., Carranza F. A., Jr, Pattison G. L. Bacterial invasion of gingiva in advanced periodontitis in humans. J Periodontol. 1982 Apr;53(4):217–222. doi: 10.1902/jop.1982.53.4.217. [DOI] [PubMed] [Google Scholar]
- Sela M. N., Kornman K. S., Ebersole J. L., Holt S. C. Characterization of treponemes isolated from human and non-human primate periodontal pockets. Oral Microbiol Immunol. 1987 Mar;2(1):21–29. doi: 10.1111/j.1399-302x.1987.tb00265.x. [DOI] [PubMed] [Google Scholar]
- Sela M. N., Weinberg A., Borinsky R., Holt S. C., Dishon T. Inhibition of superoxide production in human polymorphonuclear leukocytes by oral treponemal factors. Infect Immun. 1988 Mar;56(3):589–594. doi: 10.1128/iai.56.3.589-594.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Uitto V. J., Grenier D., Chan E. C., McBride B. C. Isolation of a chymotrypsinlike enzyme from Treponema denticola. Infect Immun. 1988 Oct;56(10):2717–2722. doi: 10.1128/iai.56.10.2717-2722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemoto T., Zambon J. J., Genco R. J., Namikawa I. Major antigens of human oral spirochetes associated with periodontal disease. Adv Dent Res. 1988 Nov;2(2):292–296. doi: 10.1177/08959374880020021401. [DOI] [PubMed] [Google Scholar]
- Weinberg A., Holt S. C. Chemical and biological activities of a 64-kilodalton outer sheath protein from Treponema denticola strains. J Bacteriol. 1991 Nov;173(21):6935–6947. doi: 10.1128/jb.173.21.6935-6947.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]