Abstract
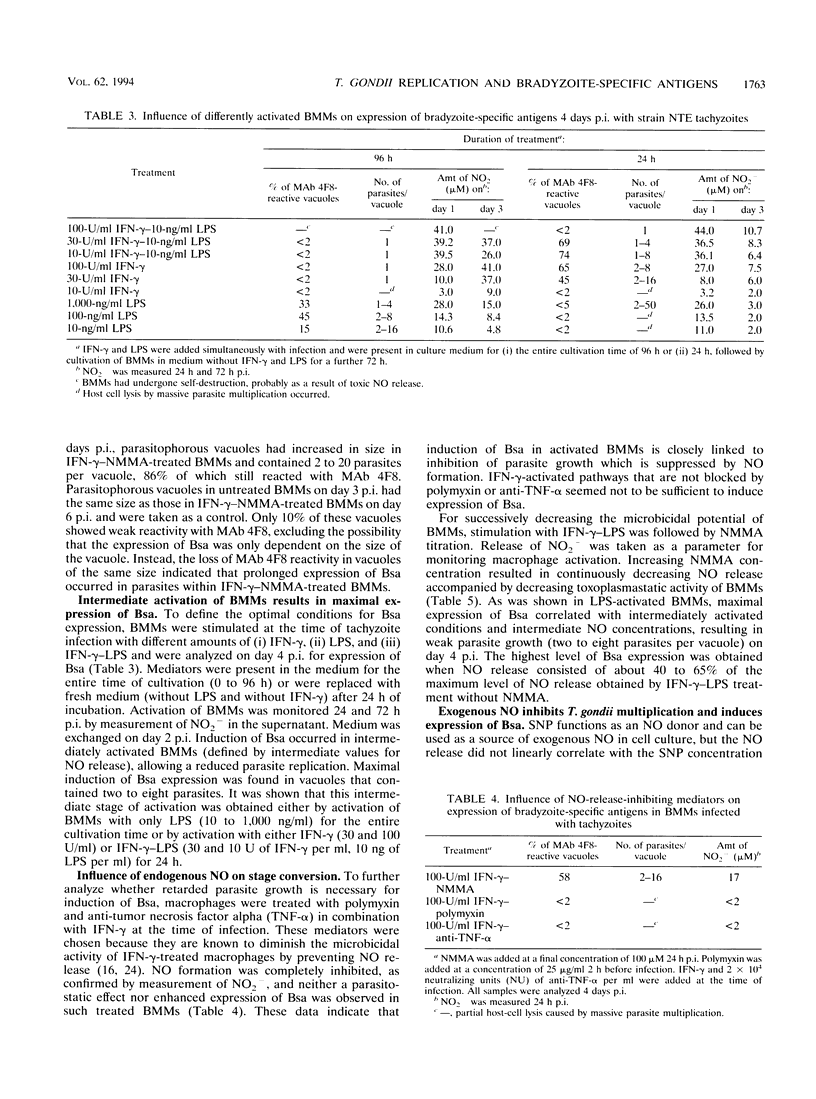
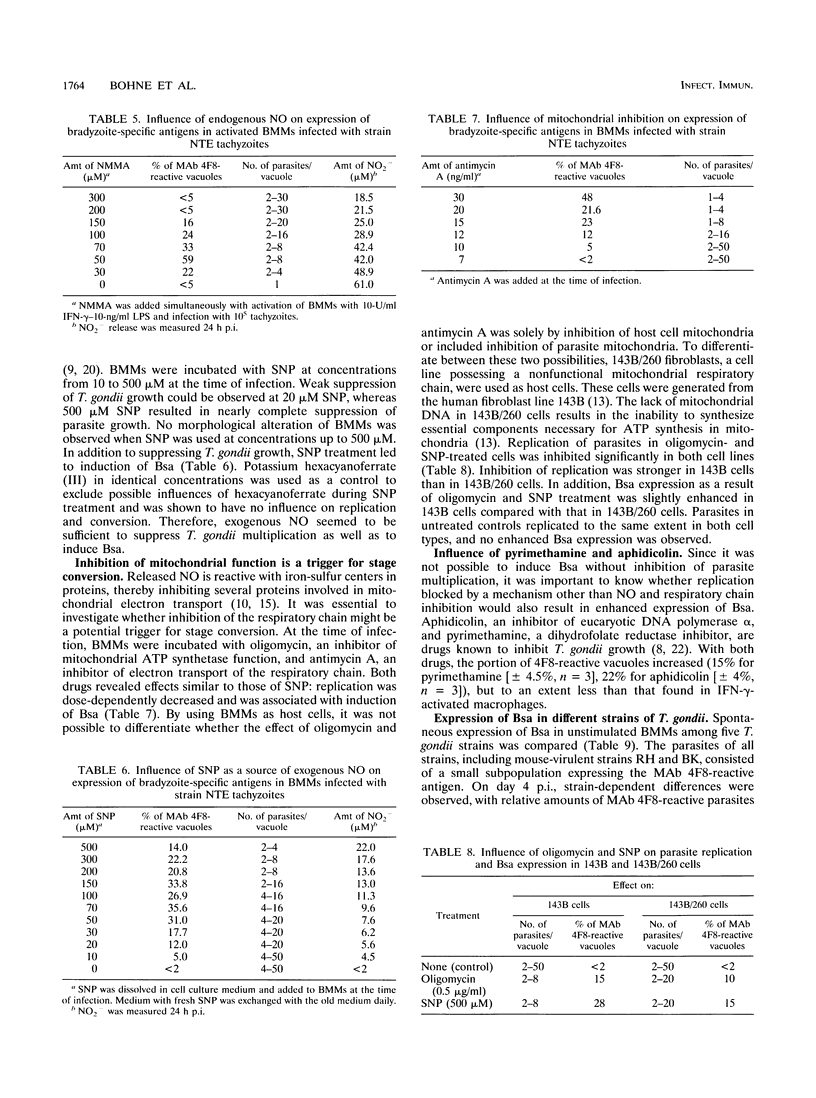
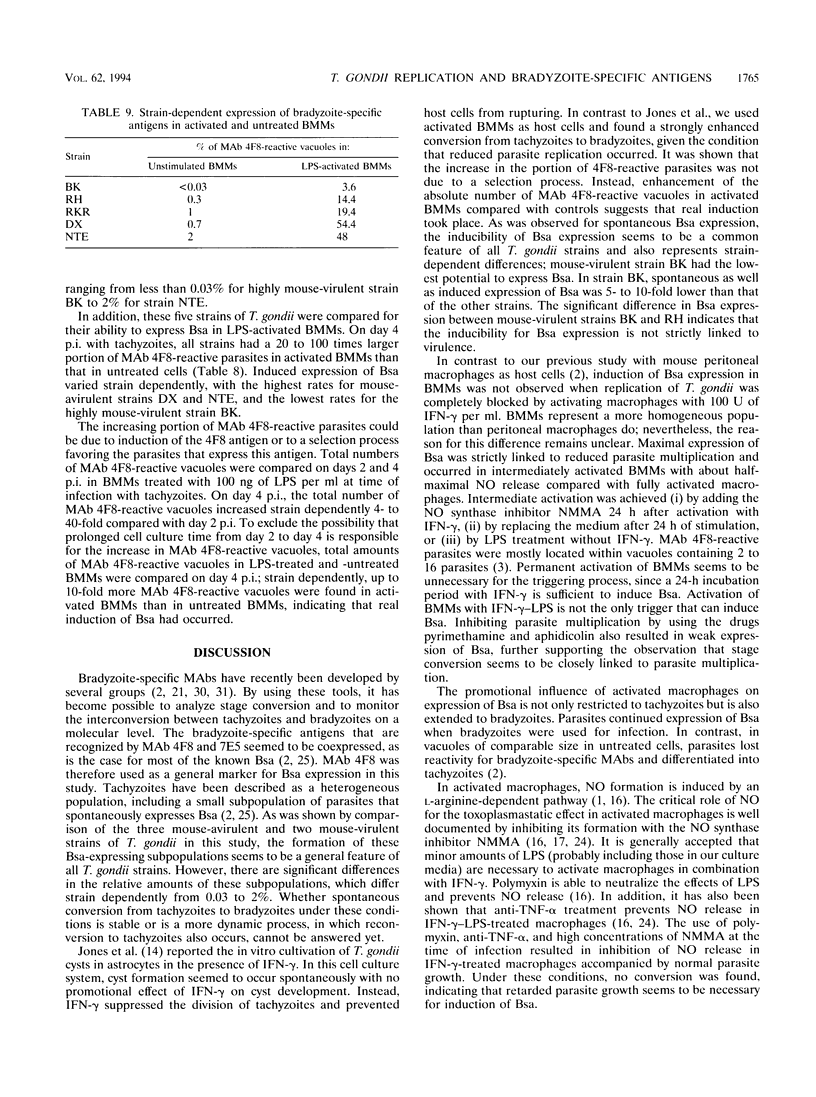
Stage conversion between tachyzoites and bradyzoites of Toxoplasma gondii was investigated in vitro by using murine bone marrow-derived macrophages (BMMs) as host cells. Following infection of untreated BMMs with tachyzoites, spontaneous expression of bradyzoite-specific antigens (Bsa) occurred at low frequency with Toxoplasma strain-dependent ratios from 0.03 to 2%. As previously described for peritoneal macrophages, activation of tachyzoite-infected BMMs with gamma interferon (IFN-gamma) or lipopolysaccharide resulted in the induction of Bsa. When bradyzoites were used for infection, prolonged expression of Bsa could be observed in IFN-gamma-activated BMMs. The induction of Bsa expression seemed to be closely linked to parasite multiplication and increased to maximal values of 50 to 70% in intermediately activated macrophages with nitric oxide (NO) levels that allowed reduced parasite replication. Identical results in stage conversion were obtained when sodium nitroprusside was used as a source of exogenous NO, indicating that NO might be a molecular trigger of stage conversion. NO is reactive with iron-sulfur centers in proteins, thereby inhibiting proteins involved in the mitochondrial respiratory chain. Using oligomycin and antimycin A as inhibitors of mitochondrial function, growth inhibition of parasites and induction of Bsa were obtained. Since microglia are the functional correlates of macrophages in the central nervous system and inhibit T. gondii upon activation with IFN-gamma, a similar mechanism might be involved during cyst development in the brain.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. B., Hibbs J. B., Jr, Taintor R. R., Krahenbuhl J. L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol. 1990 Apr 1;144(7):2725–2729. [PubMed] [Google Scholar]
- Bohne W., Heesemann J., Gross U. Coexistence of heterogeneous populations of Toxoplasma gondii parasites within parasitophorous vacuoles of murine macrophages as revealed by a bradyzoite-specific monoclonal antibody. Parasitol Res. 1993;79(6):485–487. doi: 10.1007/BF00931588. [DOI] [PubMed] [Google Scholar]
