Abstract
Wnt signals control decisive steps in development and can induce the formation of tumors. Canonical Wnt signals control the formation of the embryonic axis, and are mediated by stabilization and interaction of β-catenin with Lef/Tcf transcription factors. An alternative branch of the Wnt pathway uses JNK to establish planar cell polarity in Drosophila and gastrulation movements in vertebrates. We describe here the vertebrate protein Diversin that interacts with two components of the canonical Wnt pathway, Casein kinase Iε (CKIε) and Axin/Conductin. Diversin recruits CKIε to the β-catenin degradation complex that consists of Axin/Conductin and GSK3β and allows efficient phosphorylation of β-catenin, thereby inhibiting β-catenin/Tcf signals. Morpholino-based gene ablation in zebrafish shows that Diversin is crucial for axis formation, which depends on β-catenin signaling. Diversin is also involved in JNK activation and gastrulation movements in zebrafish. Diversin is distantly related to Diego of Drosophila, which functions only in the pathway that controls planar cell polarity. Our data show that Diversin is an essential component of the Wnt-signaling pathway and acts as a molecular switch, which suppresses Wnt signals mediated by the canonical β-catenin pathway and stimulates signaling via JNK.
Keywords: Signal transduction, embryonic axis formation, planar cell polarity, axin/conductin, Diego
Recent work shows that signals mediated by Wnt ligands branch into several intracellular pathways as follows: (1) the canonical Wnt pathway employs β-catenin/Tcf as transcription factors to activate target genes important in embryonic patterning and tumorigenesis (Behrens et al. 1996; Molenaar et al. 1996; for review, see Bienz and Clevers 2000; De Robertis et al. 2000); (2) the planar cell polarity pathway (Wnt/JNK pathway) depends on JNK activity and controls cytoskeletal rearrangements (Mlodzik 2000; Sokol 2000); (3) the Wnt/Ca2+ pathway controls PKC and CamKII and regulates cell adhesion and motility (Kuhl et al. 2000; Winklbauer et al. 2001); and (4) a not well-characterized novel pathway regulates spindle orientation and asymmetric cell division (Lu et al. 2001; for review, see Huelsken and Birchmeier 2001). Much recent work has been directed toward deciphering the molecular aspects of cytoplasmic diversification of the Wnt-signaling pathway. Separate domains of the downstream molecule Dishevelled can stimulate canonical Wnt and Wnt/JNK signaling (Sokol 2000; Wallingford et al. 2000). In contrast, particular Wnt and Frizzled molecules, like Wnt11 and Fz7, specifically activate the Wnt/JNK, but not the canonical Wnt pathway (Djiane et al. 2000; Heisenberg et al. 2000). Naked Cuticle/nkd and Strabismus bind to Dishevelled to activate the Wnt/JNK pathway at the expense of the canonical Wnt pathway. Par-1 inhibits the Wnt/JNK and activates the canonical Wnt pathway (Zeng et al. 2000; Sun et al. 2001; Yan et al. 2001; Park and Moon 2002), and Dapper inhibits both branches (Cheyette et al. 2002). The elucidation of the molecular mechanisms of the branching of the Wnt signals is a main focus of present research.
In the canonical Wnt pathway, the stability of β-catenin is controlled by a multiprotein complex that consists of Axin/Conductin, the adenomatous polyposis coli (APC) tumor suppressor protein, and glycogen synthase kinase 3β (GSK3β; Rubinfeld et al. 1993; Su et al. 1993; Zeng et al. 1997; Behrens et al. 1998; Hart et al. 1998; Ikeda et al. 1998; Sakanaka et al. 1998; von Kries et al. 2000). In this scaffolding complex, GSK3β phosphorylates the N-terminal region of β-catenin, which marks β-catenin for ubiquitination by the SCFβ-TrCP E3 ubiquitin ligase and subsequent degradation in proteasomes (Aberle et al. 1997; for review, see Polakis 2000). The importance of β-catenin phosphorylation in controlling degradation has been inferred from mutations of the N terminus of β-catenin in tumor cells, which cluster around the SCFβ-TrCP-binding site and compromise phosphorylation/ubiquitination. Many of these mutations occur at Ser/Thr residues that are phosphorylated sequentially by GSK3β (Thr 41, Ser 37, and Ser 33; Cohen and Frame 2001). However, Ser 45, a hotspot of mutation in tumors, does not conform to a GSK3β site (Polakis 2000, Wong et al. 2001). Recently, searches for additional Ser 45 kinases identified both Casein kinase Iα and Iε as priming kinases for β-catenin phosphorylation (Amit et al. 2002; Liu et al. 2002).
Ser/Thr kinases of the CKI family function in a number of cellular processes like cell cycle regulation, DNA repair, and circadian rhythms, and were also implicated in Wnt signaling (Santos et al. 1996; Kloss et al. 1998; Peters et al. 1999; Sakanaka et al. 1999; Vielhaber and Virshup 2001). Overexpression of CKIε induced secondary body axis in Xenopus embryos, and CKIε was found to interact with and promote phosphorylation of Dishevelled. However, in vivo experiments using RNA-interference in Drosophila cells and embryos revealed that both CKIα and CKIε act as inhibitors of β-catenin signaling (Liu et al. 2002; Yanagawa et al. 2002). It has also been shown that both kinases phosphorylate β-catenin at Ser 45, thereby creating a classical GSK3β-recognition motif to initiate β-catenin degradation (Amit et al. 2002; Liu et al. 2002).
In the present study, we describe a novel protein, which inhibits the canonical Wnt pathway and promotes signaling of the Wnt/JNK pathway. We named this protein Diversin, because of the diverse functions in the two branches of the Wnt pathway. Our biochemical analyses allow us to assign the molecular mechanism by which Diversin functions in the canonical Wnt pathway; Diversin recruits CKIε to the Axin/Conductin-GSK3β complex and promotes degradation of β-catenin. Through epistasis experiments in Xenopus and zebrafish, Diversin could be localized downstream of Dsh and CKIε, and upstream of GSK3β and β-catenin. We have also found that Diversin influences gastrulation movements in zebrafish embryos, which are controlled by the Wnt/JNK pathway (Yamanaka et al. 2002). Moreover, we have clarified the functional differences between Diversin and the distantly related Drosophila protein Diego (Feiguin et al. 2001): Diego does not interact with Axin and affects specifically gastrulation movements.
