Abstract
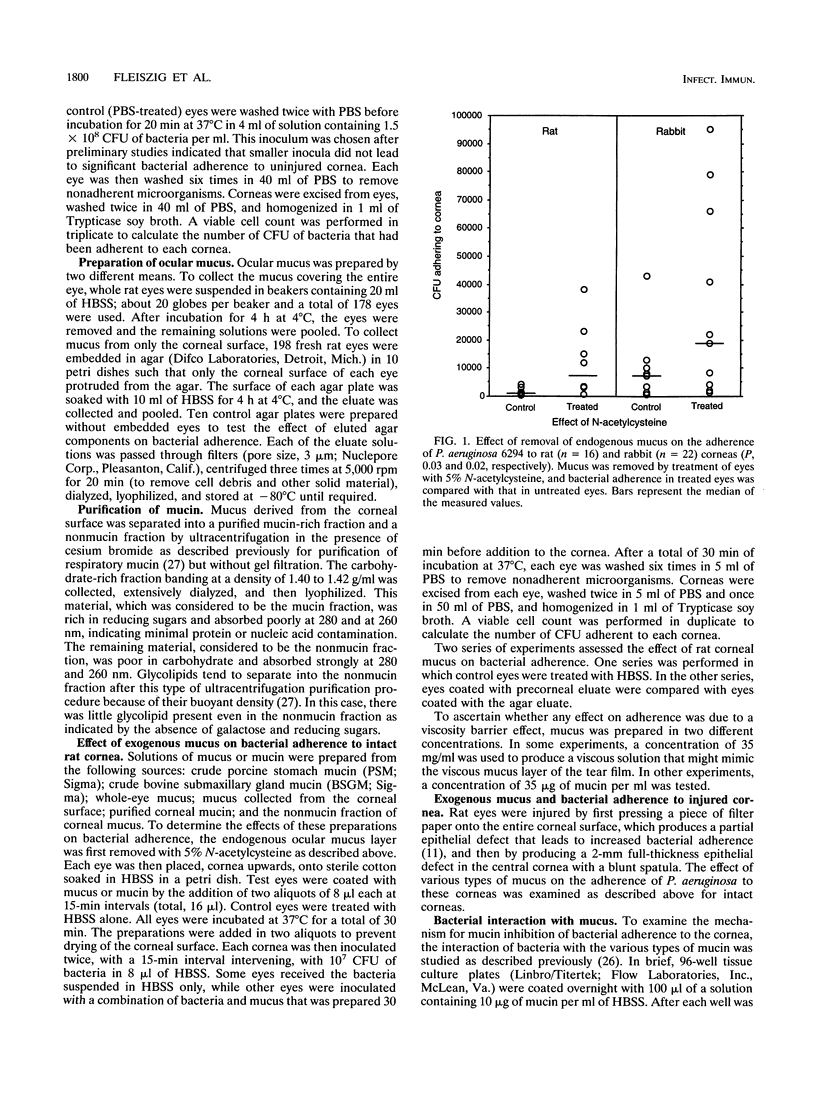
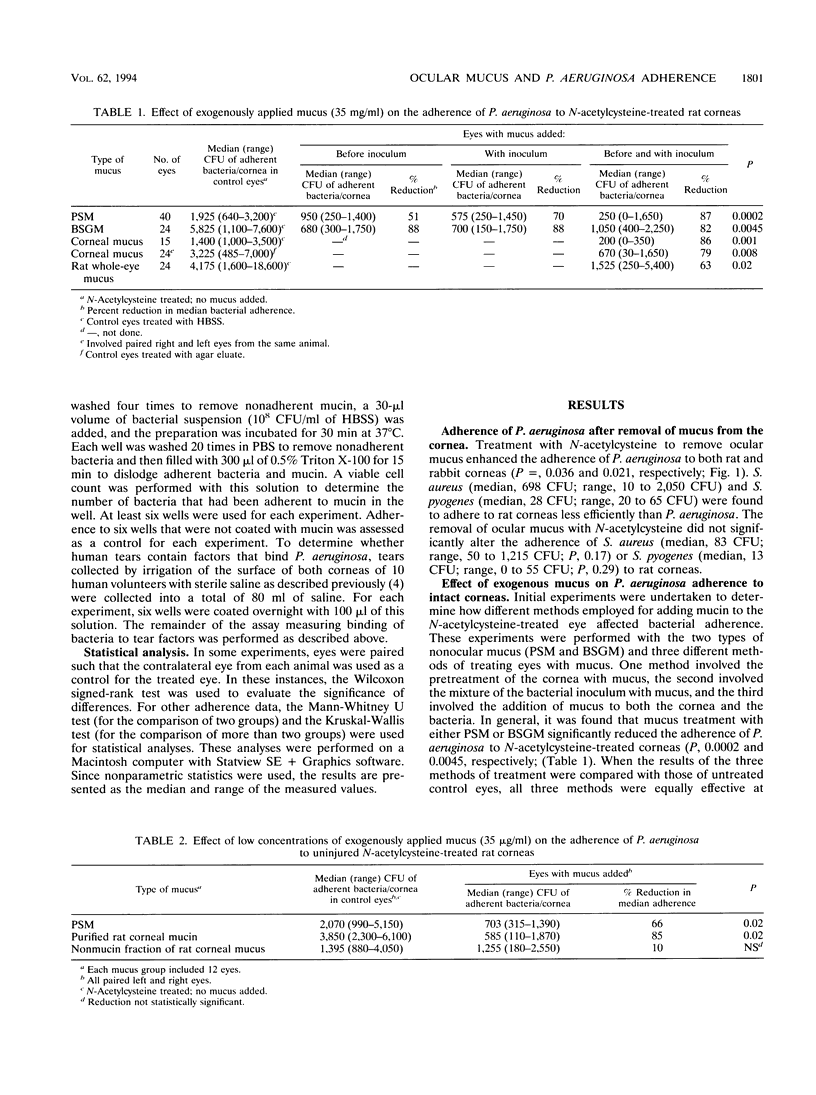
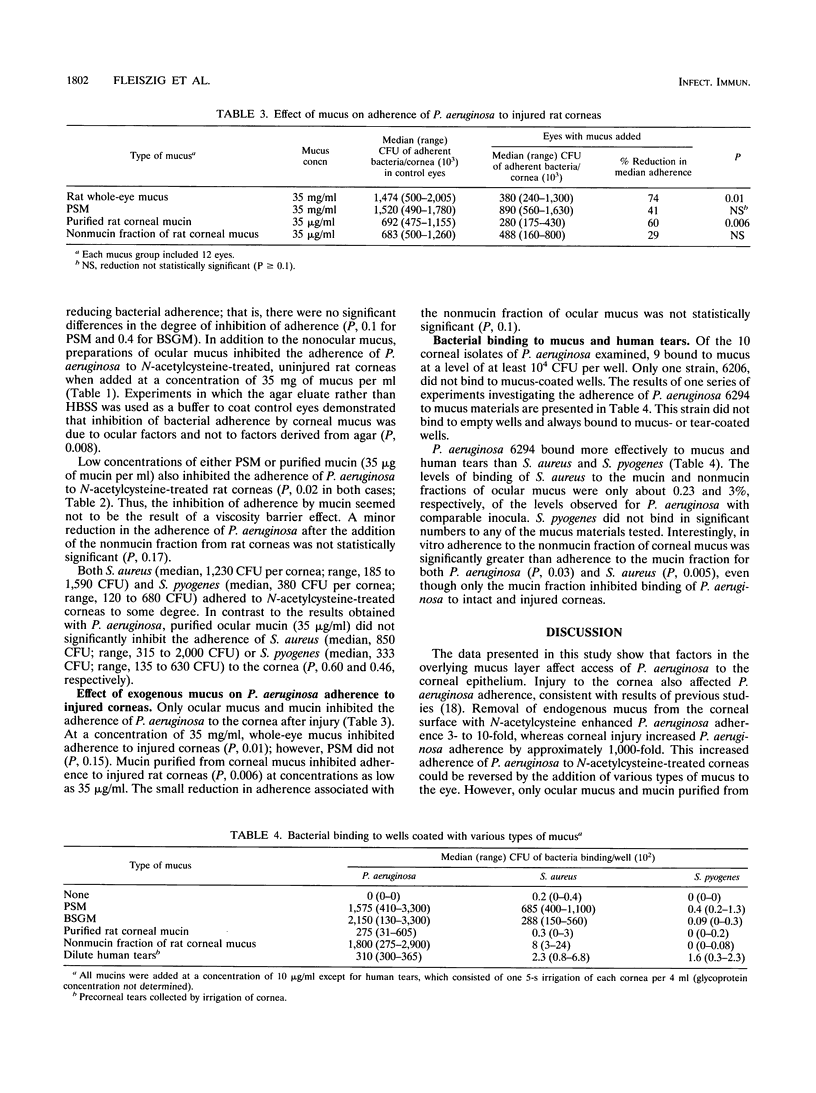
To gain access to the corneal epithelium and cause infections keratitis, bacterial pathogens must first interact with ocular surface factors that could affect bacterial adherence. In this study, we demonstrated that the mucus layer, and, in particular, the mucin fraction of mucus, modulated adherence to intact corneal epithelium of Pseudomonas aeruginosa but not that of Staphylococcus aureus or Streptococcus pyogenes. Removal of endogenous mucus from rat or rabbit eyes increased the adherence of P. aeruginosa by 3- to 10-fold. Ocular mucus obtained from rat eyes, porcine stomach mucin, or bovine submaxillary gland mucin inhibited adherence of P. aeruginosa to uninjured corneal epithelium. The mucin fraction of ocular mucus, purified by ultracentrifugation, was found to contain the inhibitory activity, and inhibition was demonstrated at concentrations of mucin as low as 35 micrograms/ml. Ocular mucin was the only material tested that inhibited adherence of P. aeruginosa to an injured cornea. However, the binding of P. aeruginosa to immobilized substrates in vitro did not predict which fraction would possess antiadherence activity: bacteria bound well to whole ocular mucus, mucin, the nonmucin fraction of ocular mucus, and dilute human tears as well as to porcine stomach mucin and bovine submaxillary gland mucin. The effectiveness of the mucin fraction of ocular mucus at inhibiting the binding of P. aeruginosa to the cornea implies that this material is a barrier that protects the surface of the eye from P. aeruginosa adherence.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfonso E., Mandelbaum S., Fox M. J., Forster R. K. Ulcerative keratitis associated with contact lens wear. Am J Ophthalmol. 1986 Apr 15;101(4):429–433. doi: 10.1016/0002-9394(86)90641-0. [DOI] [PubMed] [Google Scholar]