- Bohne W., Heesemann J., Gross U. Induction of bradyzoite-specific Toxoplasma gondii antigens in gamma interferon-treated mouse macrophages. Infect Immun. 1993 Mar;61(3):1141–1145. doi: 10.1128/iai.61.3.1141-1145.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao C. C., Hu S., Gekker G., Novick W. J., Jr, Remington J. S., Peterson P. K. Effects of cytokines on multiplication of Toxoplasma gondii in microglial cells. J Immunol. 1993 Apr 15;150(8 Pt 1):3404–3410. [PubMed] [Google Scholar]
- Chao C. C., Hu S., Molitor T. W., Shaskan E. G., Peterson P. K. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol. 1992 Oct 15;149(8):2736–2741. [PubMed] [Google Scholar]
- Cornelissen A. W., Overdulve J. P., Hoenderboom J. M. Separation of Isospora (Toxoplasma) gondii cysts and cystozoites from mouse brain tissue by continuous density-gradient centrifugation. Parasitology. 1981 Aug;83(Pt 1):103–108. doi: 10.1017/s0031182000050071. [DOI] [PubMed] [Google Scholar]
- Corradin S. B., Mauël J., Donini S. D., Quattrocchi E., Ricciardi-Castagnoli P. Inducible nitric oxide synthase activity of cloned murine microglial cells. Glia. 1993 Mar;7(3):255–262. doi: 10.1002/glia.440070309. [DOI] [PubMed] [Google Scholar]
- Derouin F., Chastang C. In vitro effects of folate inhibitors on Toxoplasma gondii. Antimicrob Agents Chemother. 1989 Oct;33(10):1753–1759. doi: 10.1128/aac.33.10.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S., Ankarcrona M., Nicotera P., Brüne B. Exogenous nitric oxide (NO) generation or IL-1 beta-induced intracellular NO production stimulates inhibitory auto-ADP-ribosylation of glyceraldehyde-3-phosphate dehydrogenase in RINm5F cells. J Immunol. 1993 Apr 1;150(7):2964–2971. [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J Immunol. 1988 Apr 15;140(8):2829–2838. [PubMed] [Google Scholar]
- Gazzinelli R. T., Eltoum I., Wynn T. A., Sher A. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-alpha and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol. 1993 Oct 1;151(7):3672–3681. [PubMed] [Google Scholar]