Results
Identification and characterization of the novel gene product Diversin
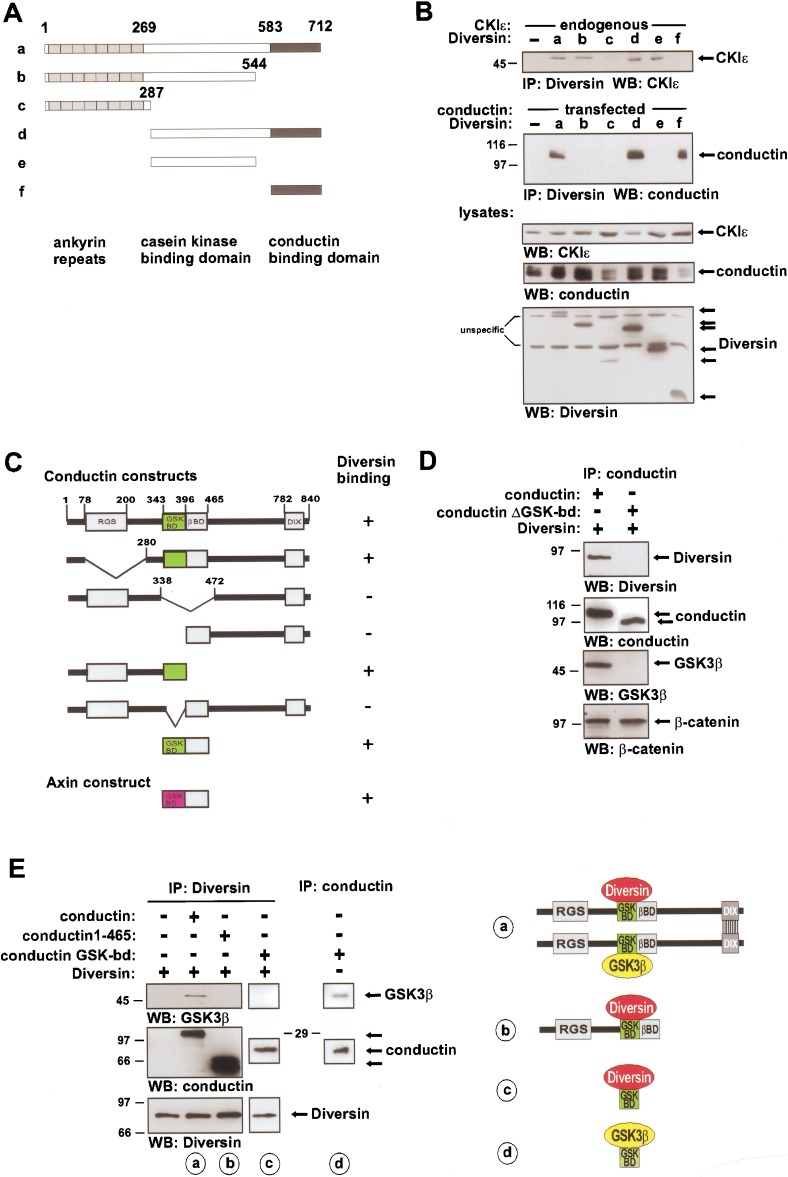
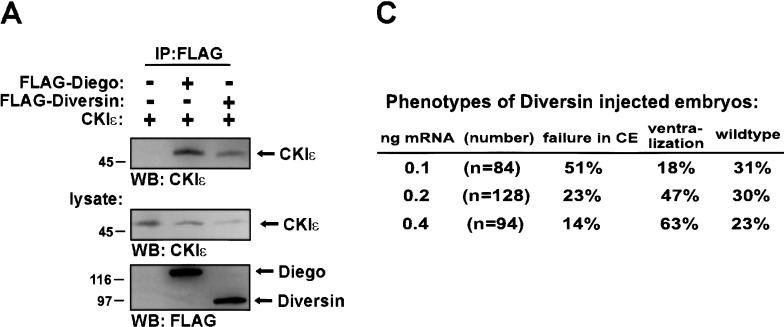
We isolated Diversin in a yeast two-hybrid screen as a binding partner of Conductin. Further analyses showed that Diversin interacts directly with Conductin or Axin and with CKIε. The cDNA of mouse Diversin encodes a protein of 712 amino acids (GenBank accession no. AY026320), with an N-terminal domain that contains eight ankyrin repeats, and central and C-terminal domains with no obvious homologies to other proteins (Fig. 1A). Closely related genes were identified in the genomes of human (GenBank accession no. AB023174) and zebrafish (GenBank accession no. AF395113). Coimmunoprecipitations and yeast two-hybrid analyses showed that the central domain of Diversin (residues 287–544) interacts with CKIε, and the C-terminal domain (residues 583–712) with Conductin or Axin. Diversin did not interact with CKIα in coimmunoprecipitation experiments (Fig. 1B; data not shown). Deletion analysis of Conductin showed that Diversin interacts with amino acids 343–396 of Conductin, which was characterized previously as the GSK3β interaction site (Fig. 1C,D; Behrens et al. 1998; Ikeda et al. 1998). Coimmunoprecipitation showed that Diversin and GSK3β exist in triple complexes with full-length, dimeric Conductin (Fig. 1E, lane a). However, in the presence of mutant Conductin that lacks the dimerization domain (Conductin 1–465; Hsu et al. 1999) or that consists of only the GSK3β-binding domain (Conductin GSK-bd), Diversin did not coprecipitate GSK3β (Fig. 1E, lanes b,c). We conclude from these data that dimeric Conductin can concomitantly bind Diversin and GSK3β, and that monomeric Conductin binds either Diversin or GSK3β (see scheme in Fig. 1F).
Figure 1.
Domain structure and biochemical interactions of Diversin. (A) Schematic representation of the domain structure of mouse Diversin and of various deletion constructs. Numbers represent amino acids of Diversin. (B) Coimmunoprecipitation analyses in transfected Neuro2A cells, showing that the central domain of Diversin (white in A) interacts with CKIε (top). The C-terminal domain (black in A) confers interaction with Conductin (middle). Similar interactions were seen with Axin. Expression of the indicated proteins was verified by Western blotting (bottom). (C,D) Yeast two-hybrid and coimmunoprecipitation analyses showing that Diversin interacts with the GSK3β-binding domain of Conductin and Axin. For constructs see Behrens et al. (1998). (+) Growth of yeast on selection medium, (−) absence of growth. The conductin ΔGSK-bd is as in C (conductin lacking amino acids 343–396). (E) Coimmunoprecipitation analyses of Diversin, Conductin, and GSK3β complexes. Full-size Conductin is able to dimerize through the DIX-domain (Hsu et al. 1999; data not shown) and allows the formation of a triple complex that contains Diversin, Conductin, and GSK3β (a). Conductin1–465 and the GSK3β-binding domain of Conductin (Conductin GSK-bd) cannot dimerize and triple complexes with Diversin and GSK3β are not observed (b,c). The conductin GSK-bd is as in C (amino acids 343–465; Behrens et al. 1998). (d) Note that amounts of Conductin GSK-bd similar to those in c are able to efficiently coprecipitate GSK3β. Schematic representations of the interactions in (a–d) are shown at right. For immunoprecipitation experiments, 6 × 105 cells per 10-cm culture dish were transfected with 5 μg of the indicated cDNAs (in the pCDNA vector, Invitrogen) and cultured for 48 h, followed by lysis and immunoprecipitation (Behrens et al. 1998). Polyacrylamide gel electophoresis and Western blot were performed with 1/5 of the immunoprecipitate from 1-ml lysate, 1/50 of the lysate was used as expression control. Concentrations of the commercial antibodies were as suggested by the manufacturers.