- Courtney H. S., Hasty D. L. Aggregation of group A streptococci by human saliva and effect of saliva on streptococcal adherence to host cells. Infect Immun. 1991 May;59(5):1661–1666. doi: 10.1128/iai.59.5.1661-1666.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarlo J. D., Van Horn D. L., Hyndiuk R. A., Davis S. D. Increased susceptibility to infection in experimental xerophthalmia. Arch Ophthalmol. 1981 Sep;99(9):1614–1617. doi: 10.1001/archopht.1981.03930020488019. [DOI] [PubMed] [Google Scholar]
- Fleiszig S. M., Efron N., Pier G. B. Extended contact lens wear enhances Pseudomonas aeruginosa adherence to human corneal epithelium. Invest Ophthalmol Vis Sci. 1992 Sep;33(10):2908–2916. [PubMed] [Google Scholar]
- Hazlett L. D., Rosen D. D., Berk R. S. Age-related susceptibility to Pseudomonas aeruginosa ocular infections in mice. Infect Immun. 1978 Apr;20(1):25–29. doi: 10.1128/iai.20.1.25-29.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett L., Dudzik D., Harries B. Development of ocular mucin: scanning EM analysis. Ophthalmic Res. 1986;18(1):28–33. doi: 10.1159/000265410. [DOI] [PubMed] [Google Scholar]
- Holly F. J., Lemp M. A. Tear physiology and dry eyes. Surv Ophthalmol. 1977 Sep-Oct;22(2):69–87. doi: 10.1016/0039-6257(77)90087-x. [DOI] [PubMed] [Google Scholar]
- Klotz S. A., Au Y. K., Misra R. P. A partial-thickness epithelial defect increases the adherence of Pseudomonas aeruginosa to the cornea. Invest Ophthalmol Vis Sci. 1989 Jun;30(6):1069–1074. [PubMed] [Google Scholar]
