Abstract
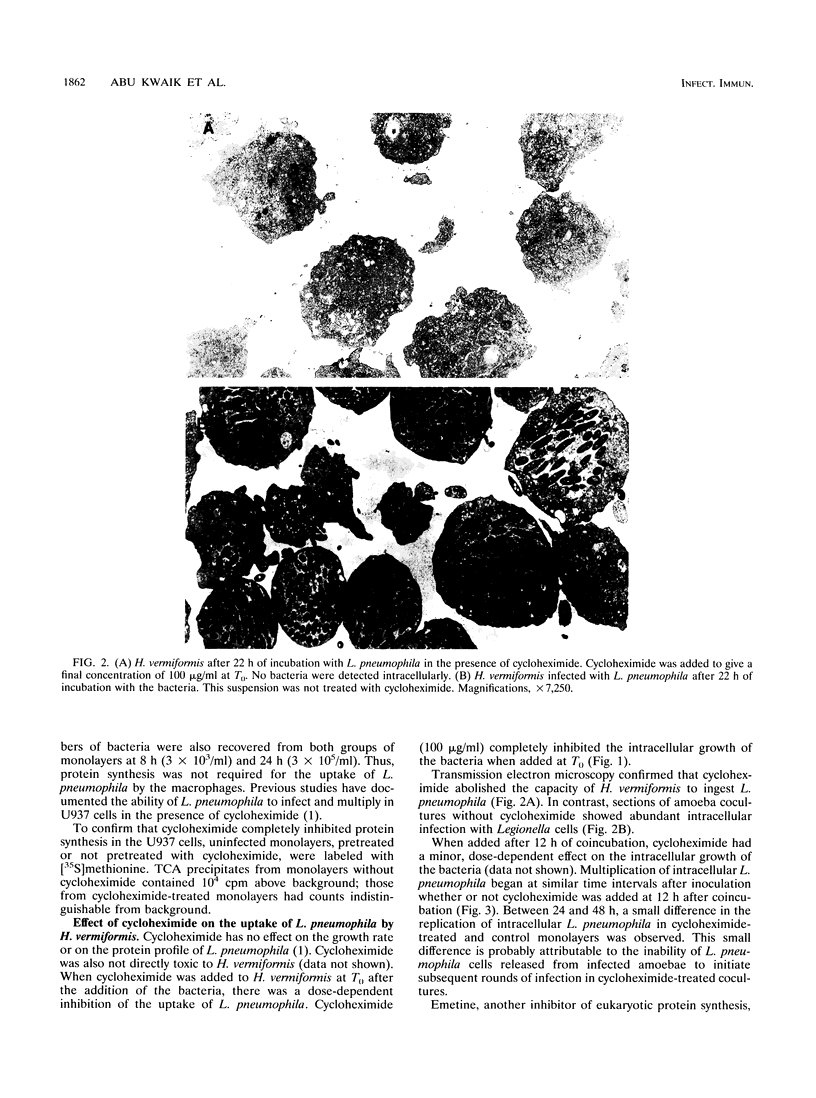
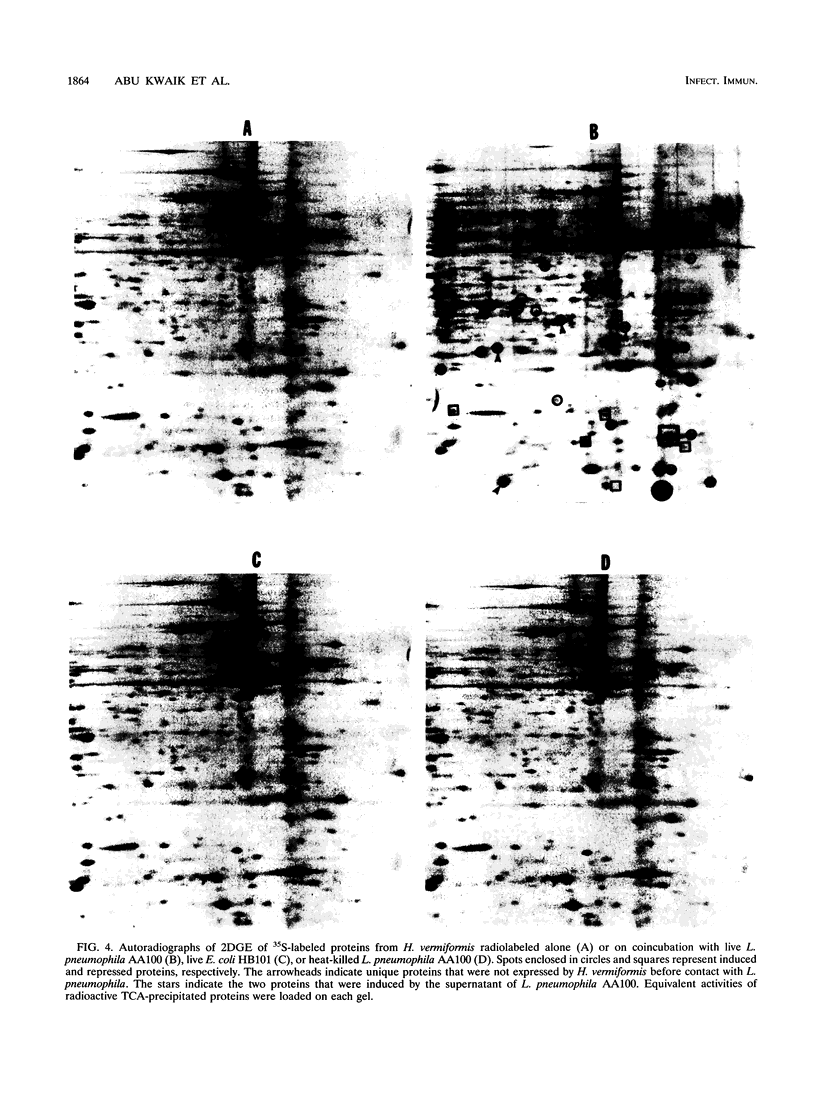
Legionella pneumophila is ingested by both human macrophages and amoebae, and it multiplies within similar endocytic compartments in both eukaryotic species. Inhibitors of eukaryotic protein synthesis, such as cycloheximide and emetine, had no effect on the uptake of L. pneumophila by macrophages but completely abolished ingestion by the amoeba Hartmannella vermiformis. Therefore, host cell protein synthesis is required for the bacterium to infect the amoeba but not human macrophages. To identify proteins expressed by H. vermiformis upon contact with L. pneumophila, we radiolabeled amoebal proteins after contact with bacteria in bacteriostatic concentrations of tetracycline to inhibit bacterial protein synthesis. We analyzed protein expression by two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis and found that 33 amoebal proteins were induced; 12 of these were not detected in resting amoebae. Eleven other amoebal proteins were repressed; four of them became undetectable. In contrast, no phenotypic changes were observed in H. vermiformis upon contact with Escherichia coli or heat-killed L. pneumophila. An isogenic, avirulent variant of L. pneumophila, incapable of infecting either macrophages or amoebae, induced a different pattern of protein expression upon contact with H. vermiformis. Our data showed that amoebae manifested a specific phenotypic response upon contact with virulent L. pneumophila. This phenotypic modulation may be necessary for uptake of the bacteria into an endocytic compartment that permits bacterial survival and multiplication.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abu Kwaik Y., Eisenstein B. I., Engleberg N. C. Phenotypic modulation by Legionella pneumophila upon infection of macrophages. Infect Immun. 1993 Apr;61(4):1320–1329. doi: 10.1128/iai.61.4.1320-1329.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baine W. B. Cytolytic and phospholipase C activity in Legionella species. J Gen Microbiol. 1985 Jun;131(6):1383–1391. doi: 10.1099/00221287-131-6-1383. [DOI] [PubMed] [Google Scholar]