- Gross U., Müller W. A., Knapp S., Heesemann J. Identification of a virulence-associated antigen of Toxoplasma gondii by use of a mouse monoclonal antibody. Infect Immun. 1991 Dec;59(12):4511–4516. doi: 10.1128/iai.59.12.4511-4516.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. D., Burne J. F., King M. P., Miyashita T., Reed J. C., Raff M. C. Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA. Nature. 1993 Jan 28;361(6410):365–369. doi: 10.1038/361365a0. [DOI] [PubMed] [Google Scholar]
- Jones T. C., Bienz K. A., Erb P. In vitro cultivation of Toxoplasma gondii cysts in astrocytes in the presence of gamma interferon. Infect Immun. 1986 Jan;51(1):147–156. doi: 10.1128/iai.51.1.147-156.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H., Kolb-Bachofen V. Nitric oxide: a pathogenetic factor in autoimmunity. Immunol Today. 1992 May;13(5):157–160. doi: 10.1016/0167-5699(92)90118-Q. [DOI] [PubMed] [Google Scholar]
- Langermans J. A., Van der Hulst M. E., Nibbering P. H., Hiemstra P. S., Fransen L., Van Furth R. IFN-gamma-induced L-arginine-dependent toxoplasmastatic activity in murine peritoneal macrophages is mediated by endogenous tumor necrosis factor-alpha. J Immunol. 1992 Jan 15;148(2):568–574. [PubMed] [Google Scholar]
- Liew F. Y., Cox F. E. Nonspecific defence mechanism: the role of nitric oxide. Immunol Today. 1991 Mar;12(3):A17–A21. doi: 10.1016/S0167-5699(05)80006-4. [DOI] [PubMed] [Google Scholar]
- Luft B. J., Brooks R. G., Conley F. K., McCabe R. E., Remington J. S. Toxoplasmic encephalitis in patients with acquired immune deficiency syndrome. JAMA. 1984 Aug 17;252(7):913–917. [PubMed] [Google Scholar]
- McCabe R. E., Luft B. J., Remington J. S. Effect of murine interferon gamma on murine toxoplasmosis. J Infect Dis. 1984 Dec;150(6):961–962. doi: 10.1093/infdis/150.6.961. [DOI] [PubMed] [Google Scholar]
- Omata Y., Igarashi M., Ramos M. I., Nakabayashi T. Toxoplasma gondii: antigenic differences between endozoites and cystozoites defined by monoclonal antibodies. Parasitol Res. 1989;75(3):189–193. doi: 10.1007/BF00931274. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn E. R. Characterization of a mutant of Toxoplasma gondii resistant to aphidicolin. J Protozool. 1984 May;31(2):306–310. doi: 10.1111/j.1550-7408.1984.tb02967.x. [DOI] [PubMed] [Google Scholar]