- Mack D. R., Sherman P. M. Mucin isolated from rabbit colon inhibits in vitro binding of Escherichia coli RDEC-1. Infect Immun. 1991 Mar;59(3):1015–1023. doi: 10.1128/iai.59.3.1015-1023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. J., Ahearn D. G. Adherence of Pseudomonas aeruginosa to hydrophilic contact lenses and other substrata. J Clin Microbiol. 1987 Aug;25(8):1392–1397. doi: 10.1128/jcm.25.8.1392-1397.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B. A., Chiappino M. L., Dawson C. R. Demonstration of the mucous layer of the tear film by electron microscopy. Invest Ophthalmol Vis Sci. 1985 Apr;26(4):464–473. [PubMed] [Google Scholar]
- Parsons C. L., Mulholland S. G. Bladder surface mucin. Its antibacterial effect against various bacterial species. Am J Pathol. 1978 Nov;93(2):423–432. [PMC free article] [PubMed] [Google Scholar]
- Prydal J. I., Artal P., Woon H., Campbell F. W. Study of human precorneal tear film thickness and structure using laser interferometry. Invest Ophthalmol Vis Sci. 1992 May;33(6):2006–2011. [PubMed] [Google Scholar]
- Ramphal R., Houdret N., Koo L., Lamblin G., Roussel P. Differences in adhesion of Pseudomonas aeruginosa to mucin glycopeptides from sputa of patients with cystic fibrosis and chronic bronchitis. Infect Immun. 1989 Oct;57(10):3066–3071. doi: 10.1128/iai.57.10.3066-3071.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Pyle M. Evidence for mucins and sialic acid as receptors for Pseudomonas aeruginosa in the lower respiratory tract. Infect Immun. 1983 Jul;41(1):339–344. doi: 10.1128/iai.41.1.339-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjan U., Reisman J., Doig P., Irvin R. T., Forstner G., Forstner J. Binding of nonmucoid Pseudomonas aeruginosa to normal human intestinal mucin and respiratory mucin from patients with cystic fibrosis. J Clin Invest. 