- Barbaree J. M., Fields B. S., Feeley J. C., Gorman G. W., Martin W. T. Isolation of protozoa from water associated with a legionellosis outbreak and demonstration of intracellular multiplication of Legionella pneumophila. Appl Environ Microbiol. 1986 Feb;51(2):422–424. doi: 10.1128/aem.51.2.422-424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman R. F., Fields B. S., Sanden G. N., Volmer L., Meier A., Spika J. S. Association of shower use with Legionnaires' disease. Possible role of amoebae. JAMA. 1990 Jun 6;263(21):2924–2926. [PubMed] [Google Scholar]
- Cianciotto N. P., Fields B. S. Legionella pneumophila mip gene potentiates intracellular infection of protozoa and human macrophages. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5188–5191. doi: 10.1073/pnas.89.11.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan J. W., Baskerville A., Ashworth L. A. Separation of Legionella pneumophila proteases and purification of a protease which produces lesions like those of Legionnaires' disease in guinea pig lung. J Gen Microbiol. 1986 Jun;132(6):1565–1574. doi: 10.1099/00221287-132-6-1565. [DOI] [PubMed] [Google Scholar]
- Feeley J. C., Gibson R. J., Gorman G. W., Langford N. C., Rasheed J. K., Mackel D. C., Baine W. B. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J Clin Microbiol. 1979 Oct;10(4):437–441. doi: 10.1128/jcm.10.4.437-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields B. S., Fields S. R., Loy J. N., White E. H., Steffens W. L., Shotts E. B. Attachment and entry of Legionella pneumophila in Hartmannella vermiformis. J Infect Dis. 1993 May;167(5):1146–1150. doi: 10.1093/infdis/167.5.1146. [DOI] [PubMed] [Google Scholar]
- Fields B. S., Nerad T. A., Sawyer T. K., King C. H., Barbaree J. M., Martin W. T., Morrill W. E., Sanden G. N. Characterization of an axenic strain of Hartmannella vermiformis obtained from an investigation of nosocomial legionellosis. J Protozool. 1990 Nov-Dec;37(6):581–583. doi: 10.1111/j.1550-7408.1990.tb01269.x. [DOI] [PubMed] [Google Scholar]
