Abstract
β-catenin and plakoglobin (γ-catenin) are homologous molecules involved in cell adhesion, linking cadherin receptors to the cytoskeleton. β-catenin is also a key component of the Wnt pathway by being a coactivator of LEF/TCF transcription factors. To identify novel target genes induced by β-catenin and/or plakoglobin, DNA microarray analysis was carried out with RNA from cells overexpressing either protein. This analysis revealed that Nr-CAM is the gene most extensively induced by both catenins. Overexpression of either β-catenin or plakoglobin induced Nr-CAM in a variety of cell types and the LEF/TCF binding sites in the Nr-CAM promoter were required for its activation by catenins. Retroviral transduction of Nr-CAM into NIH3T3 cells stimulated cell growth, enhanced motility, induced transformation, and produced rapidly growing tumors in nude mice. Nr-CAM and LEF-1 expression was elevated in human colon cancer tissue and cell lines and in human malignant melanoma cell lines but not in melanocytes or normal colon tissue. Dominant negative LEF-1 decreased Nr-CAM expression and antibodies to Nr-CAM inhibited the motility of B16 melanoma cells. The results indicate that induction of Nr-CAM transcription by β-catenin or plakoglobin plays a role in melanoma and colon cancer tumorigenesis, probably by promoting cell growth and motility.
Keywords: Nr-CAM, β-catenin, plakoglobin, melanoma, motility, colon carcinoma
β-catenin and plakoglobin (γ-catenin) are homologous proteins of the armadillo family that play a major role in cell adhesion by linking cadherin receptors to the actin cytoskeleton (Kemler 1993; Ben-Ze'ev and Geiger 1998; Zhurinsky et al., 2000a). β-catenin is also a key component of the Wnt signaling pathway, fulfilling a critical role during embryonic development (Willert and Nusse 1998). The signaling function of β-catenin in the canonical Wnt pathway is mediated by its interaction with DNA binding proteins of the LEF/TCF family, forming a bipartite complex that activates target gene transcription (Wodarz and Nusse 1998). In addition to its role in embryogenesis, β-catenin-LEF/TCF signaling is also involved in the development of a variety of human tumors (Peifer 1997; Morin 1999; Zhurinsky et al. 2000a; Polakis 2000). In normal cells, the transcriptional activity of β-catenin is constitutively inhibited by its targeting to rapid proteolytic degradation via a multiprotein complex consisting of the scaffolding proteins APC and axin/conductin and the serine–threonine kinase GSK3β that phosphorylates the N terminus of β-catenin (Peifer and Polakis 2000). Phosphorylated β-catenin is recognized by an E3 ubiquitin ligase complex containing β-TrCP that ubiquitilates β-catenin leading to its degradation by the proteasome (Hart et al. 1999; Liu et al. 1999; Sadot et al. 2000). Disruption of this complex by mutations in either APC (Polakis 2000), axin–conductin (Liu et al. 2000; Satoh et al. 2000), or the N-terminal GSK3β phosphorylation motif of β-catenin (Rubinfeld et al. 1997) leads to aberrant accumulation of β-catenin and constitutive activation of β-catenin–LEF/TCF target genes. These include positive regulators of cell proliferation such as cyclin D1 (Shtutman et al. 1999; Tetsu and McCormick 1999), c-myc (He et al. 1998), and WISP-1 (Xu et al. 2000), which may contribute to the oncogenic role of β-catenin.
Plakoglobin, similar to β-catenin, can bind to the axin/APC complex (Hulsken et al. 1994; Kodama et al. 1999), β-TrCP (Sadot et al. 2000), and LEF/TCF factors (Huber et al. 1996; Zhurinsky et al. 2000a; Williams et al. 2000). Plakoglobin also has a transactivation domain (Simcha et al. 1998; Hecht et al. 1999), but its role in Wnt signaling, activation of target genes, and oncogenesis remains controversial. For example, overexpression of a stable β-catenin mutant in the skin of transgenic mice forms hair tumors (Gat et al. 1998), whereas expression of a similar plakoglobin mutant results in decreased hair growth (Charpentier et al. 2000). The plakoglobin binding site on TCF4 is different from that of β-catenin (Miravet et al. 2002) and the plakoglobin–LEF/TCF complex is inefficient in DNA binding (Zhurinsky et al., 2000b; Williams et al. 2000). Finally, earlier reports on the capacity of exogenous plakoglobin to function in Wnt signaling (Karnovsky and Klymkowsky 1995) were attributed, at least in part, to its indirect effect on β-catenin stability, leading to signaling by the endogenous β-catenin (Miller and Moon 1997; Simcha et al. 1998; Klymkowsky et al. 1999).
To identify novel β-catenin target genes and investigate whether plakoglobin can activate such genes, we employed a human renal carcinoma cell line that does not express detectable levels of cadherins and catenins (Simcha et al. 1996). DNA microarray analysis was used to discover genes whose expression was elevated in such cells after stable overexpression of β-catenin or plakoglobin. We identified Nr-CAM, a neuronal cell adhesion molecule (Grumet et al. 1991), as a major target whose expression was dramatically induced by both β-catenin and plakoglobin. We report that the Nr-CAM promoter contains several LEF/TCF binding sites that are required for its optimal activation by β-catenin. We show that Nr-CAM is localized in the membrane and filopodia of non-neuronal cells and demonstrate that retroviral transduction of Nr-CAM into NIH3T3 cells enhances cell migration into a wound introduced in a monolayer. Moreover, such cells proliferate faster, form large foci in confluent cultures, and, when injected into nude mice, produce rapidly developing tumors. In addition, we found high levels of Nr-CAM and LEF-1 in mouse and human melanoma cells and human colon cancer tissue, but not in normal colon tissue and melanocytes. The results suggest that Nr-CAM is a target gene of the β-catenin and plakoglobin-LEF/TCF complex that contributes to oncogenesis.
Results
Nr-CAM expression is induced by β-catenin and plakoglobin and Nr-CAM is localized in membrane protrusions
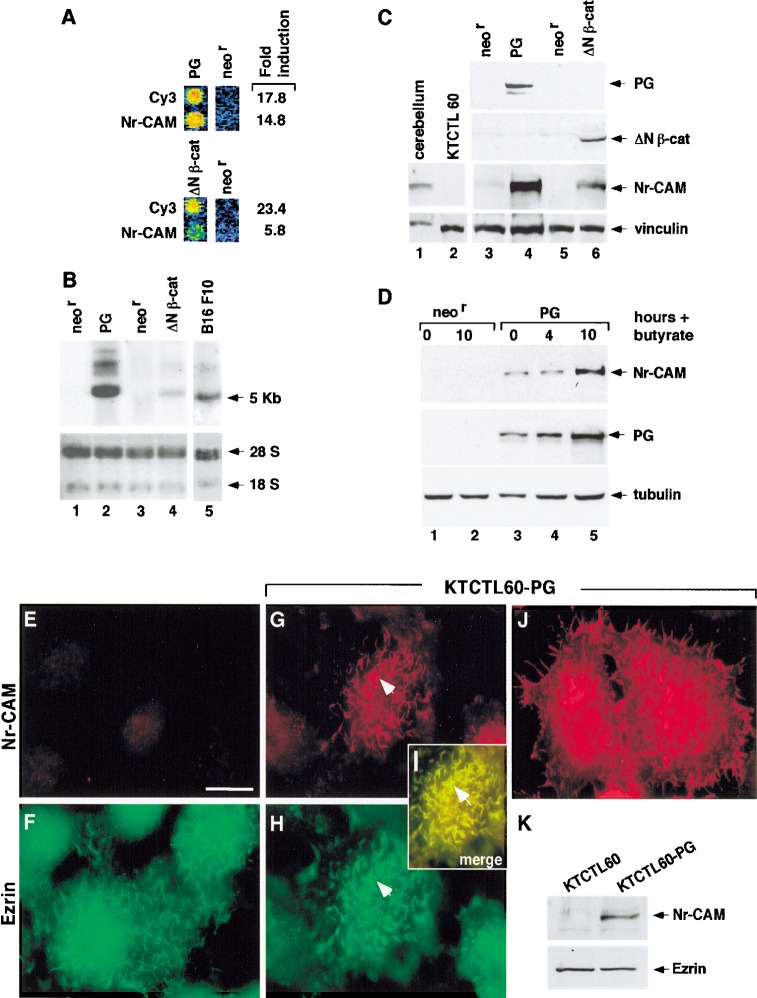
To identify novel target genes that are induced by increased expression of β-catenin or plakoglobin, we used a human renal carcinoma cell line (KTCTL60) that does not express detectable levels of cadherins and catenins (Simcha et al. 1996). RNA from a mixture of KTCTL60 clones stably transfected with either plakoglobin, a stable mutant of β-catenin (ΔN57; Salomon et al. 1997), or the neor gene, was used to prepare cDNA that was hybridized to the UniGem 1 cDNA microarray of Incyte containing 10,000 ESTs. This analysis revealed that expression of Nr-CAM, a cell adhesion molecule expressed in the nervous system (Grumet et al. 1991), was the most extensively elevated in both plakoglobin and β-catenin transfected cells (Fig. 1A). The induction of Nr-CAM expression was confirmed by Northern blot hybridization of RNA from individual clones stably expressing either plakoglobin or β-catenin (Fig. 1B). A similar Nr-CAM RNA pattern was obtained in KTCTL60 cells transfected with catenins and B16 F10 melanoma (Fig. 1B, lane 5) after hybridization with cDNA probes to the extracellular or the intracellular domains of Nr-CAM (Fig. 1B; data not shown). The elevation in Nr-CAM protein in cells expressing plakoglobin and β-catenin was confirmed by Western blot analysis with an antibody against Nr-CAM (Fig. 1C). Interestingly, only clones stably expressing low levels of ΔNβ-catenin could be isolated (high levels were toxic), whereas clones expressing high levels of plakoglobin were easily obtained. The lower level of Nr-CAM expression in ΔNβ-catenin transfected cells may therefore result from a suboptimal level of β-catenin expression for signaling in these cells. In cells treated with the histone deacetylase inhibitor sodium butyrate, which induces the expression of transfected plakoglobin (Salomon et al. 1997), we observed, as early as 4 h after treatment, an increase in plakoglobin (Fig. 1D, lane 4) that further increased by 10 h (Fig. 1D, lane 5). This was followed by a marked elevation in Nr-CAM (Fig. 1D, lane 5), whereas such treatment did not induce Nr-CAM in control cells (Fig. 1D, lanes 1,2), pointing to an association between increased plakoglobin and Nr-CAM expression. Next, we determined the localization of Nr-CAM in cells transfected with plakoglobin and β-catenin. KTCTL60 cells transfected with plakoglobin or β-catenin (not shown) displayed Nr-CAM in membrane protrusions at the cell periphery (Fig. 1J) and on the apical membrane (Fig. 1G). These structures resembled microvilli and filopodia and colocalized with actin (data not shown) and with the microvilli-associated protein ezrin (Fig. 1F,H,I).