Diversin acts in the canonical Wnt pathway by recruiting CKIε to the Axin/Conductin-complex
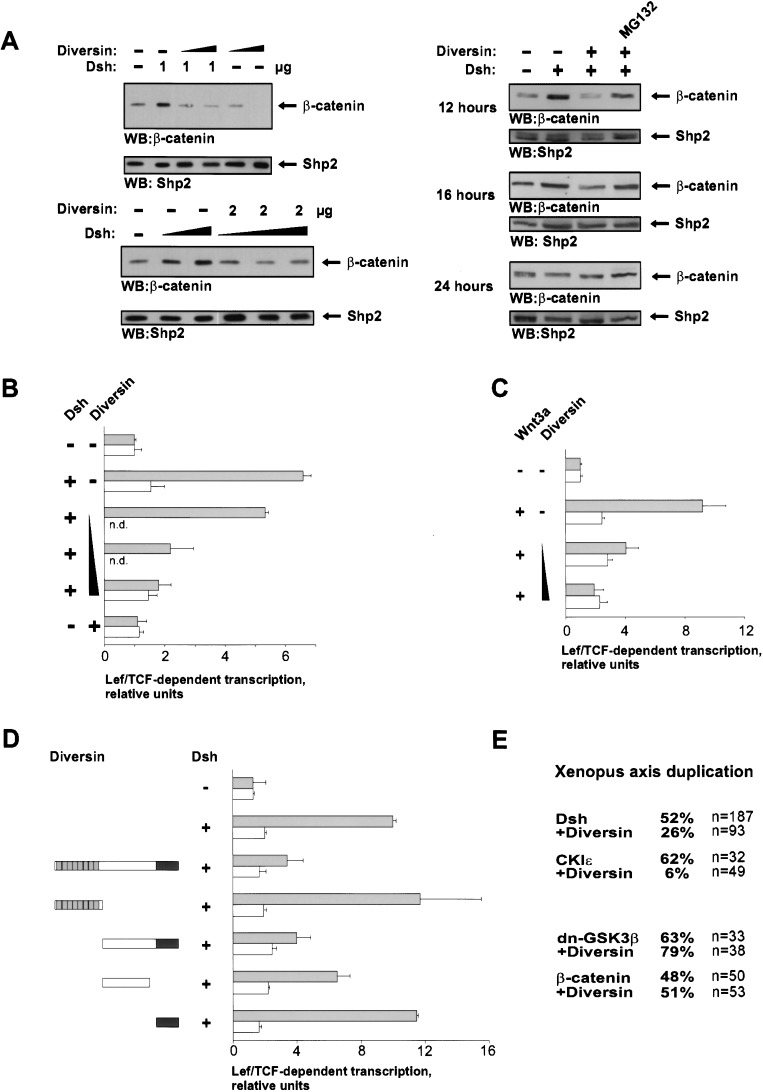
We examined the function of Diversin in cell culture and in Xenopus embryos. In 293 cells, transfection of Diversin cDNA resulted in enhanced degradation of β-catenin protein in a concentration-dependent manner (Fig. 2A). Diversin stimulated degradation of endogenous β-catenin in both untreated and Dishevelled-transfected cells, and this was blocked by the proteasome inhibitor MG132. Strong degradation of β-catenin by Diversin was seen after 12 h but not 24 h. Diversin also inhibited signaling of β-catenin that is induced by transfection of Dishevelled or Wnt3a, as determined in Lef/Tcf-dependent transcription assays (Fig. 2B,C). To address specificity, we examined deletion constructs of Diversin; wild-type Diversin and the fragment containing the CKIε- and Conductin-binding domains efficiently blocked signaling of β-catenin (Fig. 2D), whereas the other domains of Diversin were inactive or significantly less active.
Figure 2.
Diversin inhibits Wnt signaling in cell culture and in Xenopus embryos. (A) Diversin promotes degradation of β-catenin in 293 cells, as analyzed by Western blot analysis of cytoplasmic β-catenin. Cells were mildly lysed using 0.1% Triton-X 100, and amounts of cell lysates were normalized using Shp2. (Top, left) Transfection of increasing amounts (2 and 5 μg) of Diversin cDNA destabilized endogenous β-catenin and blocked β-catenin that is induced by transfection of 1.5 μg of Dishevelled cDNA. (Bottom, left) Diversin inhibits the stabilization of β-catenin induced by transfection of increasing amounts (0.25–2 μg) of Dishevelled cDNA. (Right) The activity of Diversin is blocked by addition of 20 μM MG132, and is observable up to 16 h after transient transfection. Dishevelled (1.5 μg) and Diversin (3 μg) were transfected, and expression was monitored by Western blotting (data not shown). The proteasome inhibitor MG132 was added 2 h before cell lysis. (B,C) Diversin blocks Dishevelled- and Wnt-induced transcription of a Lef/Tcf-dependent luciferase reporter gene in a dose-dependent manner. A total of 1–3 μg of Diversin cDNA and 1.5 μg of Dishevelled or Wnt3a cDNA were cotransfected in 293 cells with the TOP (gray bars) or the control FOP reporter (white bars). (D) Analysis of the functionally important domains of Diversin in Lef/Tcf-dependent transcriptional assays. Full-length Diversin and a fragment able to interact with CKIε and Axin/Conductin inhibit transcription. Expression of similar levels of Diversin and Diversin fragments were verified by Western blot analysis. Reporter assays were performed as in B and C. (E) Epistasis analysis of Diversin in Xenopus embryos. Ventral injection of 1 ng of Dishevelled, CKIε, (dn)GSK3β, or β-catenin mRNA induced secondary body axes (percent axis duplication shown). Coinjection of 3 ng of Diversin mRNA blocked axis duplication by Dishevelled and CKIε, but not dn-GSK3β or β-catenin.
Components of the Wnt-signaling pathway are known to control axis formation in Xenopus embryos (De Robertis et al. 2000). Ventral injection of Diversin mRNA into 4-cell stage embryos blocked the formation of secondary body axes that are induced by Dishevelled or CKIε (Fig. 2E). Diversin did not inhibit axis duplication that is induced by dominant-negative GSK3β or β-catenin. These epistasis experiments indicate that Diversin acts downstream of Dishevelled and CKIε, but upstream of GSK3β and β-catenin.
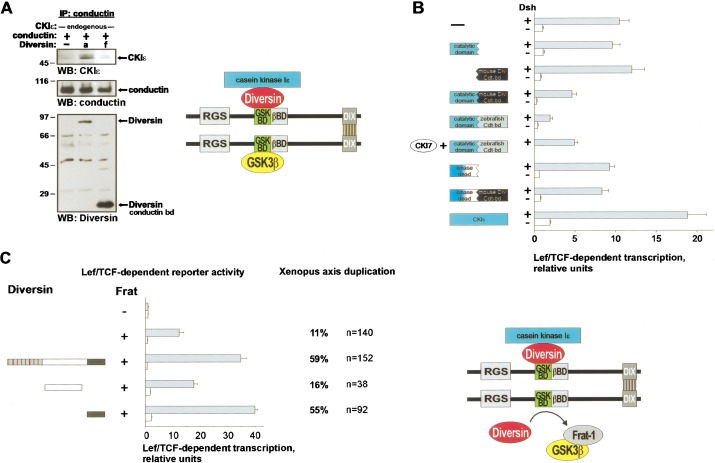
We addressed the mechanism of Diversin‘s action in the canonical Wnt-signaling pathway. Coimmunoprecipitation experiments showed that full-length Diversin promotes the association of CKIε with Conductin in 293 cells (Fig. 3A). GSK3β is also part of this complex (see Fig. 1E; data not shown). We conclude from these experiments that Diversin links CKIε to the Axin/Conductin-GSK3β complex (see scheme in Fig. 3A). CKIε consists of the catalytic domain at the N terminus and a C-terminal tail, which is necessary for the function of CKIε in Wnt signaling (Sakanaka et al. 1999). We examined whether this C terminus of CKIε could be replaced with the Conductin-binding domain of Diversin. These CKIε-Diversin fusion constructs inhibited β-catenin signaling, whereas the separate domains were inactive (Fig. 3B). A fusion construct containing the Axin/Conductin-binding domain of zebrafish Diversin was also inhibitory, and could be inactivated by the CKI-inhibitor CKI-7 (Chijiwa et al. 1989). A fusion construct containing kinase-inactive CKIε (K38R) was ineffective. Thus, Diversin can recruit CKIε to the Axin/Conductin-GSK3β complex to inhibit β-catenin signaling.
Figure 3.