- Fliermans C. B., Cherry W. B., Orrison L. H., Thacker L. Isolation of Legionella pneumophila from nonepidemic-related aquatic habitats. Appl Environ Microbiol. 1979 Jun;37(6):1239–1242. doi: 10.1128/aem.37.6.1239-1242.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C. H., Fields B. S., Shotts E. B., Jr, White E. H. Effects of cytochalasin D and methylamine on intracellular growth of Legionella pneumophila in amoebae and human monocyte-like cells. Infect Immun. 1991 Mar;59(3):758–763. doi: 10.1128/iai.59.3.758-763.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade J. E., Shepard C. C. Virulent to avirulent conversion of Legionnaires' disease bacterium (Legionella pneumophila)--its effect on isolation techniques. J Infect Dis. 1979 Jun;139(6):707–711. doi: 10.1093/infdis/139.6.707. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Payne N. R., Horwitz M. A. Phagocytosis of Legionella pneumophila is mediated by human monocyte complement receptors. J Exp Med. 1987 Nov 1;166(5):1377–1389. doi: 10.1084/jem.166.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman E., Jiwa A. H., Engleberg N. C., Eisenstein B. I. Growth of Legionella pneumophila in a human macrophage-like (U937) cell line. Microb Pathog. 1988 Aug;5(2):87–95. doi: 10.1016/0882-4010(88)90011-3. [DOI] [PubMed] [Google Scholar]
- Rowbotham T. J. Current views on the relationships between amoebae, legionellae and man. Isr J Med Sci. 1986 Sep;22(9):678–689. [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- VanBogelen R. A., Neidhardt F. C. Ribosomes as sensors of heat and cold shock in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5589–5593. doi: 10.1073/pnas.87.15.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadowsky R. M., Butler L. J., Cook M. K., Verma S. M., Paul M. A., Fields B. S., Keleti G., Sykora J. L., Yee R. B. Growth-supporting activity for Legionella pneumophila in tap water cultures and implication of hartmannellid amoebae as growth factors. Appl Environ Microbiol. 1988 Nov;54(11):2677–2682. doi: 10.1128/aem.54.11.2677-2682.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadowsky R. M., Wilson T. M., Kapp N. J., West A. J., Kuchta J. M., States S. J., Dowling J. N., Yee R. B. Multiplication of Legionella spp. in tap water containing Hartmannella vermiformis. Appl Environ Microbiol. 1991 Jul;57(7):1950–1955. doi: 10.1128/aem.57.7.1950-1955.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]