Figure 1.
Induction of Nr-CAM expression by β-catenin and plakoglobin. (A) RNA was prepared from a mixture of three independent human renal carcinoma cell clones each of: cells stably expressing plakoglobin (PG), ΔNβ-catenin, or the neor gene alone. The RNA was tested, after Cy3 (PG and β-cat)- and Cy5 (neor)-labeling (following RT), for the expression of genes using an Incyte DNA microarray containing 10,000 ESTs. Fold induction of Nr-CAM expression compared with the positive (Cy3) control is shown. The blue color indicates no hybridization; the yellow and red area of the spectrum represent strong hybridization. (B) Northern blot hybridization for Nr-CAM using renal carcinoma cells stably expressing the genes described in A. B16 F10 melanoma cells served as positive control for Nr-CAM expression. (C) Western blot analysis for Nr-CAM in the cell clones indicated in A. Mouse cerebellum served as positive control for Nr-CAM and untransfected renal carcinoma cells (KTCTL60) as negative control. (D) KTCTL60 cells stably expressing plakoglobin (PG) and control neorcells were treated for different times with sodium butyrate to enhance the expression of the transgene (PG) and the expression of plakoglobin and Nr-CAM was determined by Western blotting. The levels of 18S and 28S rRNA in B, vinculin in C, and tubulin in D were used as controls for gel loading. (E–J) The localization of Nr-CAM and ezrin in cells stably transfected with plakoglobin. Untransfected (KTCTL60; E,F) and cells stably transfected with plakoglobin (KTCTL60-PG; G–J) were immunostained with anti Nr-CAM antibodies (E,G,J) and doubly stained with an antibody for ezrin (F,H). (I) Merged image of ezrin and Nr-CAM staining. The cells in J were stained for Nr-CAM and the image focuses at the cell periphery close to the substrate. (K) Expression of ezrin and Nr-CAM was determined by Western blotting. Note colocalization of Nr-CAM with ezrin-containing cellular protrusions (arrowhead). Bar in E, 10 μm.
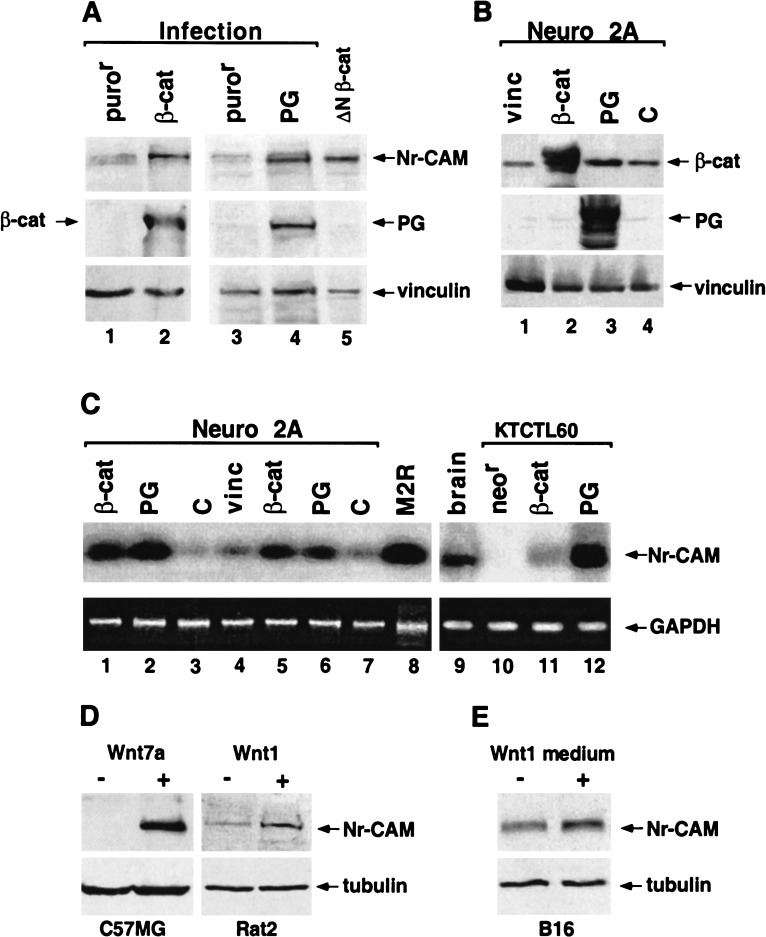
To confirm that the induction of Nr-CAM by catenins did not result from clonal variation in stable transfections, we employed retrovirus transduction of plakoglobin and β-catenin into KTCTL60 cells. This method produced pools of cells infected by each catenin that displayed elevated Nr-CAM expression (Fig. 2A, lanes 2,4). Next, we transiently transfected Neuro 2A cells with either plakoglobin and β-catenin, or vinculin, and an empty vector as control (Fig. 2B). RT-PCR analysis revealed that expression of both β-catenin (Fig. 2C, cf. lanes 1,5 and 3,7) and plakoglobin (Fig. 2C, lanes 2,6), but not vinculin (Fig. 2C, lane 4), dramatically induced Nr-CAM RNA in Neuro 2A cells. KTCTL60 cells transfected with both catenins (Fig. 2C, cf. lanes 11,12 and 10) and mouse brain tissue (Fig. 2C, lane 9) were used as positive controls. Interestingly, melanoma cells also expressed high levels of Nr-CAM RNA (Figs. 1B, lane 5, and 2C, lane 8). Nr-CAM expression was also elevated by various Wnt proteins in different cell types (by Wnt7A in C57MG, Wnt1 in Rat2 cells, Fig. 2D) and by Wnt1-conditioned medium in B16 melanoma (Fig. 2E), indicating that Nr-CAM expression is regulated by Wnt signaling. These results demonstrate that various Wnt proteins, or an increase in either plakoglobin or β-catenin, can induce the expression of Nr-CAM in different cell types.
Figure 2.
Retroviral transduction and transient transfection of β-catenin or plakoglobin induce the expression of Nr-CAM in different cell types. (A) KTCTL60 cells were infected with retroviruses expressing either a stable, HA-tagged β-catenin mutant S33Y (β-cat; lane 2), HA-tagged plakoglobin (PG; lane 4), or the puror gene (lanes 1,3). Cells stably expressing ΔNβ-catenin (lane 5) served as positive control. Expression of Nr-CAM and HA-tagged proteins was determined by Western blotting. The level of vinculin served as loading control. (B) Expression of the transfected genes in Neuro 2A cells was verified by Western blotting with antibodies to β-catenin, plakoglobin and vinculin. (C) Expression of Nr-CAM RNA in Neuro 2A cells transiently transfected either with a stable β-catenin mutant S33Y (β-cat; lanes 1,5), plakoglobin (PG; lanes 2,6), vinculin (vinc; lane 4), or untrasfected cells (c; lanes 3,7) was determined by RT-PCR and compared with that of B16 M2R melanoma cells (lane 8), mouse brain tissue (lane 9), and KTCTL60 cells transfected with β-catenin (lane 11), plakoglobin (lane 12), or the neor gene (lane 10). (D) Expression of Nr-CAM was also determined in C57MG and C57MG-Wnt7A and Rat2 and Rat2-Wnt-1 cells. (E) B16 M2R cells were incubated for 24 h with control medium and conditioned medium from Rat2–Wnt-1 cells and the level of Nr-CAM was determined by Western blotting.