Diversin recruits CKIε to the β-catenin degradation complex. (A) Diversin promotes association of endogenous CKIε with Conductin. The Conductin-binding domain of Diversin did not mediate this interaction. Note that the Diversin constructs (lanes a,f) are as in Fig. 1A. Immunoprecipitations were performed as described in Fig. 1. A scheme of Diversin linking CKIε to the Axin/Conductin-GSK3β complex is shown at right. (B) Hybrid molecules that contain the catalytic domain of CKIε and the carboxy-termini of murine or zebrafish Diversin inhibit Dishevelled-induced Lef/Tcf-dependent transcription. cDNAs that encode the individual domains have no effect. The catalytic activity of CKIε is essential, as shown by a kinase-inactive fusion construct or addition of 50 μM CKI-7, a specific CKI inhibitor. The indicated cDNAs (3 μg) were transfected into 293 cells either with Dishevelled (1.5 μg) cDNA (gray bars) or transfected alone (white bars). Reporter assays are as described in Fig. 2, hybrid constructs are specified in Materials and Methods. (C) Diversin cooperates with Frat-1 in displacing GSK3β from Axin/Conductin complex. Expression of Frat-1 (1.5 μg) in 293 cells activates Lef/Tcf-dependent transcription. Cotransfection of Diversin cDNA (3 μg) enhances this further. The C-terminal, but not the central domain of Diversin is responsible for this activity. Reporter assays were performed as in Fig. 2. Gray bars represent the TOP and white bars the control FOP reporters. A cooperative effect of Diversin and Frat-1 was also observed in axis duplication of Xenopus embryos. For this, Diversin (3 ng) and Frat-1 mRNA (1 ng) were injected ventrally into the 4–8-cell embryo. Note that limited amounts of Frat-1 mRNA were injected to visualize cooperation. A schematic model of the action of Diversin in displacing GSK3β from the Axin/Conductin complex, which leads to enhanced signaling of β-catenin, is presented at right.
Frat-1/GBP (GSK3β-binding protein) binds and displaces GSK3β from the Axin/Conductin complex and thus activates β-catenin signaling (Farr et al. 2000). Here, we have used Frat-1/GBP to examine the contribution of GSK3β to the function of Diversin. In 293 cells, transfection of Frat-1 cDNA increased β-catenin/Tcf-dependent transcription (Fig. 3C; Li et al. 1999), by removing GSK3β from the β-catenin degradation complex. Cotransfection of full-size Diversin enhanced this signaling, possibly by synergizing GSK3β-displacement. The C-terminal domain of Diversin, which specifically binds to the GSK3β-binding domain of Axin/Conductin, was sufficient for this activity. Similarly, coinjection of Diversin mRNA into Xenopus embryos potentiated Frat-1-dependent formation of a secondary body axis, which also required the presence of the C terminus of Diversin. A mutant Conductin that lacks the dimerization domain did not allow cooperation of Diversin and Frat-1 (data not shown). These combined data indicate that (1) Diversin/CKIε and GSK3β need to cooperate in β-catenin degradation, and (2) the active β-catenin degradation complex consists of dimeric Axin/Conductin, which carries Diversin/CKIε on one subunit and GSK3β on the other.
Diversin controls axis formation in zebrafish embryos
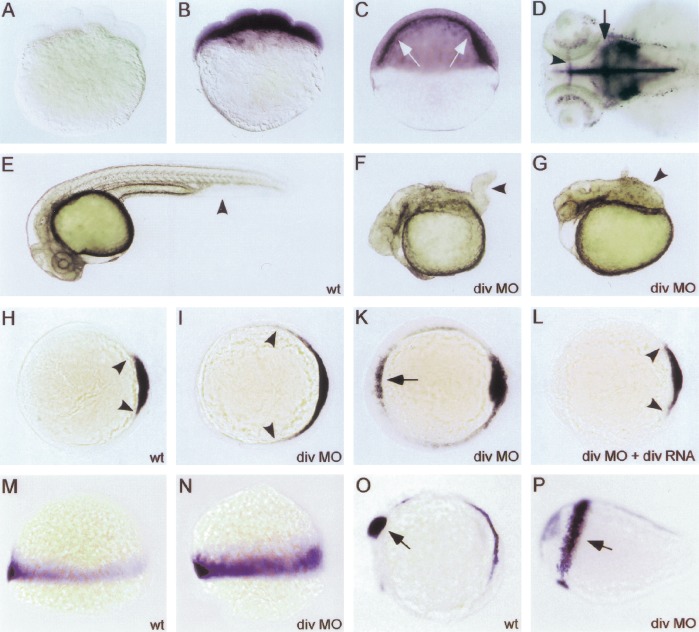
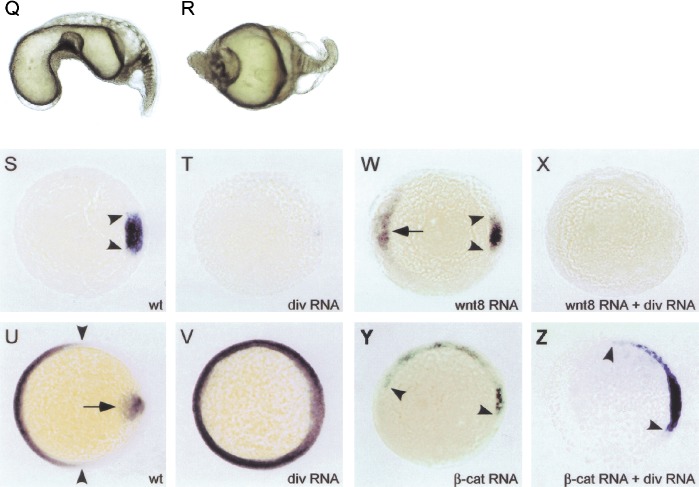
We next determined the role of Diversin in early zebrafish development. The establishment of the dorsal organizer and the subsequent formation of the embryonic axis is an early event in zebrafish embryogenesis and requires Wnt/β-catenin signals (De Robertis et al. 2000; Schier 2001). Interference with these early processes perturbs subsequent development. We found that the Diversin gene is expressed maternally in zebrafish embryos, and expression is ubiquitous during early development (Fig. 4 A,B). In the blastula and early gastrula, Diversin expression is high in cells located between yolk and blastoderm, in which inductive processes occur that drive axis and mesoderm formation (Fig. 4C). At later stages, Diversin expression becomes restricted to retinal ganglion cells, otic vesicles, roof plate of the brain, and the forebrain-midbrain and midbrain-hindbrain boundaries (Fig. 4D). Antisense morpholino oligonucleotides (MO) are an effective tool in zebrafish that reduce or eliminate gene functions (Nasevicius and Ekker 2000). We injected MOs directed against the 5′-untranslated region and the first ATG of fish Diversin into the yolk of 1–4-cell stage zebrafish embryos, which resulted in strong dorsalization of the embryos, as assessed by the absence of the ventral tail fin and a wound-up trunk (Fig. 4F,G, and control embryo in E). Both MOs resulted in a significant broadening of the goosecoid (gsc) expression domain and ectopic gsc expression ventrally (Fig. 4I,K, see control in H). Coinjection of mouse Diversin mRNA (which is not recognized by the fish MOs) rescued the embryos (Fig. 4L), indicating that the MOs specifically target endogenous zebrafish Diversin. MOs that contain four mismatches had no effect (data not shown). In addition, injection of MOs led to expansion of the expression domain of the ventro-lateral mesoderm marker tbx6 (Fig. 4M,N), which was found previously to be reduced in Wnt8-mutant zebrafish (Lekven et al. 2001). At later stages of development, the injected embryos were hyperdorsalized, that is, Pax2.1, which is expressed only dorsally at the presumptive midbrain-hindbrain boundary in control embryos, was also expressed ventrally (Fig. 4O,P).
Figure 4.