- Schulze-Osthoff K., Beyaert R., Vandevoorde V., Haegeman G., Fiers W. Depletion of the mitochondrial electron transport abrogates the cytotoxic and gene-inductive effects of TNF. EMBO J. 1993 Aug;12(8):3095–3104. doi: 10.1002/j.1460-2075.1993.tb05978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley L. D., Adams L. B., Fukutomi Y., Krahenbuhl J. L. Tumor necrosis factor-alpha triggers antitoxoplasmal activity of IFN-gamma primed macrophages. J Immunol. 1991 Oct 1;147(7):2340–2345. [PubMed] [Google Scholar]
- Soete M., Fortier B., Camus D., Dubremetz J. F. Toxoplasma gondii: kinetics of bradyzoite-tachyzoite interconversion in vitro. Exp Parasitol. 1993 May;76(3):259–264. doi: 10.1006/expr.1993.1031. [DOI] [PubMed] [Google Scholar]
- Subauste C. S., Remington J. S. Role of gamma interferon in Toxoplasma gondii infection. Eur J Clin Microbiol Infect Dis. 1991 Feb;10(2):58–67. doi: 10.1007/BF01964408. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Conley F. K., Remington J. S. Importance of endogenous IFN-gamma for prevention of toxoplasmic encephalitis in mice. J Immunol. 1989 Sep 15;143(6):2045–2050. [PubMed] [Google Scholar]
- Suzuki Y., Orellana M. A., Schreiber R. D., Remington J. S. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988 Apr 22;240(4851):516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Remington J. S. The effect of anti-IFN-gamma antibody on the protective effect of Lyt-2+ immune T cells against toxoplasmosis in mice. J Immunol. 1990 Mar 1;144(5):1954–1956. [PubMed] [Google Scholar]
- Tomavo S., Fortier B., Soete M., Ansel C., Camus D., Dubremetz J. F. Characterization of bradyzoite-specific antigens of Toxoplasma gondii. Infect Immun. 1991 Oct;59(10):3750–3753. doi: 10.1128/iai.59.10.3750-3753.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L. M., LaPlace D., Tanowitz H. B., Wittner M. Identification of Toxoplasma gondii bradyzoite-specific monoclonal antibodies. J Infect Dis. 1992 Jul;166(1):213–215. doi: 10.1093/infdis/166.1.213. [DOI] [PubMed] [Google Scholar]