Characterization of the Nr-CAM promoter and identification of LEF/TCF binding sites in the Nr-CAM promoter
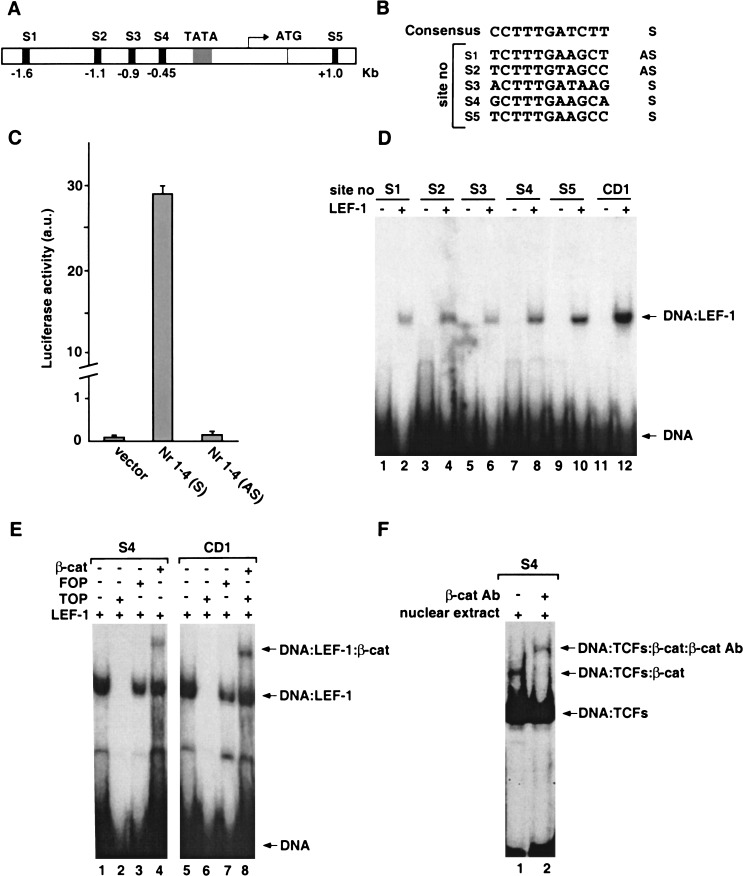
Based on published DNA sequences of full-length human Nr-CAM cDNA from several laboratories and sequence data available from the Human Genome project, we identified the Nr-CAM promoter that contains a TATA box 25 bp upstream of the transcription start site (Fig. 3A). Analysis of the Nr-CAM locus revealed four putative LEF/TCF binding sequences in the promoter region and one in the first intron (Fig. 3A,B). We cloned by genomic PCR of HeLa cell DNA a 1.8-kb fragment of the Nr-CAM promoter and inserted it in both sense (Nr 1–4 S) and antisense (Nr 1–4 AS) orientation into the pA3Luc plasmid. Transient transfection of the reporter plasmid containing the Nr-CAM promoter in the sense orientation into 293T cells resulted in an about 70-fold higher level of luciferase activity compared with either the empty reporter plasmid or the antisense construct (Fig. 3C). This indicates that the cloned Nr-CAM genomic fragment contained the functional Nr-CAM promoter. Using electrophoretic mobility shift analysis (Zhurinsky et al. 2000b) we examined whether the 32P-labeled LEF/TCF sites in the Nr-CAM promoter bind to in vitro-translated LEF-1 and found that when each of the five DNA sequences were incubated with LEF-1, a single LEF-1–DNA band was observed (Fig. 3D, lanes 2,4,6,8,10), similar to the one obtained with the LEF-1 binding site of the cyclin D1 promoter (Fig. 3D, lane 12; Shtutman et al. 1999). The assembly of such complexes was inhibited by excess unlabeled consensus LEF/TCF binding sequences (Fig. 3E, TOP, cf. lanes 1 and 2), but not by a mutant DNA sequence (Fig. 3E, FOP, lane 3). When in vitro-translated LEF-1 and β-catenin were incubated together with LEF/TCF site number 4 (S4) of the Nr-CAM promoter, a ternary complex was detected between this DNA, LEF-1, and β-catenin (Fig. 3E, lane 4). The LEF/TCF site of the cyclin D1 promoter that served as control (Fig. 3E, lanes 5–8) and the other LEF/TCF sites in the Nr-CAM promoter (data not shown) displayed a similar behavior. Furthermore, incubation of nuclear extracts from human SW480 colon cancer cells, which are rich in LEF/TCF-β-catenin complexes (Morin et al. 1997), with 32P-labeled S4 of the Nr-CAM promoter resulted in the formation of a ternary complex consisting of S4, LEF/TCF, and β-catenin (Fig. 3F, lane 1). The presence of β-catenin in this complex was demonstrated by its efficient supershift by an antibody against β-catenin (Fig. 3F, lane 2).
Figure 3.
The Nr-CAM gene promoter contains several LEF-1 binding sites that can complex with LEF-1 and β-catenin. Schematic presentation of LEF-1 binding sites in the Nr-CAM gene promoter (A) and their nucleotide sequence compared to the consensus LEF/TCF binding sequence (B). (C) Activation of a synthetic 1.8-kb Nr-CAM promoter reporter containing the sense (S), but not the antisense (AS), sequence in 293T cells. (D) Binding of the different 32P-labeled LEF-1 sites in the Nr-CAM promoter to in vitro-transcribed–translated LEF-1 determined by the DNA mobility shift assay. The LEF-1 site of the cyclin D1 promoter (CD1) served as positive control. (E) Ternary complex formation by the LEF-1 binding site no. 4 (S4) of the Nr-CAM promoter with in vitro-translated LEF-1 and β-catenin was determined as in D. (F) Nuclear extracts from SW480 colon cancer cells displaying constitutive β-catenin-LEF/TCF signaling were incubated with 32P-labeled LEF-1 site no. 4 (S4) and analyzed as in D, with (lane 2), or without (lane 1) incubation with anti β-catenin antibody. Note the supershift in the band representing the ternary complex in the presence of anti β-catenin antibody.
LEF/TCF-dependent activation of the Nr-CAM promoter by β-catenin and plakoglobin
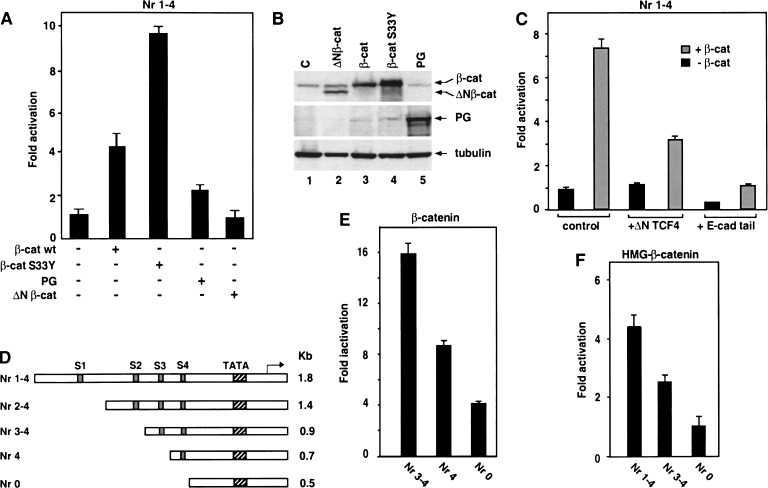
To determine whether β-catenin and plakoglobin can activate the Nr-CAM promoter, the reporter plasmid containing the Nr-CAM promoter (Fig. 3C) was cotransfected with either β-catenin or plakoglobin into 293T cells and its activity was determined (Fig. 4A). The results presented in Figure 4A demonstrate that β-catenin S33Y activates the Nr-CAM promoter about 10-fold, whereas plakoglobin was much weaker in this capacity. The oncogenic stable β-catenin (S33Y) mutant, as expected, was more potent than wild-type β-catenin, plakoglobin, or the mutant ΔNβ-catenin (Fig. 4A), at least in part because of its more efficient accumulation in the transfected cells (Fig. 4B). Activation of the Nr-CAM promoter by β-catenin was reduced by a dominant negative TCF4 construct (Fig. 4C, ΔN TCF4) and blocked when the E-cadherin cytoplasmic domain containing the β-catenin binding site (Sadot et al. 1998; Simcha et al. 2001) was cotransfected (Fig. 4C, E-cad tail). The involvement of the various LEF/TCF binding sites in Nr-CAM promoter activation was analyzed in deletion mutants progressively removing these sites (Fig. 4D). Activation of the 1.8-kb Nr-CAM promoter by cotransfected β-catenin showed no significant change when the first two TCF sites were removed (data not shown). Removal of TCF site number 3 resulted in a reduction in promoter activation by β-catenin (Fig. 4E, Nr 4) that further decreased when TCF S4 was also deleted (Fig. 4E, Nr 0). The importance of LEF/TCF sites for Nr-CAM promoter activation by β-catenin was also supported by the use of a dominant positive chimeric plasmid containing the C-terminal β-catenin transactivation domain linked to the DNA binding domain of LEF-1 (Fig. 4F). Activation of the Nr-CAM promoter by this chimeric molecule was reduced when either one or all four TCF sites were deleted (Fig. 4F). These results demonstrate that β-catenin can activate the Nr-CAM promoter and this activation involves the LEF/TCF sites in the Nr-CAM promoter.
Figure 4.
. Activation of the Nr-CAM promoter by β-catenin involves LEF-1 binding sites. (A) 293T cells were transfected with the Nr-CAM promoter reporter plasmid containing LEF-1 sites 1–4 (Nr 1–4) and with either wild-type β-catenin, the β-catenin mutant S33Y, ΔNβ-catenin, or plakoglobin (PG), and luciferase activity determined. (B) The levels of the proteins transfected in A were determined by Western blotting. (C) The Nr-CAM reporter plasmid was cotransfected with a dominant negative TCF4 (ΔNTCF4), or with a plasmid expressing the cytoplasmic domain of E-cadherin (E-cad tail), and luciferase activity determined. (D) Schematic representation of Nr-CAM promoter constructs containing different LEF-1 sites. The activities of these Nr-CAM promoter constructs was determined in cells cotransfected with β-catenin (E), or with a chimeric dominant positive construct containing the C terminus of β-catenin and the DNA binding domain of LEF-1 (HMG-β-catenin; F). Note the weak ability of plakoglobin and inability of ΔNβ-catenin to activate the Nr-CAM promoter in 293T cells and the dependence on LEF-1 sites of Nr-CAM promoter activation by the dominant positive HMG-β-catenin chimera.