Diversin affects the formation of the dorsal organizer in zebrafish embryos. (A–D) In situ hybridization of diversin in zebrafish embryos at different developmental stages. Diversin is expressed ubiquitously (B) in the blastomeres at the 4–8-cell stage and (C) in shield-stage embryos. Pronounced expression in the yolk-blastoderm interphase is indicated by arrows. (D) Specific expression of diversin in ganglial layers of the retina, otic vesicles, the roof plate of the brain, and in the forebrain-midbrain (arrowhead) and midbrain-hindbrain boundaries (arrow) at 72 h post fertilization (hpf). A shows the sense control. (E–P) Down-regulation of Diversin by the injection of antisense morpholinos leads to a dorsalization of zebrafish embryos and the appearance of an enlarged or ectopically localized dorsal organizer. (E) Wild-type embryo at 30 hpf (dorsal up, arrowhead indicates ventral tail fin). (F,G) Embryos at 30 hpf after injection with 4–6 ng of Diversin antisense morpholino oligonucleotides (divMO) that display class 3 (F, arrowhead indicates missing ventral tail fin) and class 4 dorsalization (G, arrowhead indicates wound-up trunk). (H,I,K,L) In situ hybridization of gsc at the shield stage (animal view, dorsal to the right; arrowheads indicate the size of the gsc expression domain; arrow indicates ectopic gsc expression). (H) Uninjected control; (I,K) embryo injected with Diversin MOs; (L) embryo coinjected with Diversin MOs and mouse Diversin mRNA. (M,N) In situ hybridization of tbx6 at 70% epiboly (lateral view, anterior up, dorsal to the right). (M) Control; (N) embryo injected with Diversin MOs; note the expansion of the tbx6 expression domain. (O,P) In situ hybridization of pax2.1 at the 5-somite stage (lateral view, anterior to the left, dorsal up; arrows indicate the midbrain-hindbrain boundary region). (O) Control; (P) embryo injected with Diversin MOs; note the ventral expansion of the pax2.1 expression domain. (Q–Z) Overexpression of Diversin ventralizes zebrafish embryos. (Q,R) Strongly ventralized embryos (Q, class 3, and R, class 4) at 30 hpf after injection with full-length Diversin mRNA; (S,T) In situ hybridization of embryos at shield stage for gsc (arrowheads in S). (S) Uninjected control. (T) Embryo injected with Diversin mRNA loses gsc expression at the dorsal side. (U,V) In situ hybridization of embryos at 70% epiboly stained for eve1 in the ventral mesoderm (arrowheads) and gsc in the prechordal plate (arrow). (U) Uninjected control. (V) Embryo injected with Diversin mRNA shows an expansion of the eve1 expression domain and looses gsc expression at the dorsal side. (W–Z) Diversin acts downstream of Wnt and upstream of β-catenin, as shown on injected embryos by in situ hybridization with gsc that marks the dorsal organizer. (W) Embryo injected with Wnt8 mRNA; (X) embryo coinjected with Diversin and Wnt8 mRNA; (Y) embryo injected with β-catenin mRNA; (Z) embryo coinjected with Diversin and β-catenin mRNA. Arrowheads indicate lateral borders of gsc expression domains, arrow indicates ectopic gsc expression. All embryos are shown as lateral views (dorsal to the right and animal pole up).
In contrast, microinjection of Diversin mRNA induced a strong ventralization of the zebrafish embryos, which lacked heads and dorsal midline structures (Fig. 4Q,R). Embryos lost gsc expression in the prospective dorsal organizer region (Fig. 4, cf. T and control in S) and displayed a broadened expression of eve1, which is only expressed ventrally in control embryos (Fig. 4, cf. V and control in U; arrowheads mark the boundaries of expression in the wild-type embryo). Injection of Wnt8 or constitutively active β-catenin mRNA induce enlarged or ectopic organizers, as assessed by the analysis of gsc expression (Fig. 4W,Y, and control in S; Kelly et al. 2000; Lekven et al. 2001). Coinjection of Diversin blocked the formation of enlarged or ectopic organizers that were induced by Wnt8, but not those induced by β-catenin (Fig. 4X,Z, and control in T).
Diversin enhances JNK activation and modulates gastrulation movements in zebrafish embryos
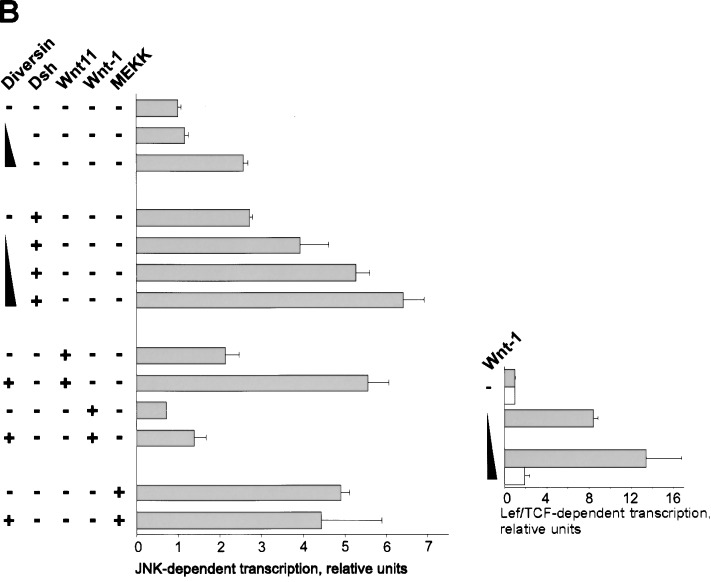
Diversin is distantly related to Diego of Drosophila (Feiguin et al. 2001). Compared with Diversin, Diego contains six ankyrin repeats instead of eight, and has 35% amino acid sequence identity within the ankyrin repeats, but little identity (18%) in the residual domains. Diego acts downstream of Frizzled and controls planar polarization of epithelial cells in the eye and wing, which depends on JNK activity (Boutros et al. 1998). Our coimmunoprecipitation experiments show that Diego interacts with mammalian CKIε but not with Drosophila Axin (Fig. 5A; data not shown). Further, Diversin stimulates JNK-dependent transcription in 293 cells (Fig. 5B). Diversin also promotes JNK activation that is induced by Dishevelled or Wnt11. In zebrafish embryos, injection of 0.1 ng of Diversin mRNA resulted in abnormal gastrulation movements, that is, the convergence and extension of injected embryos were defective (Fig. 5D). These low amounts of Diversin mRNA induced failure of gastrulation movements, but little ventralization, whereas at higher dosages, ventralization was predominant (Fig. 5C). Similarly, injection of low amounts of Diversin MOs also interfered with gastrulation movements and induced a general undulation of the embryo along its anterior-posterior axis, as revealed by in situ hybridization for myoD at the 5–10 somite stage (Fig. 5G, and control in F). Thus, both activation and inhibition of the Wnt/JNK pathway perturb gastrulation movements (for review, see Sokol 2000). Injection of Diego mRNA also induced deficits in convergence and extension movements, but did not affect axis formation at any concentration tested (Fig. 5E). Diego could not rescue the Diversin MO-induced dorsalization (data not shown). Taken together, our data indicate that Diversin functions both in the Wnt/β-catenin and the Wnt/JNK pathway, and that Diego acts only in the Wnt/JNK pathway (Feiguin et al. 2001). Diego and Diversin are therefore structurally and functionally not entirely homologous.
Figure 5.
