Abstract
The RecBCD enzyme from Escherichia coli is an ATP-dependent helicase and an ATP-stimulated nuclease. The 3′ → 5′ exonuclease activity on double-stranded DNA is suppressed when the enzyme encounters a recombinational hot spot, called chi (χ). We have prepared a RecB deletion mutant (RecB1–929) by using results of limited proteolysis experiments that indicated that the RecB subunit consists of two main domains. The RecB1–929 protein, comprising the 100-kDa N-terminal domain of RecB, is an ATP-dependent helicase and a single-stranded DNA-dependent ATPase. Reconstitution of RecB1–929 with RecC and RecD leads to processive unwinding of a linearized plasmid. However, the reconstituted RecB1–929CD enzyme has lost the single-strand endo- and exonuclease and the double-strand exonuclease activities of the RecBCD enzyme. These results show that the 30-kDa C-terminal domain of RecB has an important role in the nuclease activity of RecBCD. On the basis of these findings, we propose the RecB C-terminal domain swing model to explain RecBCD’s transformation from a 3′ → 5′ exonuclease to a helicase when it meets a χ site.
RecBCD enzyme (exonuclease V) from Escherichia coli is a 330-kDa enzyme consisting of three nonidentical subunits encoded by the recB, recC, and recD genes (1). It is an ATP-dependent helicase and an ATP-stimulated nuclease. The χ sequence (5′-GCTGGTGG-3′) plays an important role in inhibiting the 3′ → 5′ exonuclease activity of RecBCD (2–4). A χ site is recognized by RecBCD if the enzyme enters a DNA molecule on the 3′ side of χ (5). The enzyme degrades the χ-containing DNA with a 3′ → 5′ polarity, until χ is recognized (2–4). RecBCD cuts the DNA strand containing the χ sequence 4, 5, or 6 nucleotides to the 3′ side of χ as it is written above (5). The 3′ → 5′ exonuclease activity of the enzyme is then attenuated either by changing the nuclease polarity to a 5′ → 3′ direction as suggested (2, 3) or changing its function from a nuclease to a helicase. χ-modified RecBCD produces a long 3′ single-stranded overhang that the E. coli RecA protein binds for recombinational and repair processes (6–8).
The RecD ejection model (9, 10) proposes that a χ sequence affects the function of RecD or causes its ejection from RecBC, thereby suppressing the nonspecific exonuclease function of RecBCD (6, 11). The RecD ejection model has been proposed and supported by several important observations both in vivo and in vitro. Two of these observations are (i) that cells with null mutations in the recD gene do not have exonuclease activity and χ-specific recombination but are highly recombination-proficient (12, 13) and (ii) that χ-encountered RecBCD becomes “enzymatically equivalent” (6) to RecBC(D−) both in vivo (14–17) and in vitro (18–21). RecD clearly has an important role in the double-stranded DNA (dsDNA) exonuclease activity of RecBCD (12, 13), but a nuclease active site has not been located in RecD. Rather, RecD is homologous to several helicases (22–24).
The nonspecific dsDNA exonuclease activity of RecBCD can also be suppressed in vitro by reducing the free Mg2+ concentration (8). At low [Mg2+], RecBCD unwinds a linear dsDNA substrate, producing full-length single-stranded DNA (ssDNA) (25–29). A χ sequence and Mg2+ thus have opposite effects on the functions of RecBCD: Mg2+ shifts RecBCD toward its nuclease form, whereas χ shifts RecBCD toward its helicase form. RecD can be dissociated from RecBC with incubation of RecBCD in 4 M NaCl (13, 30), but limiting Mg2+ does not cause RecD to dissociate from RecBC, as observed during RecBCD purification. The RecBCD holoenzyme can be purified in the absence of any Mg2+ in the purification buffer. Thus, it is not clear why RecBCD is a helicase instead of a nuclease at limiting free Mg2+ concentration.
Herein we report the construction of a RecB truncation mutant (RecB1–929) by deleting the DNA encoding the C-terminal 251 amino acids of RecB. The RecB1–929 protein is an ATP-dependent helicase and a ssDNA-dependent ATPase. The RecB1–929 protein associates with the RecC subunit and the histidine-tagged RecD subunit (hRecD or hD; ref. 31). The reconstituted RecB1–929ChD enzyme is a processive helicase that is able to unwind linearized plasmid DNA. However, the RecB1–929ChD enzyme is not able to cut any of the three substrates (circular ssDNA, linear ssDNA, and linear dsDNA) that are degraded by RecBCD. These findings suggest an alternative model to explain the RecBC(D−) phenotypic behavior of χ-modified RecBCD: we propose a C-terminal 30-kDa domain swing model in which the C-terminal 30-kDa domain of RecB swings away from the DNA upon encountering the χ site, a recombinational hot spot, thereby attenuating the 3′ → 5′ exonuclease activity of RecBCD.