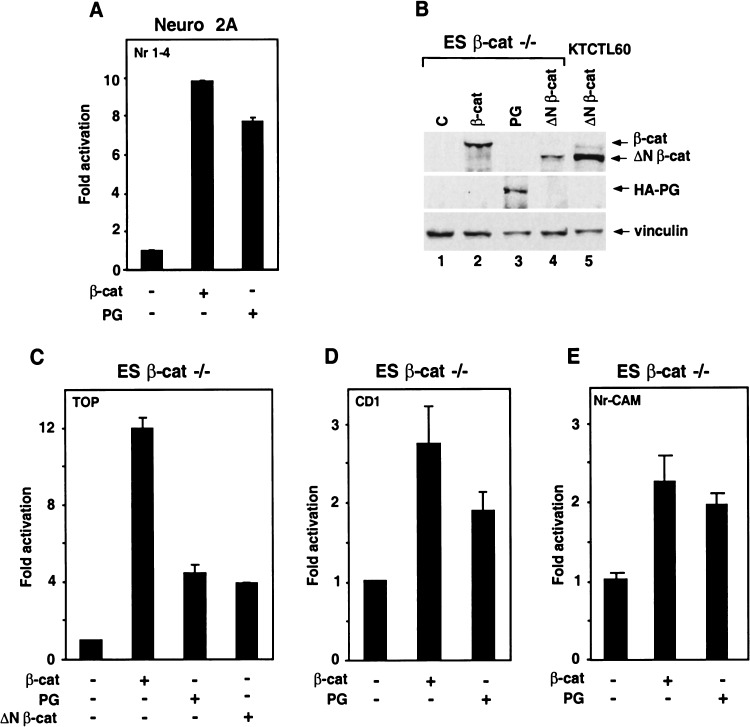
Because activation of the Nr-CAM promoter by plakoglobin in 293T cells was weak (Fig. 4A), and previous studies suggested that induction of LEF/TCF-dependent transactivation in plakoglobin-transfected cells may result from its indirect effect on β-catenin stability (Simcha et al. 1998; Zhurinsky et al. 2000a), we examined whether plakoglobin-mediated transcription occurs in Neuro 2A and in cells lacking β-catenin. First, we demonstrated that plakoglobin can effectively activate the Nr-CAM promoter in Neuro 2A cells (Fig. 5A). Next, we employed the recently generated β-catenin-null ES cells (Huelsken et al. 2000) and found that plakoglobin can activate a LEF/TCF-responsive reporter (though to a lesser extent than β-catenin; Fig. 5C) and also the promoters of cyclin D1 (Fig. 5D) and Nr-CAM (Fig. 5E). Expression of the various transfected proteins is shown in Figure 5B (lanes 1–4). Taken together, these results demonstrate the ability of plakoglobin to activate LEF/TCF-dependent transcription (including the promoters of target genes such as cyclin D1 and Nr-CAM) in the absence of β-catenin.
Figure 5.
Plakoglobin can activate the Nr-CAM promoter in Neuro 2A cells and the cyclin D1 and Nr-CAM promoters in β-catenin−/− ES cells. (A) Neuro 2A cells were transfected with the Nr-CAM promoter plasmid together with either β-catenin (β-cat) or plakoglobin (PG) and luciferase activity was determined. (B,C) β-catenin−/− ES cells were transfected with the synthetic LEF/TCF reporter plasmid TOPFLASH (TOP) and either with β-catenin, plakoglobin or ΔNβ-catenin, and luciferase activity (C) and expression of the transfected proteins by Western blotting (B) were determined. (D,E) The abilities of β-catenin and plakoglobin to activate a cyclin D1 (CD1) promoter reporter (D) and the Nr-CAM promoter reporter (E) in β-catenin-null ES cells were determined. Note that plakoglobin can efficiently activate the Nr-CAM promoter in Neuro 2A cells, and the CD1 and Nr-CAM promoters and TOPFLASH in β-catenin−/− ES cells.
Expression of Nr-CAM in NIH3T3 cells increases motility and confers cell transformation and tumorigenesis
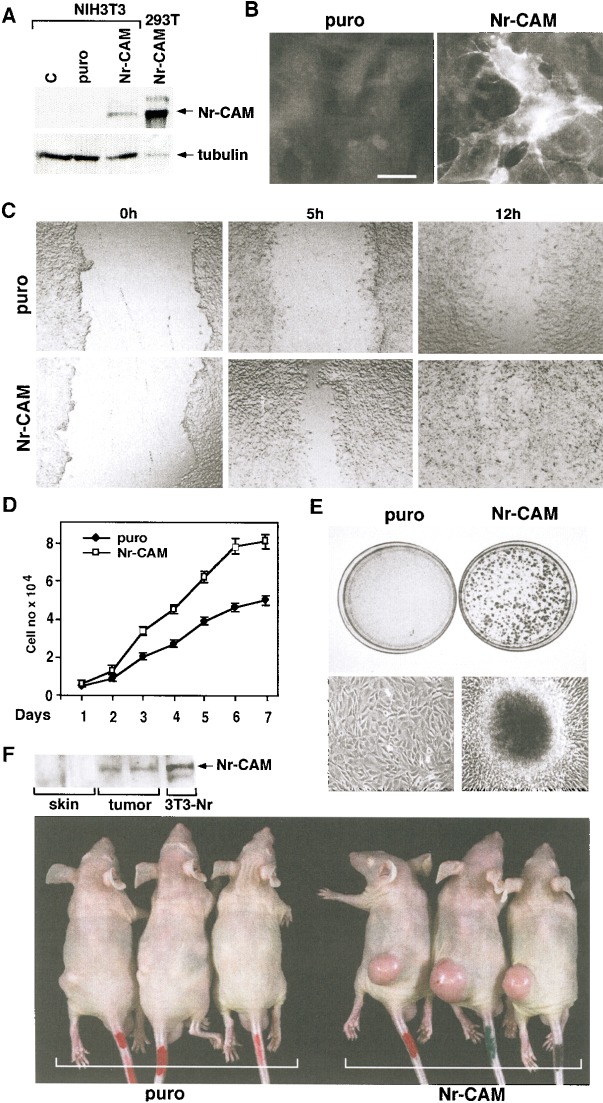
To examine the effects of elevated Nr-CAM expression on cell behavior, NIH3T3 cells that lacked Nr-CAM (Fig. 6A) were infected with a retrovirus expressing Nr-CAM and the puror gene, or with the puror gene alone, and puromycin-resistant cultures were isolated. Retrovirus-mediated expression of Nr-CAM in NIH3T3 cells was much lower (and thus probably more representative of the physiological level) than in 293T cells transiently transfected with a CMV-driven plasmid expressing Nr-CAM (Fig. 6A). The localization of Nr-CAM in these 3T3 cells was mostly in the membrane and membrane protrusions (Fig. 6B). Most significantly, when an artificial wound was introduced into a confluent monolayer, NIH3T3 cells expressing Nr-CAM (Fig. 6C, bottom) were much faster in closing the wound than the puror control NIH3T3 cells (Fig. 6C, top).
Figure 6.
Retroviral infection of NIH3T3 cells with Nr-CAM enhances their capacity to migrate and close an artificial wound and confers tumorigenesis. (A) NIH3T3 cells were infected with a retrovirus coding for Nr-CAM and the puror gene, or the puror vector alone. Puromycin resistant cultures were isolated and expression of Nr-CAM compared by Western blotting to 293T cells transiently transfected with Nr-CAM. (B) Immunofluorescence detection of Nr-CAM localization in retrovirus infected NIH3T3 cells. (C) An artificial wound was introduced with a micropipette tip into confluent cultures of puror control and Nr-CAM infected NIH3T3 cells and the closure of this wound was followed for different times. (D) The proliferation of NIH3T3 cells expressing puror and Nr-CAM was compared. (E) Nr-CAM expressing 3T3 cells formed many large foci visualized at low magnification by Giemsa staining of the cells (top), or at higher magnification in live cultures (bottom). (F) Retrovirus-mediated transduction of Nr-CAM conferred the ability to form fast growing tumors when 106 cells were injected subcutaneously into nude mice. The picture was taken 14 d after injection and expression of Nr-CAM in tumor tissue and in normal skin and muscle was determined by Western blotting with anti Nr-CAM antibodies.
In addition to enhanced motility, Nr-CAM expressing NIH3T3 cells displayed a faster growth rate (Fig. 6D) and formed numerous large foci (Fig. 6E) after reaching confluence, indicative of the transforming capacity of Nr-CAM. Furthermore, injection of NIH3T3 cells expressing Nr-CAM into nude mice resulted in rapidly growing tumors in all the animals (Fig. 6F), whereas the puror NIH3T3 cells either formed no tumors or occasionally produced small tumors (Fig. 6F). The tumors formed by NIH3T3–Nr-CAM cells maintained the expression of Nr-CAM as indicated by Western blot analysis (Fig. 6F). These results suggest that relatively low levels of Nr-CAM expression (Fig. 6A) have dramatic effects on motility and growth of NIH3T3 cells and can cause cell transformation and tumorigenesis.
Nr-CAM expression and β-catenin signaling in melanoma and colon cancer
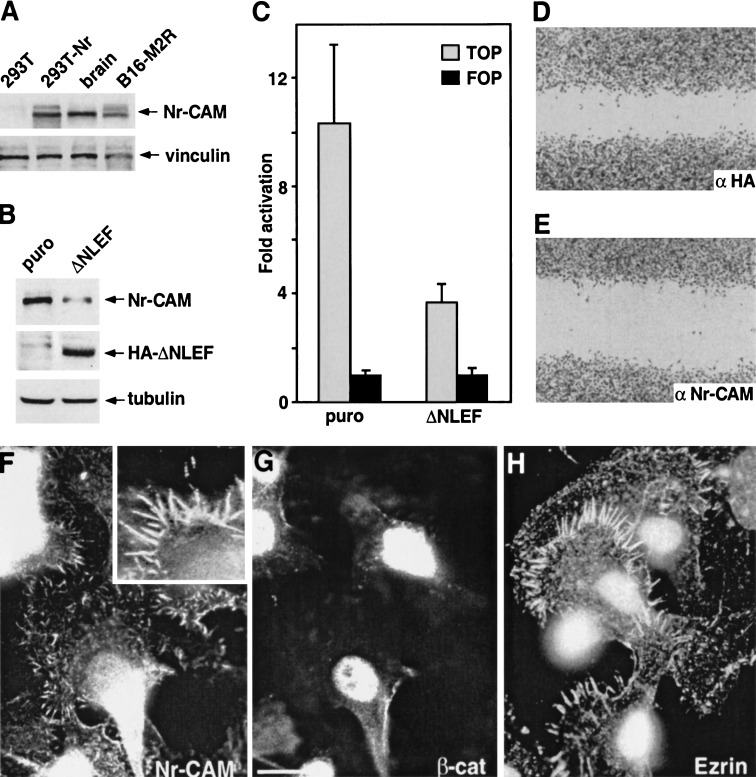
Because Nr-CAM is expressed mostly by cells of the nervous system (Grumet 1997), and β-catenin signaling is often elevated in melanoma cells that are derived from the neural crest (Rubinfeld et al. 1997; Rimm et al. 1999; Murakami et al. 2001), we investigated the expression of Nr-CAM in human and mouse melanoma cells and in normal melanocytes. We found that mouse B16 melanoma cells express high levels of Nr-CAM RNA (Figs. 1B, lane 5, and 2B, lane 8) and protein (Fig. 7A). High levels of Nr-CAM were also displayed in several sub-lines of B16 melanoma (Figs. 1B and 7A) and K1375 mouse melanoma (data not shown). In B16 F10 melanoma and its derivative B16 M2R, Nr-CAM was localized in conspicuous membrane protrusions, mostly filopodia and microvilli (Fig. 7F) that were also stained by an antibody against ezrin (Fig. 7H). Interestingly, double fluorescent staining revealed that B16 M2R cells display strong nuclear β-catenin (Fig. 7G) and constitutive β-catenin-LEF/TCF transactivation (Fig. 7C), indicative of the signaling activity of β-catenin in these cells. We examined the possibility that the high Nr-CAM levels in these melanoma cells result from β-catenin-LEF/TCF transcription, by retroviral transduction of a dominant negative LEF-1 (ΔNLEF) and analysis of Nr-CAM expression in such cells (Fig. 7B). The results demonstrated that melanoma cells expressing ΔNLEF displayed decreased β-catenin-LEF-mediated transcription (Fig. 7C) and produced lower levels of Nr-CAM compared with control puror cells (Fig. 7B), suggesting that inhibition of constitutive β-catenin signaling by ΔNLEF reduces the expression of endogenous Nr-CAM, a target gene of β-catenin in melanoma cells.