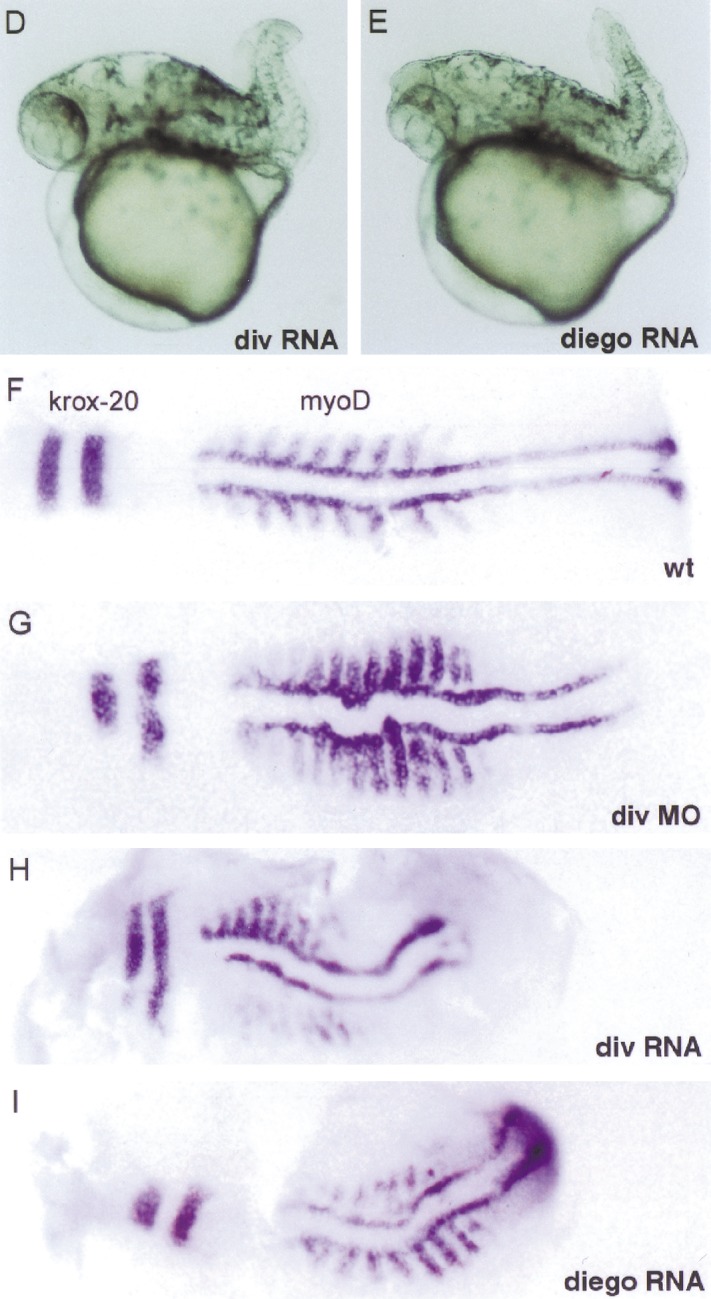
Diversin activates the JNK branch of the Wnt-signaling pathway. (A) Coimmunoprecipitation analyses of CKIε, Diego and Diversin, which show that both Diego and Diversin interact with CKIε. Note that CKIε was transfected here. Immunoprecipitations were performed as described in Fig. 1. Note equal expression of Diego and Diversin. (B) Transfection of 1- and 3-μg Diversin cDNA activate transcription from a JNK-dependent luciferase reporter gene. Diversin (1–3 μg) also enhances Dishevelled (1.5 μg) and Wnt11 (1.5 μg)-induced JNK activity. Wnt-1 (1.5 and 3 μg) do not activate JNK-dependent, but activate Lef/Tcf-dependent transcription (inset). The indicated cDNAs were transfected into 293 cells, and MEKK (3 μg) served as control. (C) Diversin affects gastrulation movements in zebrafish embryos. Diversin induces failure of convergence and extension (CE), or affects dorso-ventral patterning, depending on the amounts of injected mRNA. (D,E) Zebrafish embryos injected with low amounts (0.1 ng) of Diversin and standard amounts (0.4 ng) of Diego mRNA show a shortened body axis and a curled tail, typical for defects in gastrulation movements. (F–I) In situ hybridization of flattened embryos injected with either Diversin MO (1.5 ng), Diversin mRNA (0.1 ng), or Diego mRNA (0.4 ng), respectively, at the 5–10 somite stage. Embryos are stained for myoD to visualize somites. Note the undulation of the body axis, the compression of the anterior-posterior axis, and the shortening of the intersomitic distances in the injected embryos. In situ hybridization with krox20 expressed in the hindbrain (see the two stripes at left) shows that the embryos are not ventralized.
Discussion
We identify here the novel ankyrin repeat protein Diversin that contains three domains, a central domain that interacts with CKIε, a C-terminal domain that mediates interaction to Axin/Conductin, and an ankyrin repeat-containing domain with unknown function. Diversin regulates the Wnt/β-catenin and the Wnt/JNK pathway in negative and positive manners, respectively. This dual function is observed in cultured cells and in the embryo. In the canonical Wnt pathway, Diversin reduces cytoplasmic concentrations of β-catenin and β-catenin-dependent transcription in cultured cells, blocks the formation of the body axis in Xenopus embryos, and controls size and position of the dorsal organizer and the subsequent axis formation in zebrafish embryos. We further show, by epistasis experiments in embryos, that Diversin acts downstream of Dishevelled and CKIε and upstream of GSK3β and β-catenin. In Xenopus embryos, the function of β-catenin in axis formation is well documented (for review, see De Robertis et al. 2000). Previous work also implicated β-catenin as an important signaling molecule during axis formation in zebrafish; inhibition of β-catenin signaling by expression of dominant-negative Frizzled decreased the size of the dorsal organizer, and the ichabod mutation, which interferes with nuclear localization of β-catenin, causes a lack of the dorsal organizer (Nasevicius et al. 1998). In contrast, increased β-catenin signaling by expression of activated β-catenin or dominant-negative GSK3β, or the inhibition of Axin function, increase the size of the organizer and/or induce it at ectopic positions (Nasevicius et al. 1998; Kelly et al. 2000; Heisenberg et al. 2001). Our data show that Diversin is a new negative regulator of the canonical Wnt pathway and is essential for axis formation in early zebrafish development.
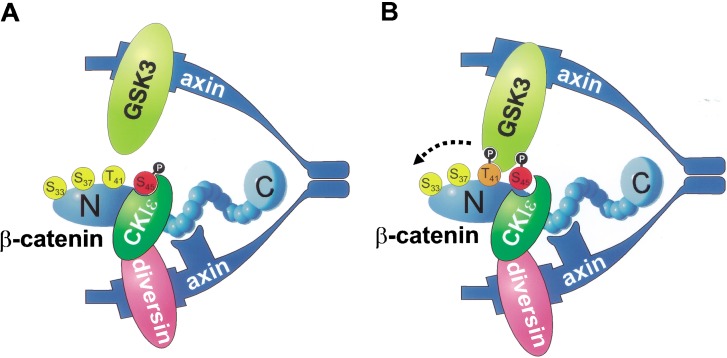
Our biochemical analysis allows us to assign the molecular mechanism by which Diversin functions in the canonical Wnt pathway. Efficient β-catenin degradation requires a two-step mechanism, a priming phosporylation at Ser 45 catalyzed by CKIε or CKIα, and subsequent phosphorylation on three equally spaced serine/threonine residues by GSK3β (Amit et al. 2002; Liu et al. 2002). We show that Diversin recruits the priming kinase CKIε to the Axin/Conductin-GSK3β complex (see scheme in Fig. 6A). Separate domains of Diversin, the central and C-terminal regions, mediate these two interactions. We also show that both Diversin and GSK3β bind simultaneously to dimeric Axin/Conductin, and that they use identical binding sites. Diversin-mediated recruitment of CKIε allows phosporylation of Ser 45 of β-catenin, thus creating a classical GSK3β recognition motif and initiating the subsequent phosphorylation cascade (Fig. 6B; Fiol et al. 1987). A minimal fusion molecule that contains the catalytic domain of CKIε and the Axin/Conductin-binding domain of Diversin is fully functional in β-catenin signaling, showing the role of Diversin as a molecular linker. Diversin is inactive in the presence of Frat-1/GBP, which displaces GSK3β, showing the importance of GSK3β in the complex. Taken together, these data demonstrate that Diversin functions in the canonical Wnt pathway by engaging CKIε to the β-catenin degradation complex, and allows priming phosphorylation and degradation of β-catenin.
Figure 6.