1992 Feb;89(2):657–665. doi: 10.1172/JCI115632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Hazlett L. D., Berk R. S. Characterization of Pseudomonas aeruginosa adherence to mouse corneas in organ culture. Infect Immun. 1990 May;58(5):1301–1307. doi: 10.1128/iai.58.5.1301-1307.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern G. A., Lubniewski A., Allen C. The interaction between Pseudomonas aeruginosa and the corneal epithelium. An electron microscopic study. Arch Ophthalmol. 1985 Aug;103(8):1221–1225. doi: 10.1001/archopht.1985.01050080133033. [DOI] [PubMed] [Google Scholar]
- Thermes F., Molon-Noblot S., Grove J. Effects of acetylcysteine on rabbit conjunctival and corneal surfaces. A scanning electron microscopy study. Invest Ophthalmol Vis Sci. 1991 Oct;32(11):2958–2963. [PubMed] [Google Scholar]
- Valenton M. J., Tan R. V. Secondary ocular bacterial infection in hypovitaminosis a xerophthalmia. Am J Ophthalmol. 1975 Oct;80(4):673–677. doi: 10.1016/0002-9394(75)90399-2. [DOI] [PubMed] [Google Scholar]
- Versura P., Maltarello M. C., Cellini M., Marinelli F., Caramazza R., Laschi R. Detection of mucus glycoconjugates in human conjunctiva by using the lectin-colloidal gold technique in TEM. III. A quantitative study in asymptomatic contact lens wearers. Acta Ophthalmol (Copenh) 1987 Dec;65(6):661–667. doi: 10.1111/j.1755-3768.1987.tb07060.x. [DOI] [PubMed] [Google Scholar]
- Vishwanath S., Ramphal R. Adherence of Pseudomonas aeruginosa to human tracheobronchial mucin. Infect Immun. 1984 Jul;45(1):197–202. doi: 10.1128/iai.45.1.197-202.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward H., Horsey B., Bhavanandan V. P., Davidson E. A. Isolation, purification, and properties of respiratory mucus glycoproteins. Biochemistry. 1982 Feb 16;21(4):694–701. doi: 10.1021/bi00533a017. [DOI] [PubMed] [Google Scholar]