Figure 7.
Melanoma cells express elevated Nr-CAM levels in membrane protrusions and nuclear β-catenin, and ΔNLEF reduces β-catenin/TCF activity and Nr-CAM expression in these cells. (A) Levels of Nr-CAM were determined by Western blotting in B16 M2R melanoma, brain tissue, 293T cells, and 293T cells transfected with Nr-CAM. (B) B16 M2R melanoma cells were infected with a retrovirus coding for dominant negative LEF-1 (ΔNLEF) and puror and expression (in puror cultures) of Nr-CAM and HA-ΔNLEF was detected by Western blotting. (C) β-catenin-TCF-mediated transcription activity in puror control and ΔNLEF cultures was determined by cotransfection of TOPFLASH (TOP) or the mutant FOPFLASH (FOP). The ratio between luciferase activity with TOP vs. FOP, after normalizing for transfection efficiency with co-transfected β-galactosidase, is presented. (D,E) The ability of B16 M2R melanoma to close a wound introduced in a monolayer was determined in cultures incubated for 14 h with a 1:20 dilution of polyclonal antibodies to HA (D) or Nr-CAM (E). (F) Endogenous Nr-CAM localized to membrane protrusions in B16 M2R cells that displayed nuclear β-catenin by double immunofluorescence staining (G). The localization of Nr-CAM was similar to that of ezrin, a marker of microvilli (H).
To address the possible role of Nr-CAM in melanoma cell motility, a wound was introduced in cultures of melanoma cells and their ability to close the wound in the presence of an antibody to the extracellular domain of Nr-CAM was determined. The results shown in Figure 7, D and E, demonstrate that the extent of wound closure was reduced significantly in B16 melanoma cells incubated with anti Nr-CAM antibodies (Fig. 7E) compared with cells incubated with control anti HA antibodies (Fig. 7D).
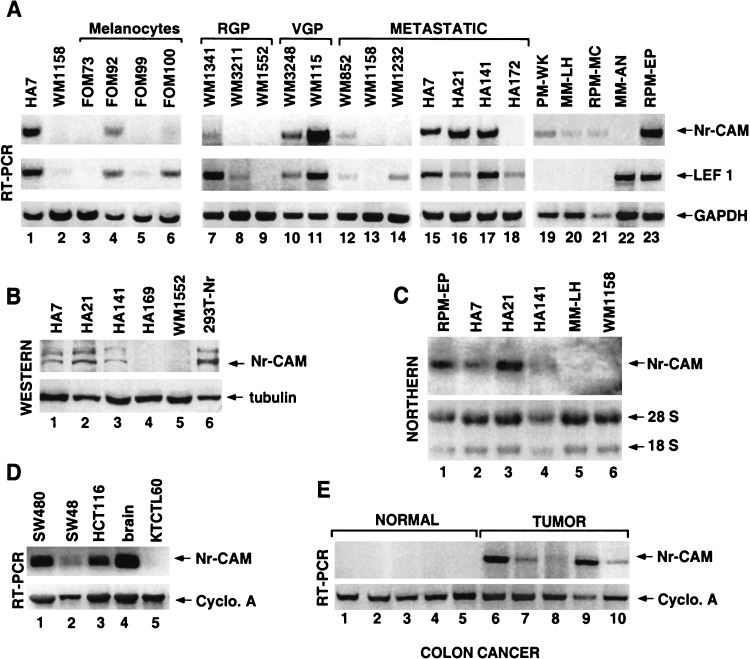
In addition to high Nr-CAM expression levels in mouse melanoma cells, we also found in a series of human cell lines established from malignant melanoma at various phases of melanoma development increased levels of Nr-CAM RNA (Fig. 8A,C) and protein (Fig. 8B). Although increased Nr-CAM was observed in a number of malignant melanoma cells (Fig. 8A, lanes 10,11,15–17,23, and 8B,C, lanes 1–3), this was not observed in several melanocyte cell lines (Fig. 8A, lanes 3–6) and in primary melanoma of the radial growth phase (Fig. 8A, lanes 7–9, RGP). Moreover, the human melanoma cells displaying increased Nr-CAM expression also showed higher LEF-1 RNA levels (another β-catenin target gene; Hovanes et al. 2001; Fig. 8A) and nuclear β-catenin staining, whereas melanocytes showed no nuclear β-catenin (data not shown). Furthermore, injection of several melanoma cell lines into nude mice demonstrated that cells expressing both high levels of LEF-1 and Nr-CAM formed rapidly developing tumors (Table 1, WM115 and RPM-EP), but those lacking (WM3211 and WM1552) or expressing very low levels of Nr-CAM (WM 1341) did not form tumors even a long time after injection.
Figure 8.
Expression of Nr-CAM is increased in human malignant melanoma and colon carcinoma tissue and cell lines. (A) Expression of Nr-CAM, LEF-1, and GAPDH, which served as control, was determined by RT-PCR in human melanocytes and human melanoma cell lines from different sources and stages of melanoma development, including the radial growth phase (RGP), vertical growth phase (VGP), and different metastatic loci (METASTATIC; see Materials and Methods). The level of Nr-CAM was also determined in some of these cell lines by Western (B) or Northern (C) blotting. (D) Nr-CAM levels in human colon cancer cell lines and (E) colon carcinoma and normal colon tissue samples was determined by RT-PCR using cyclophilin A as loading control. Note that whereas primary melanocytes and melanoma from the radial growth phase display almost no Nr-CAM expression, several metastatic melanoma cell lines show high levels of both Nr-CAM and LEF-1 expression. Also, note that although Nr-CAM was detected in all colon tumor tissue samples and cell lines, it was not detected in normal colon tissue.
Table 1.
Tumor formation in nude mice by human melanoma cell lines
| Cell
line
|
WM1552
|
WM3211
|
WM1341
|
WM115
|
RPM-EP
|
|---|---|---|---|---|---|
| Tumor Ø | 0 | 0 | 0 | 0.4 ± 0.2 cm | 1.5 ± 0.5 cm |
| Nr-CAM | − | − | +/− | ++ | ++ |
| LEF-1 | − | + | ++ | ++ | ++ |
The tumorigenicity of human melanoma cell lines was determined by s.c. injection of 4 × 106 cells into five nude mice with each cell line. The average tumor diameter 2 wk after injection is shown. The expression of LEF-1 and Nr-CAM was determined by RT-PCR as shown in Fig. 8A. Animals injected with cells that did not form tumors after 2 wk remained tumor-free after 2 mo.
Because excessive activation of β-catenin signaling is characteristic of colon cancer, we also analyzed the expression of Nr-CAM in colon cancer tissue and cell lines and compared it with that of normal colon tissue. The results shown in Figure 8, D and E, demonstrate significant expression of Nr-CAM in the human colon cancer cell lines SW48, SW480, and HCT116 (Fig. 8D), and more importantly also in colon carcinoma tissue samples (Fig. 8E) but no detectable Nr-CAM RNA in normal colon tissue samples (Fig. 8E). Taken together, these results imply that Nr-CAM may play a key role in the oncogenesis of melanoma and colon cancer by being a target gene of the β-catenin-LEF-1 pathway.
Discussion
Aberrant elevation in β-catenin expression is believed to be involved in the development of a variety of human cancers by inducing β-catenin-LEF/TCF-dependent activation of genes contributing to tumor progression (Peifer 1997; Polakis 2000). In this study, we have shown that Nr-CAM, a transmembrane cell adhesion protein mostly expressed in normal cells of the nervous system (Grumet 1997), is a novel target gene of β-catenin and plakoglobin and the increase in its level may promote malignant melanoma and colon cancer development. Using a renal carcinoma cell line that does not express cadherins or catenins (Simcha et al. 1996), we found that the level of Nr-CAM is elevated in these cells by plakoglobin or β-catenin expression. The significance of Nr-CAM induction is underlined by the fact that Nr-CAM was induced by both catenins and to the greatest extent among the 10,000 genes examined in this DNA microarray. This result was obtained by stable or transient transfection, or retroviral transduction of both catenins, in several cell types. Moreover, Nr-CAM expression was found to be high in human melanoma cells and colon cancer tissue that also showed increased levels of nuclear β-catenin and high levels of LEF-1. The induction of Nr-CAM expression by β-catenin and plakoglobin resulted from the ability of both catenins to activate the Nr-CAM promoter in complex with LEF/TCF. Supporting this view was the discovery of several LEF/TCF binding sites in the Nr-CAM promoter that complexed with LEF-1 and β-catenin, and the activation of an Nr-CAM promoter reporter plasmid by β-catenin, plakoglobin, or a dominant positive β-catenin-LEF-1 chimera.