Schematic model of the action of Diversin in β-catenin degradation. (A) Diversin recruits CKIε to the Axin/Conductin complex, and this leads to priming phosphorylation of β-catenin on Ser 45. Dimeric Axin/Conductin allows simultaneous binding of Diversin/CKIε and GSK3β in the complex. (B) GSK3β mediates subsequent phosphorylation of β-catenin on Thr 41, Ser 37, and Ser 33. Phosphorylated Ser 37 and Ser 33 are binding sites for the E3ubiquitin ligase β-TrCP (Amit et al. 2002), which promotes degradation of β-catenin.
We also observed that Diversin activates the JNK branch of the Wnt-signaling pathway, which controls the establishment of planar cell polarity in Drosophila and gastrulation movements in vertebrates (Boutros et al. 1998; Sokol 2000). In zebrafish, inhibition and overexpression of Diversin cause defects in gastrulation movements, that is, a reduction in body length and undulation of the body axis, which are similar to those observed in pipetail (Wnt5a) mutants (Hammerschmidt et al. 1996). Thus, Diversin controls gastrulation movements, as does the Drosophila protein Diego. However, Diego is only in part a functional homolog of Diversin, because it does not interact with Drosophila Axin and has not been implicated in Wnt/β-catenin signaling (Feiguin et al. 2001). Specific to Diversin in vertebrates is its role at a branchpoint of intracellular Wnt signaling, where it represses the canonical Wnt/β-catenin pathway and simultaneously activates the JNK pathway.
Materials and methods
Yeast two-hybrid analysis, plasmids and expression analysis
Yeast two-hybrid screens were performed with members of the Wnt pathway that were cloned into the DNA-binding domain vector and a cDNA library from 10.5-d mouse embryos (Behrens et al. 1998). Full-length Diversin was isolated from a λgt10 mouse embryo cDNA library (Stratagene), by use of a probe that was identified initially in the yeast two-hybrid screen. Deletion mutants of Diversin and CKIε/Diversin-fusions consisting of amino acids 1–301 of human CKIε and amino acids 583–712 of murine or amino acids 595– 729 of zebrafish Diversin, respectively, were constructed by PCR and restriction digests. The Diego and Drosophila Axin cDNAs were cloned by RT–PCR. Transfection of mammalian cells, immunoprecipitations and Western blotting were performed as described (Behrens et al. 1998), using antibodies against β-catenin, GSK3β, CKIε (Transduction Laboratories), Flag (Kodak), and HA (Roche). In situ hybridizations of zebrafish embryos were performed as described (Hammerschmidt et al. 1996).
Lef/Tcf and JNK-dependent reporter assays
The Lef/Tcf reporter assay was essentially performed as described (Korinek et al. 1997) with the following modifications: 293 cells were transfected with 1–5 μg of the indicated plasmids using the standard calcium phosphate method. Cells were harvested 20 h after transfection, and luciferase values were normalized against β-galactosidase activity. The JNK-dependent reporter assay was performed using the PathDetect Kit (Stratagene) according to manufacturer’s instructions. Experiments were carried out in triplicate and repeated at least three times.
mRNA and morpholino injections
Xenopus embryos at the 4-cell stage were injected ventrally with 1–3 ng of the indicated mRNA (Behrens et al. 1998). Zebrafish embryos at the 1–4-cell stage were injected into the yolk with 0.1–0.4 ng of mRNA (TL strain) or 1–6 ng of antisense morpholinos (AB strain; Hammerschmidt et al. 1996; Nasevicius and Ekker 2000). The mRNAs for injection were prepared from linearized plasmid DNAs using the SP6 or T7 mMessage Machine kit (Ambion). Antisense morpholinos were designed and obtained from Gene Tools, LLC Sequences were as follows: Diversin-5′UTR, 5′-CAGCCCTCATGTCCTGAAGA GAATC-3′; Diversin-ATG, 5′-CATCGTGCTGGCTTATGAA TCAGGG-3′; Diversin-5′UTR mismatch control, 5′-CAGCG CTCAAGTCCTCAAGACAATC-3′.
Acknowledgments
We thank Carmen Birchmeier for helpful discussion and critical reading of the manuscript. We thank Drs. H. Clevers, D.J. Sussman, D.M. Virshup, J.M. Graff, and A. Berns for the generous gift of plasmids. We also thank K. Kramer, C. Köster, and W. Herzog for performing expression analyses on zebrafish embryos. This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 366 to W.B. and SFB 271 to M.K.).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL wbirch@mdc-berlin.de; FAX 49-30-94-062-656.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.230402.
References
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. β-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit S, Hatzubai A, Birman Y, Andersen JS, Ben Shushan E, Mann M, Ben Neriah Y, Alkalay I. Axin-mediated CKI phosphorylation of β-catenin at Ser 45: A molecular switch for the Wnt pathway. Genes & Dev. 2002;16:1066–1076. doi: 10.1101/gad.230302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W. Functional interaction of an axin homolog, conductin, with β-catenin, APC, and GSK3β. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–118. doi: 10.1016/s0092-8674(00)81226-x. [DOI] [PubMed] [Google Scholar]
- Cheyette BN, Waxman JS, Miller JR, Takemaru KI, Sheldahl LC, Khlebtsova N, Fox EP, Earnest T, Moon RT. Dapper, a Dishevelled-associated antagonist of β-Catenin and JNK signaling, is required for Notochord formation. Dev Cell. 2002;2:449–461. doi: 10.1016/s1534-5807(02)00140-5. [DOI] [PubMed] [Google Scholar]
- Chijiwa T, Hagiwara M, Hidaka H. A newly synthesized selective casein kinase I inhibitor, N-(2-aminoethyl)-5-chloroisoquinoline-8-sulfonamide, and affinity purification of casein kinase I from bovine testis. J Biol Chem. 1989;264:4924–4927. [PubMed] [Google Scholar]
- Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Larrain J, Oelgeschlager M, Wessely O. The establishment of Spemann's organizer and patterning of the vertebrate embryo. Nat Rev Genet. 2000;1:171–181. doi: 10.1038/35042039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djiane A, Riou J, Umbhauer M, Boucaut J, Shi D. Role of frizzled 7 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development. 2000;127:3091–3100. doi: 10.1242/dev.127.14.3091. [DOI] [PubMed] [Google Scholar]
- Farr GH, III, Ferkey DM, Yost C, Pierce SB, Weaver C, Kimelman D. Interaction among GSK-3, GBP, axin, and APC in Xenopusaaxis specification. J Cell Biol. 2000;148:691–702. doi: 10.1083/jcb.148.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiguin F, Hannus M, Mlodzik M, Eaton S. The ankyrin repeat protein Diego mediates Frizzled-dependent planar polarization. Dev Cell. 2001;1:93–101. doi: 10.1016/s1534-5807(01)00010-7. [DOI] [PubMed] [Google Scholar]
- Fiol CJ, Mahrenholz AM, Wang Y, Roeske RW, Roach PJ. Formation of protein kinase recognition sites by covalent modification of the substrate. Molecular mechanism for the synergistic action of casein kinase II and glycogen synthase kinase 3. J Biol Chem. 1987;262:14042–14048. [PubMed] [Google Scholar]
- Hammerschmidt M, Pelegri F, Mullins MC, Kane DA, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Haffter P, Heisenberg CP, et al. Mutations affecting morphogenesis during gastrulation and tail formation in the zebrafish, Danio rerio. Development. 1996;123:143–151. doi: 10.1242/dev.123.1.143. [DOI] [PubMed] [Google Scholar]