Plakoglobin contains a C-terminal transactivation domain (Simcha et al. 1998; Hecht et al. 1999) and activates the c-myc gene in some cells (Kolligs et al. 2000), but LEF/TCF-signaling in such cells was mostly attributed to the indirect effect of plakoglobin on the stability of endogenous β-catenin (Miller and Moon 1997; Simcha et al. 1998; Klymkowsky et al. 1999). Our results showing that plakoglobin can induce the Nr-CAM and cyclin D1 promoters and a synthetic LEF/TCF-responsive reporter in β-catenin-null ES cells are the first demonstration of the capacity of plakoglobin to transactivate LEF/TCF-responsive genes in mammalian cells lacking β-catenin. Nr-CAM was initially believed to be present exclusively in the nervous system (Grumet 1997), but recent studies indicated that it is expressed in a variety of tissues and cells including endothelial cells (Glienke et al. 2000), pancreatic cells (Dhodapkar et al. 2001), lens fiber cells (More et al. 2001), and other cell types (Wang et al. 1998). A major role attributed to Nr-CAM and other members of the L1 family (L1 and Ng-CAM) is to promote motility. Nr-CAM can enhance neurite outgrowth (Volkmer et al. 1996; Sakurai et al. 1997) and sensory axon guidance by binding to axonin-1 or F11 (Stoeckli et al. 1997; Lustig et al. 1999; Perrin et al. 2001). L1, a close homolog of Nr-CAM expressed in the nervous system (Sakurai et al. 2001), promotes the motility of fibroblasts and melanoma cells by binding to integrins (Mechtersheimer et al. 2001; Voura et al. 2001). Moreover, shedding of the ectodomain of L1 can enhance cell migration on fibronectin and laminin and it was detected in a variety of human carcinoma (Mechtersheimer et al. 2001). Furthermore, we identified several consensus LEF/TCF sites in the L1 gene promoter (M. Conacci-Sorrell, J. Zhurinsky, and A. Ben-Ze'ev, unpubl.), and a recent DNA microarray analysis showed that adenoviral infection of APC into SW480 cells leads to a decrease in L1 RNA levels (Lin et al. 2001). Our results describing enhanced motility in cells expressing retrovirally transduced Nr-CAM and the inhibition of melanoma cell motility in the presence of anti Nr-CAM antibodies are in line with these studies and underscore the regulation of cell migration by L1 family proteins in the nervous system and in fibroblasts. Increased Nr-CAM levels were also correlated with the invasive/metastatic behavior of pancreatic cancer (Dhodapkar et al. 2001) and in glioblastoma (Sehgal et al. 1998). Moreover, antisense Nr-CAM was shown to decrease the tumorigenic capacity of human glioblastoma cells (Sehgal et al. 1999). Analysis of genes whose levels are elevated during the morphogenetic changes involved in new blood vessel formation by endothelial cells discovered Nr-CAM as one of these genes (Aitkenhead et al. 2002). These results are compatible with our study directly demonstrating that retroviral transduction of Nr-CAM is sufficient to significantly enhance the motile properties of NIH3T3 cells and confers tumorigenesis. In melanoma cells, retrovirus transduced 3T3 cells, and in renal carcinoma cells overexpressing catenins, Nr-CAM was localized in filopodia and other membrane protrusions known to be involved in cell motility. Interestingly, in a recent study, human melanoma cells displaying increased β-catenin-LEF-1 signaling were more motile than melanoma cells expressing lower levels of β-catenin and lacking LEF-1 (Murakami et al. 2001). We propose that the increase in Nr-CAM expression in malignant melanoma cells and the enhanced motility, growth, and tumorigenesis of 3T3 cells expressing Nr-CAM contribute to the promotion of tumorigenesis in both cases.
The relevance of increased Nr-CAM expression to cancer is also supported by our finding that human melanoma cells expressing both high LEF-1 and Nr-CAM are highly tumorigenic in nude mice, but those lacking Nr-CAM are not (Table 1). Moreover, our observation that human colon cancer tissue and colon cancer cell lines display detectable Nr-CAM expression whereas normal colon tissue are negative for Nr-CAM is expected based on the aberrant activation of β-catenin signaling in the majority of colon cancer patients (Polakis 2000). The direct molecular mechanism(s) underlying the signaling pathway(s) by which Nr-CAM causes enhanced motility and tumorigenesis remain to be determined. These may include the ability of Nr-CAM to affect cell motility via signaling from the Nr-CAM intracellular domain to the actin cytoskeleton, as suggested for growth cones (Faivre-Sarrailh et al. 1999). An interaction between Nr-CAM and the actin cytoskeleton can be mediated by proteins that bind to the Nr-CAM cytoplasmic tail, including ankyrin (Davis and Bennett 1994) and PDZ-containing proteins. Such selective binding of the Nr-CAM cytoplasmic tail to various proteins can probably be regulated by phosphorylation, as described for its homolog in Caenorhabditis elegans (Chen et al. 2001). The various mechanisms regulating these Nr-CAM-mediated effects on motility and oncogenesis are currently examined in our laboratory.
Materials and methods
Cell lines and cell culture
293T, NIH3T3, Neuro 2A, SW48, SW480, and HCT116 cells were maintained in DMEM with 10% bovine calf serum (BCS). NIH3T3 cells expressing puror constructs were cultured with 10 μg/mL puromycin. KTCTL60 clones were grown in RPMI medium with 10% BCS (Simcha et al. 1996). The β-catenin−/− ES cells obtained from Dr. W. Birchmeier and J. Huelsken (Max-Delbrueck-Center, Berlin, Germany) were cultured as described (Huelsken et al. 2000). Mouse B16 F10 and M2R melanoma cells were grown in DMEM/F12 (1:1 dilution), 10% BCS, and 1% glutamine. Human primary cutaneous melanoma PM-WK, RPM-MC, and RPM-EP recurrent primary cutaneous melanoma and MM-LH and MM-AN metastatic melanoma, obtained from Dr. R. Byers (Boston University School of Medicine, MA) were cultured as described (Murakami et al. 2001). The human melanocytes FOM 73, FOM 92, FOM 99, FOM 100, and human melanoma cell lines from the radial growth phase (RGP) WM1341, WM 3211, WM1552, vertical growth phase (VGP) WM3248, WM115, and metastatic melanoma WM852, WM1158, WM 278, and WM1232 were provided by Dr. M. Herlyn (Wistar Institute, Philadelphia, PA). Melanocytes were cultured in melanocyte growth medium (Clonetics) and melanoma cells as described by Hsu et al. (1999). The human malignant melanoma cell lines HA7, HA21, HA141, and HA172 metastasizing to different sites were provided by Dr. M. Lotan (Hadassah Medical School, Jerusalem, Israel). Human colon cancer and normal colon tissue was provided by Dr. N. Arber (Tel Aviv Sourasky Medical Center, Israel). C57MG and C57MG-Wnt7A cells were from Dr. J. Kitajewsky (Columbia University, New York, NY) and Rat2 and Rat2-Wnt-1 cells were from Dr. A. Brown (Cornell Medical Center, New York, NY). In some experiments B16 M2R mouse melanoma cells were incubated for 24 h with conditioned medium from Rat2–Wnt-1 cells.
Transfections and retroviral infections
Transient transfection of Neuro 2A and 293T cells was carried out using the calcium phosphate method. β-Catenin−/− ES cells were transfected using lipofectamin plus (GIBCO-BRL). In transactivation assays, 0.5 μg of β-galactosidase plasmid was cotransfected with 1 μg reporter plasmids and 3.5 μg catenin constructs in duplicate plates and cell lysates prepared after 48 h were used to determine luciferase and β-galactosidase activities as described (Shtutman et al. 1999). Retroviral infections were carried out as described previously by Damalas et al. (2001) using the pBABE puro-based constructs. Selection with 10 μg/mL puromycin was for 7 d.
Plasmids
The 1.8-kb Nr-CAM promoter (Nr 1–4) containing the TATA box and upstream regulatory sequences, including the four putative LEF/TCF binding sites, was amplified by PCR from genomic DNA of HeLa cells using primers that were designed based on the genomic sequence of the Nr-CAM locus (7q21) and upstream of the 5′ UTR, 5′-ACCTAAGCTTCGGGGAACTGA AAAAGGATAGAGTA-3′ and 5′-ACCTGGTACCGCACTAT TCCACACAGAGACATGAT-3′. The PCR products were digested with KpnI/HindIII and cloned into the pA3Luc plasmid upstream of the luciferase reporter gene. Deletion constructs of the Nr-CAM promoter were constructed by PCR amplification from the full-length promoter using primers containing KpnI and HindIII sites and the PCR products cloned into the pA3Luc plasmid. The full-length human Nr-CAM cDNA KIAA0343 obtained from Dr. T. Nagase (Kazusa DNA Research Institute, Chiba, Japan) was subcloned into the SalI/NotI sites of the pCIneo plasmid and into the SnabI site of the pBABE puro plasmid. Mutant β-catenin S33Y, wild-type plakoglobin, and dominant negative HA-ΔNLEF, obtained from Dr. A. Hecht and R. Kemler (Max-Planck Institute, Freiburg, Germany) were also subcloned into the SnabI site of pBABE puro. To prepare the dominant positive LEF-1 chimera containing of the C-terminal transactivation domain of β-catenin and the DNA binding domain of LEF-1 (HMG-β-cat) the HMG domain was amplified from LEF-1, inserted into the pCGN plasmid using the XbaI/KpnI sites and an SpeI site was added to its 3′ end. The C-terminal transactivation domain of β-catenin was cloned into pHMG using the SpeI/BamHI sites. Dominant negative TCF4 (ΔN TCF4) was provided by Dr. H. Clevers (University Medical Center, Utrecht, The Netherlands) and the plasmid coding for the E-cadherin cytoplasmic domain (E-cad tail) was described previously (Sadot et al. 1998). The cyclin D1 promoter reporter plasmid was as described (Shtutman et al. 1999).