- Hart MJ, de los SR, Albert IN, Rubinfeld B, Polakis P. Downregulation of β-catenin by human Axin and its association with the APC tumor suppressor, β-catenin and GSK3β. Curr Biol. 1998;8:573–581. doi: 10.1016/s0960-9822(98)70226-x. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Houart C, Take-Uchi M, Rauch GJ, Young N, Coutinho P, Masai I, Caneparo L, Concha ML, Geisler R, et al. A mutation in the Gsk3-binding domain of zebrafish Masterblind/Axin1 leads to a fate transformation of telencephalon and eyes to diencephalon. Genes & Dev. 2001;15:1427–1434. doi: 10.1101/gad.194301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu W, Zeng L, Costantini F. Identification of a domain of Axin that binds to the serine/threonine protein phosphatase 2A and a self-binding domain. J Biol Chem. 1999;274:3439–3445. doi: 10.1074/jbc.274.6.3439. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Birchmeier W. New aspects of Wnt signaling pathways in higher vertebrates. Curr Opin Genet Dev. 2001;11:547–553. doi: 10.1016/s0959-437x(00)00231-8. [DOI] [PubMed] [Google Scholar]
- Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3β and β-catenin and promotes GSK-3β-dependent phosphorylation of β-catenin. EMBO J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, Chin AJ, Leatherman JL, Kozlowski DJ, Weinberg ES. Maternally controlled (β)-catenin-mediated signaling is required for organizer formation in the zebrafish. Development. 2000;127:3899–3911. doi: 10.1242/dev.127.18.3899. [DOI] [PubMed] [Google Scholar]
- Kloss B, Price JL, Saez L, Blau J, Rothenfluh A, Wesley CS, Young MW. The Drosophilaclock gene double-time encodes a protein closely related to human casein kinase Iε. Cell. 1998;94:97–107. doi: 10.1016/s0092-8674(00)81225-8. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Kuhl M, Sheldahl LC, Park M, Miller JR, Moon RT. The Wnt/Ca2+ pathway: A new vertebrate Wnt signaling pathway takes shape. Trends Genet. 2000;16:279–283. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- Lekven AC, Thorpe CJ, Waxman JS, Moon RT. Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev Cell. 2001;1:103–114. doi: 10.1016/s1534-5807(01)00007-7. [DOI] [PubMed] [Google Scholar]
- Li L, Yuan H, Weaver CD, Mao J, Farr GH, III, Sussman DJ, Jonkers J, Kimelman D, Wu D. Axin and Frat1 interact with dvl and GSK, bridging Dvl to GSK in Wnt-mediated regulation of LEF-1. EMBO J. 1999;18:4233–4240. doi: 10.1093/emboj/18.15.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- Lu B, Roegiers F, Jan LY, Jan YN. Adherens junctions inhibit asymmetric division in the Drosophilaepithelium. Nature. 2001;409:522–525. doi: 10.1038/35054077. [DOI] [PubMed] [Google Scholar]
- Mlodzik M. Spiny legs and prickled bodies: New insights and complexities in planar polarity establishment. Bioessays. 2000;22:311–315. doi: 10.1002/(SICI)1521-1878(200004)22:4<311::AID-BIES1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Molenaar M, van de WM, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopusembryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Hyatt T, Kim H, Guttman J, Walsh E, Sumanas S, Wang Y, Ekker SC. Evidence for a frizzled-mediated wnt pathway required for zebrafish dorsal mesoderm formation. Development. 1998;125:4283–4292. doi: 10.1242/dev.125.21.4283. [DOI] [PubMed] [Google Scholar]
- Park M, Moon RT. The planar cell-polarity gene stbm regulates cell behaviour and cell fate in vertebrate embryos. Nat Cell Biol. 2002;4:20–25. doi: 10.1038/ncb716. [DOI] [PubMed] [Google Scholar]
- Peters JM, McKay RM, McKay JP, Graff JM. Casein kinase I transduces Wnt signals. Nature. 1999;401:345–350. doi: 10.1038/43830. [DOI] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes & Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P. Association of the APC gene product with β-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- Sakanaka C, Weiss JB, Williams LT. Bridging of β-catenin and glycogen synthase kinase-3β by axin and inhibition of β-catenin-mediated transcription. Proc Natl Acad Sci. 1998;95:3020–3023. doi: 10.1073/pnas.95.6.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanaka C, Leong P, Xu L, Harrison SD, Williams LT. Casein kinase Iε in the wnt pathway: regulation of β-catenin function. Proc Natl Acad Sci. 1999;96:12548–12552. doi: 10.1073/pnas.96.22.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos JA, Logarinho E, Tapia C, Allende CC, Allende JE, Sunkel CE. The casein kinase 1 α gene of Drosophila melanogasteris developmentally regulated and the kinase activity of the protein induced by DNA damage. J Cell Sci. 1996;109:1847–1856. doi: 10.1242/jcs.109.7.1847. [DOI] [PubMed] [Google Scholar]
- Schier AF. Axis formation and patterning in zebrafish. Curr Opin Genet Dev. 2001;11:393–404. doi: 10.1016/s0959-437x(00)00209-4. [DOI] [PubMed] [Google Scholar]
- Sokol S. A role for Wnts in morphogenesis and tissue polarity. Nat Cell Biol. 2000;2:E124–E125. doi: 10.1038/35017136. [DOI] [PubMed] [Google Scholar]
- Su LK, Vogelstein B, Kinzler KW. Association of the APC tumor suppressor protein with catenins. Science. 1993;262:1734–1737. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- Sun TQ, Lu B, Feng JJ, Reinhard C, Jan YN, Fantl WJ, Williams LT. PAR-1 is a Dishevelled-associated kinase and a positive regulator of Wnt signalling. Nat Cell Biol. 2001;3:628–636. doi: 10.1038/35083016. [DOI] [PubMed] [Google Scholar]
- Vielhaber E, Virshup DM. Casein kinase I: From obscurity to center stage. IUBMB Life. 2001;51:73–78. doi: 10.1080/15216540117461. [DOI] [PubMed] [Google Scholar]
- von Kries JP, Winbeck G, Asbrand C, Schwarz-Romond T, Sochnikova N, Dell'Oro A, Behrens J, Birchmeier W. Hot spots in β-catenin for interactions with LEF-1, conductin and APC. Nat Struct Biol. 2000;7:800–807. doi: 10.1038/79039. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopusgastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- Winklbauer R, Medina A, Swain RK, Steinbeisser H. Frizzled-7 signalling controls tissue separation during Xenopusgastrulation. Nature. 2001;413:856–860. doi: 10.1038/35101621. [DOI] [PubMed] [Google Scholar]
- Wong CM, Fan ST, Ng IO. β-Catenin mutation and overexpression in hepatocellular carcinoma: Clinicopathologic and prognostic significance. Cancer. 2001;92:136–145. doi: 10.1002/1097-0142(20010701)92:1<136::aid-cncr1301>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Moriguchi T, Masuyama N, Kusakabe M, Hanafusa H, Takada R, Takada S, Nishida E. JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 2002;3:69–75. doi: 10.1093/embo-reports/kvf008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Wallingford JB, Sun TQ, Nelson AM, Sakanaka C, Reinhard C, Harland RM, Fantl WJ, Williams LT. Cell autonomous regulation of multiple Dishevelled-dependent pathways by mammalian Nkd. Proc Natl Acad Sci. 2001;98:3802–3807. doi: 10.1073/pnas.071041898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa S, Matsuda Y, Lee J, Matsubayashi H, Sese S, Kadowaki T, Ishimoto A. Casein kinase I phosphorylates the Armadillo protein and induces its degradation in Drosophila. EMBO J. 2002;21:1733–1742. doi: 10.1093/emboj/21.7.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL, III, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- Zeng W, Wharton KA, Jr, Mack JA, Wang K, Gadbaw M, Suyama K, Klein PS, Scott MP. naked cuticle encodes an inducible antagonist of Wnt signalling. Nature. 2000;403:789–795. doi: 10.1038/35001615. [DOI] [PubMed] [Google Scholar]