DNA microarrays, northern blot hybridization and RT-PCR
The UniGem-1 DNA microarray (Incyte Genomics) was used to screen for genes induced by β-catenin or plakoglobin. It consisted of 9790 human EST clones. The cDNA probes were synthesized using 50 μg of RNA from KTCTL60 cells stably expressing plakoglobin or ΔNβ-catenin using reverse transcriptase (Superscript, GIBCO-BRL) and an 18-mer oligo-dT primer, in the presence of Cy3-dCTP, whereas with the control cells (neor) Cy5-dCTP labeling was used. Hybridization and posthybridization DNA microarray processes were as described by Schena et al. (1996). Image and quantitative analysis employed the GEMTools software (Incyte Genomics). Northern blotting of 40 μg of total RNA was followed by hybridization with 32P-labeled mouse Nr-CAM cDNA probes for the extracellular and the intracellular domains of the molecule obtained from Drs. M. Grumet and S. Takeshi (NYU Medical Center, New York, NY). RT-PCR was performed using the following primers for Nr-CAM, 5′-AGGTGGATATTGCAACTCAGGGCTGG-3′ and 5′CAAAGGAAT-3′; for LEF-1, 5′-CTACCACGACAAGGC CAGAG-3′ and 5′-CAGTGAGGATGGGTAGGGTTG-3′; for GAPDH, 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TC CACCCTGTTGCTGTA-3′; for cyclophilin A, 5′-ATGGC TAACCCCACCGTG-3′ and 5′-TGCAATCCAGCTAGGCA TG-3′. RT-PCR for Nr-CAM was followed by Southern blot hybridization with a probe containing the 5′-CTCCTTCAGA CAGGACTGTG-3′ sequence. RT-PCR for Nr-CAM in colon cancer cell lines was carried out using the primers described by Sehgal et al. (1998).
In vitro translation, nuclear extracts, and electrophoretic mobility shift assays
These assays were performed essentially as described by Zhurinsky et al. (2000a) using double-stranded DNA oligonucleotides containing the putative LEF/TCF binding sites of the Nr-CAM promoter (Fig. 3B) and 10 adjacent nucleotides upstream and 10 downstream.
Immunofluorescence
Cells were cultured on glass coverslips, fixed with 3% paraformaldehyde in PBS, and permeabilized with 0.5% Triton X-100. The coverslips were incubated with the polyclonal antibodies against the extracellular domain of mouse Nr-CAM, provided by Dr. M. Grumet, anti-ezrin antibodies obtained from Dr. M. Arpin (Curie Institute, Paris, France), and anti-β-catenin antibodies (Transduction Laboratories). The secondary antibodies were as described by Simcha et al. (1998).
Western blotting
The antibodies used for Western blotting were anti-vinculin and anti-tubulin (Sigma-Israel, Nes Ziona), anti-plakoglobin 11E4 from Dr. M. Wheelock (University of Toledo, OH) and the antibodies described above. Western blots were developed using the ECL method (Amersham).
Cell growth rate and wound healing
Cells (5 × 103) were plated into 24-well dishes and cell number was determined every 24 h for 7 d in quadruplicates. A “wound” was introduced into a confluent monolayer of NIH3T3 cells with a tip of a micropipette and the culture medium was replaced with fresh medium as described (Rodriguez Fernandez et al. 1993). Photographs of the wounded areas were taken at different times after wounding the monolayer.
Tumorigenicity assays
NIH3T3 cells (106) expressing retrovirally transduced Nr-CAM, or the empty vector, were injected subcutaneously into 6-week-old CD1 nude male mice. Groups of 8 mice were followed for 2 wk when the size of tumors in mice injected with cells expressing Nr-CAM reached about 1–2 cm in diameter.
Acknowledgments
We thank B. Geiger for his interest and support of this project, J. Zhurinsky and D. Ginsberg for illuminating discussions, and L. Novak and Y. Shibolet for technical assistance with microarray analysis. We are grateful to Drs. M. Grumet, S. Takeshi, M. Herlyn, R. Byers, J. Kitajewsky, A. Brown, H. Clevers, A. Raz, M. Lotan, W. Birchmeier, J. Huelsken, T. Nagase, M. Arpin, R. Kemler, C. Albanese, and N. Arber for cells, tissue, plasmids, and antibodies. These studies were supported by grants from the Israel Science Foundation, The M.D. Moross Institute for Cancer Research, and The Yad Abraham Center for Cancer Research and Diagnosis at the Weizmann Institute of Science.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL avri.ben-zeev@weizmann.ac.il; FAX 97-28-946-5261.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.227502.
References
- Aitkenhead M, Wang S-H, Nakatsu M, Mestas J, Heard C, Hughes C. Identification of endothelial cell genes expressed in an in vitro model of angiogenesis: induction of ESM-1 big-h3, and NrCAM. Microvascular Res. 2002;63:159–171. doi: 10.1006/mvre.2001.2380. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A, Geiger B. Differential molecular interactions of β-catenin an plakoglobin in adhesion, signaling and cancer. Curr Opin Cell Biol. 1998;10:629–639. doi: 10.1016/s0955-0674(98)80039-2. [DOI] [PubMed] [Google Scholar]
- Charpentier E, Lavker R, Acquista E, Cowin P. Plakoglobin suppresses epithelial proliferation and hair growth in vivo. J Cell Biol. 2000;149:503–520. doi: 10.1083/jcb.149.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Ong B, Bennett V. LAD-1, the Caenorhabditis elegansL1CAM homologue, participates in embryonic and gonadal morphogenesis and is a substrate for fibroblast growth factor receptor pathway-dependent phosphotyrosine-based signaling. J Cell Biol. 2001;154:841–855. doi: 10.1083/jcb.200009004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damalas A, Kahan S, Shtutman M, Ben-Ze'ev A, Oren M. Deregulated β-catenin induces a p53– and ARF-dependent growth arrest and cooperates with Ras in transformation. EMBO J. 2001;20:4912–4922. doi: 10.1093/emboj/20.17.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JQ, Bennett V. Ankyrin binding activity shared by the neurofascin/L1/NrCAM family of nervous system cell adhesion molecules. J Biol Chem. 1994;269:27163–27166. [PubMed] [Google Scholar]
- Dhodapkar K, Friedlander D, Scholes J, Grumet M. Differential expression of the cell-adhesion molecule Nr-CAM in hyperplastic and neoplastic human pancreatic tissue. Hum Pathol. 2001;32:396–400. doi: 10.1053/hupa.2001.23526. [DOI] [PubMed] [Google Scholar]
- Faivre-Sarrailh C, Falk J, Pollerberg E, Schachner M, Rougon G. NrCAM, cerebellar granule cell receptor for the neuronal adhesion molecule F3, displays an actin-dependent mobility in growth cones. J Cell Sci. 1999;112:3015–3027. doi: 10.1242/jcs.112.18.3015. [DOI] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Glienke J, Schmitt A, Pilarsky C, Hinzmann B, Weiss B, Rosenthal A, Thierauch K. Differential gene expression by endothelial cells in distinct angiogenic states. Eur J Biochem. 2000;267:2820–2830. doi: 10.1046/j.1432-1327.2000.01325.x. [DOI] [PubMed] [Google Scholar]
- Grumet M. Nr-CAM: A cell adhesion molecule with ligand and receptor functions. Cell Tissue Res. 1997;290:423–428. doi: 10.1007/s004410050949. [DOI] [PubMed] [Google Scholar]
- Grumet M, Mauro V, Burgoon MP, Edelman GM, Cunningham BA. Structure of a new nervous system glycoprotein, Nr-CAM, and its relationship to subgroups of neural cell adhesion molecules. J Cell Biol. 1991;113:1399–1412. doi: 10.1083/jcb.113.6.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart M, Concordet J, Lassot I, Albert I, del los Santos R, Durand H, Perret C, Rubinfeld B, Margottin F, Benarous R, et al. The F-box protein β-TrCP associates with phosphorylated β-catenin and regulates its activity in the cell. Curr Biol. 1999;9:207–210. doi: 10.1016/s0960-9822(99)80091-8. [DOI] [PubMed] [Google Scholar]
- He T, Sparks A, Rago C, Hermeking H, Zawel L, da Costa L, Morin P, Vogelstein B, Kinzler K. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Hecht A, Litterst C, Huber O, Kemler R. Functional characterization of multiple transactivating elements in β-catenin, some of which interact with the TATA-binding protein in vitro. J Biol Chem. 1999;274:18017–18025. doi: 10.1074/jbc.274.25.18017. [DOI] [PubMed] [Google Scholar]
- Hovanes K, Li T, Munguia J, Truong T, Milovanovic T, Lawrence Marsh J, Holcombe R, Waterman M. β-Catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat Genet. 2001;28:53–57. doi: 10.1038/ng0501-53. [DOI] [PubMed] [Google Scholar]
- Hsu MY, Elder D, Herlyn M. In: Melanoma: The Wistar (WM) melanoma cell lines. In Human cell culture. Masters JRW, Palson B, editors. London, UK: Kluwer Academic Publishers; 1999. pp. 259–274. [Google Scholar]
- Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann B, Kemler R. Nuclear localization of β-catenin by interaction with transcription factor LEF-1. Mech Dev. 1996;59:3–10. doi: 10.1016/0925-4773(96)00597-7. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W. Requirement for β-catenin in anterior-posterior axis formation in mice. J Cell Biol. 2000;148:567–578. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsken J, Birchmeier W, Behrens J. E-cadherin and APC compete for the interaction with β-catenin and the cytoskeleton. J Cell Biol. 1994;127:2061–2069. doi: 10.1083/jcb.127.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky A, Klymkowsky M. Anterior axis duplication in Xenopusinduced by the over-expression of the cadherin-binding protein plakoglobin. Proc Natl Acad Sci. 1995;92:4522–4526. doi: 10.1073/pnas.92.10.4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler R. From cadherins to catenins: Cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993;9:317–321. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- Klymkowsky M, Williams B, Barish G, Varmus H, Vourgourakis Y. Membrane-anchored plakoglobins have multiple mechanisms of action in Wnt signaling. Mol Biol Cell. 1999;10:3151–3169. doi: 10.1091/mbc.10.10.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama S, Ikeda S, Asahara T, Kishida M, Kikuchi A. Axin directly interacts with plakoglobin and regulates its stability. J Biol Chem. 1999;274:27682–27688. doi: 10.1074/jbc.274.39.27682. [DOI] [PubMed] [Google Scholar]
- Kolligs F, Kolligs B, Hajra K, Hu G, Tani M, Cho K, Fearon ER. γ-Catenin is regulated by the APC tumor suppressor and its oncogenic activity is distinct from that of β-catenin. Genes & Dev. 2000;14:1319–1331. [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Ono K, Satoh S, Ishiguro H, Fugita M, Miwa N, Tanaka T, Tsunoda T, Yang K, Nakamura Y, et al. Identification of AF17 as a downstream gene of the β-catenin/T-cell factor pathway and its involvement in colorectal carcinogenesis. Cancer Res. 2001;61:6345–6349. [PubMed] [Google Scholar]
- Liu C, Kato Y, Zhang Z, Do V, Yankner B, He X. β-Trcp couples β-catenin phosphorylation-degradation and regulates Xenopusaxis formation. Proc Natl Acad Sci. 1999;96:6273–6278. doi: 10.1073/pnas.96.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Dong X, Mai M, Seelan R, Taniguchi K, Krishnadath K, Halling K, Cunningham J, Boardman L, Qian C, et al. Mutations in AXIN2 cause colorectal cancer with defective mismatch repair by activating β-catenin/TCF signaling. Nat Genet. 2000;26:146–147. doi: 10.1038/79859. [DOI] [PubMed] [Google Scholar]
- Lustig M, Sakurai T, Grumet M. Nr-CAM promotes neurite outgrowth from peripheral ganglia by a mechanism involving axonin-1 as a neuronal receptor. Dev Biol. 1999;209:340–351. doi: 10.1006/dbio.1999.9250. [DOI] [PubMed] [Google Scholar]
- Mechtersheimer S, Gutwein P, Agmon-Levin N, Stoeck A, Oleszewski M, Riedle S, Fogel M, Lemmon V, Altevogt P. Ectodomain shedding of L1 adhesion molecule promotes cell migration by autocrine binding to integrins. J Cell Biol. 2001;155:661–673. doi: 10.1083/jcb.200101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J, Moon R. Analysis of the signaling activities of localization mutants of β-catenin during axis specification in Xenopus. J Cell Biol. 1997;139:229–243. doi: 10.1083/jcb.139.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miravet S, Piedra J, Miro F, Itarte E, Garcia de Herreros A, Dunach M. The transcriptional factor Tcf-4 contains different binding sites for β-catenin and plakoglobin. J Biol Chem. 2002;277:1884–1891. doi: 10.1074/jbc.M110248200. [DOI] [PubMed] [Google Scholar]
- More M, Kirsch F, Rathjen F. Targeted ablation of NrCAM or ankyrin-B results in disorganized lens fibers leading to cataract formation. J Cell Biol. 2001;154:187–196. doi: 10.1083/jcb.200104038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin P. β-Catenin signaling and cancer. BioEssays. 1999;21:1021–1030. doi: 10.1002/(SICI)1521-1878(199912)22:1<1021::AID-BIES6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Morin P, Sparks A, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler K. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Murakami T, Toda S, Fujimoto M, Ohtsuki M, Byers H, Etoh T, Nakagawa H. Constitutive activation of Wnt/β-catenin signaling pathway in migration-active melanoma. Biochem Biophys Res Commun. 2001;288:8–15. doi: 10.1006/bbrc.2001.5719. [DOI] [PubMed] [Google Scholar]
- Peifer M. β-Catenin as oncogene: The smoking gun. Science. 1997;275:1752–1753. doi: 10.1126/science.275.5307.1752. [DOI] [PubMed] [Google Scholar]
- Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- Perrin F, Rathjen F, Stoeckli E. Distinct subpopulations of sensory afferents require F11 or axonin-1 for growth to their target layers within the spinal cord of the chick. Neuron. 2001;30:707–723. doi: 10.1016/s0896-6273(01)00315-4. [DOI] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes & Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- Rimm D, Caca K, Hu G, Harrison F, Fearon ER. Frequent nuclear/cytoplasmic localization of β-catenin without exon 3 mutations in malignant melanoma. Am J Pathol. 1999;154:325–329. doi: 10.1016/s0002-9440(10)65278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Fernandez J, Geiger B, Salomon D, Ben-Ze'ev A. Suppression of vinculin expression by antisense transfection confers changes in cell morphology, motility, and anchorage-dependent growth of 3T3 cells. J Cell Biol. 1993;122:1285–1294. doi: 10.1083/jcb.122.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of β-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- Sadot E, Simcha I, Shtutman M, Ben-Ze'ev A, Geiger B. Inhibition of β-catenin-mediated transactivation by cadherin derivatives. Proc Natl Acad Sci. 1998;95:15339–15344. doi: 10.1073/pnas.95.26.15339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadot E, Simcha I, Iwai K, Ciechanover A, Geiger B, Ben-Ze'ev A. Differential interaction of plakoglobin and β-catenin with the ubiquitin–proteasome system. Oncogene. 2000;19:1992–2001. doi: 10.1038/sj.onc.1203519. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Lustig M, Nativ M, Hemperly J, Schlessinger J, Peles E, Grumet M. Induction of neurite outgrowth through contactin and Nr-CAM by extracellular regions of glial receptor tyrosine phosphatase β. J Cell Biol. 1997;136:907–918. doi: 10.1083/jcb.136.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Lustig M, Babiarz J, Furley A, Tait S, Brophy P, Brown S, Brown L, Mason C, Grumet M. Overlapping functions of the cell adhesion molecules Nr-CAM and L1 in cerebellar granule cell development. J Cell Biol. 2001;154:1259–1273. doi: 10.1083/jcb.200104122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon D, Sacco P, Roy S, Simcha I, Johnson K, Wheelock M, Ben-Ze'ev A. Regulation of β-catenin levels and localization by overexpression of plakoglobin and inhibition of the ubiquitin-proteasome system. J Cell Biol. 1997;139:1325–1335. doi: 10.1083/jcb.139.5.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, et al. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet. 2000;24:245–250. doi: 10.1038/73448. [DOI] [PubMed] [Google Scholar]
- Schena M, Shalon D, Heller R, Chai A, Brown P, Davis R. Parallel human genome analysis: Microarray-based expression monitoring of 1000 genes. Proc Nat Acad Sci. 1996;93:10614–10619. doi: 10.1073/pnas.93.20.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal A, Boynton AL, Young RF, Vermeulen SS, Yonemura KS, Kohler EP, Aldape HC, Simrell CR, Murphy GP. Cell adhesion molecule Nr-CAM is over-expressed in human brain tumors. Int J Cancer. 1998;76:451–458. doi: 10.1002/(sici)1097-0215(19980518)76:4<451::aid-ijc1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Sehgal A, Ricks S, Warrick J, Boynton AL, Murphy GP. Antisense human neuroglia related cell adhesion molecule hNr-CAM, reduces the tumorigenic properties of human glioblastoma cells. Anticancer Res. 1999;19:4947–4953. [PubMed] [Google Scholar]
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the β-catenin/LEF-1 pathway. Proc Natl Acad Sci. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simcha I, Geiger B, Yehuda-Levenberg S, Salomon D, Ben-Ze'ev A. Suppression of tumorigenicity by plakoglobin: An augmenting effect of N-cadherin. J Cell Biol. 1996;133:199–209. doi: 10.1083/jcb.133.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simcha I, Shtutman M, Salomon D, Zhurinsky J, Sadot E, Geiger B, Ben-Ze'ev A. Differential nuclear translocation and transactivation potential of β-catenin and plakoglobin. J Cell Biol. 1998;141:1433–1448. doi: 10.1083/jcb.141.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simcha I, Kirkpatrick C, Sadot E, Shtutman M, Polevoy G, Geiger B, Peifer M, Ben-Ze'ev A. Cadherin sequences that inhibit β-catenin signaling: A study in yeast and mammalian cells. Mol Biol Cell. 2001;12:1177–1188. doi: 10.1091/mbc.12.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckli E, Sonderegger P, Pollerberg G, Landmesser L. Interference with axonin-1 and NrCAM interactions unmasks a floor-plate activity inhibitory for commissural axons. Neuron. 1997;18:209–221. doi: 10.1016/s0896-6273(00)80262-7. [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Volkmer H, Leuschner R, Zacharias U, Rathjen F. Neurofascin induces neurites by heterophilic interactions with axonal NrCAM while NrCAM requires F11 on the axonal surface to extend neurites. J Cell Biol. 1996;135:1059–1069. doi: 10.1083/jcb.135.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voura E, Ramjeesingh R, Montgomery A, Siu C. Involvement of integrin α(v)β(3) and cell adhesion molecule L1 in transendothelial migration of melanoma cells. Mol Biol Cell. 2001;12:2699–2710. doi: 10.1091/mbc.12.9.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Williams H, Du JS, Terrett J, Kenwrick S. Alternative splicing of human NrCAM in neural and nonneural tissues. Mol Cell Neurosci. 1998;10:287–295. doi: 10.1006/mcne.1997.0658. [DOI] [PubMed] [Google Scholar]
- Willert K, Nusse R. β-Catenin: A key mediator of Wnt signaling. Curr Opin Genet Dev. 1998;8:95–102. doi: 10.1016/s0959-437x(98)80068-3. [DOI] [PubMed] [Google Scholar]
- Williams B, Barish G, Klymkowsky M, Varmus HE. A comparative evaluation of β-catenin and plakoglobin signaling activity. Oncogene. 2000;19:5720–5728. doi: 10.1038/sj.onc.1203921. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Xu L, Corcoran RB, Welsh JW, Pennica D, Levine AJ. WISP-1 is a Wnt-1– and β-catenin-responsive oncogene. Genes & Dev. 2000;14:585–595. [PMC free article] [PubMed] [Google Scholar]
- Zhurinsky J, Shtutman M, Ben-Ze'ev A. Plakoglobin and β-catenin: protein interactions, regulation and biological roles. J Cell Sci. 2000a;113:3127–3139. doi: 10.1242/jcs.113.18.3127. [DOI] [PubMed] [Google Scholar]
- Zhurinsky J, Shtutman M, Ben-Ze'ev A. Differential mechanisms of LEF/TCF family-dependent transcriptional activation by β-catenin and plakoglobin. Mol Cell Biol. 2000b;20:4238–4252. doi: 10.1128/mcb.20.12.4238-4252.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]