MATERIALS AND METHODS
Limited Proteolysis of RecB and N-Terminal Sequencing.
The purified RecB protein was treated with 1–2 μg of subtilisin Carlsberg (Sigma) per mg of RecB in 0.2 M potassium phosphate, pH 6.8/5 mM DTT at 37°C. Samples were removed at the indicated times, quenched with 5 mM phenylmethylsulfonyl fluoride (Sigma), and analyzed by SDS/PAGE. To prepare the samples for N-terminal sequencing, the digested protein bands in the SDS/PAGE gel were transferred to a poly(vinylidene difluoride) membrane (Millipore) before they were stained. The N-terminal sequencing was performed with an Applied Biosystems model 470 gas-phase protein sequencer.
Expression and Purification of the RecB1–929 Deletion Mutant and the RecB Protein.
The plasmid pPB700 (32) was digested with PstI and the 7354-bp fragment was recircularized. The resulting plasmid (pMY100) encodes the first 929 amino acids of RecB followed by 7 amino acids (KLLFWRM) encoded by the pKK223–3 vector sequence. The plasmid pMY100 was transformed into V186 cells (Δ)recBCD, ref. 33). The RecB1–929 deletion mutant and the RecB protein were purified as described (32) except for the following modifications: the cells were lysed by sonication, the DNA precipitation step was omitted, and a DEAE-cellulose column (Whatman DE52) was added to remove the DNA. The purification of the target protein (the RecB subunit or the RecB1–929 deletion mutant) was continued by using chromatography on hydroxylapatite, followed by a heparin-agarose column (Sigma) or a ssDNA-agarose column (Sigma).
RecBCD (34) and RecC (32) were purified as described. RecBC and RecB1–929C were prepared by mixing purified RecB1–929 (or RecB) and RecC proteins as described (20). The hRecD protein was purified in denatured form, renatured, and reconstituted with the other subunits as described (31).
Helicase Assays.
Two substrates were used in the DNA unwinding assays. The first substrate, a 21-bp oligomer with 4-nt 3′ overhangs at both ends, was prepared by annealing two 25-mers [5′-TGCACATCGACATCCAGCCGTAGTA-3′ and 5′-ACGGCTGGATGTCGATGTGCATGTGCAAGTA-3′ (DNA International; Lake Oswego, OR)]. The second strand was 32P-end-labeled at the 5′ terminus with T4 polynucleotide kinase. Reactions containing 4 nM 5′-32P-labeled DNA molecules and 1 mM ATP in buffer A [25 mM Mops⋅KOH, pH 7.0/10 mM MgCl2/10 mM KCl/1 mM DTT/acetylated nuclease free BSA (0.1 mg/ml)] at 37°C were started by adding the indicated enzymes. Samples were removed at the indicated times, quenched by adding 0.25 vol of quenching/loading dye (65 mM EDTA/30% glycerol/0.25% bromophenol blue), and analyzed by electrophoresis in a 15% polyacrylamide gel in TBE (89 mM Tris borate, pH 8.0/2 mM EDTA) at room temperature. The helicase assay with the plasmid DNA, pBR322χ+FH (obtained from Gerald Smith, Fred Hutchinson Cancer Research Center, Seattle, WA) was performed as described (31).
Nuclease Assays.
Three different substrates were used in the nuclease assays. The ssDNA exonuclease reaction using 100 nM single-stranded 5′-32P-labeled 25-mer (the second strand in the preparation of the 21-bp oligomer) and 200 μM ATP in buffer A at 37°C was initiated by adding the indicated enzymes. Two-microliter samples were removed, quenched with 2 μl of quench/loading dye solution (70% formamide/80 mM EDTA/0.2% bromophenol blue), and loaded on 20% nondenaturing polyacrylamide gels. The radioactivity in the gel was analyzed on a Molecular Dynamics PhosphorImager with imagequant software. The ssDNA endonuclease reaction containing 6 nM circular M13 ssDNA molecules as the substrate in 50 mM Mops⋅KOH, pH 7.0/10 mM MgCl2/0.5 mM ATP at 37°C, was initiated by adding the indicated enzymes. Samples were removed at the indicated times, quenched by adding 6X stop buffer (40% glycerol/0.125% bromophenol blue/0.12 M EDTA), and loaded on a 0.8% agarose gel in TBE. After electrophoresis, the gels were stained with ethidium bromide (5 μg/ml). The dsDNA exonuclease assay using [3H]pTZ19R DNA linearized with AvaI (specific radioactivity of 3.7 × 107 cpm/μmol of nucleotide; pTZ19R is from U.S. Biochemicals) was performed as described (34).
RESULTS
Presence of Two Main Domains in RecB.
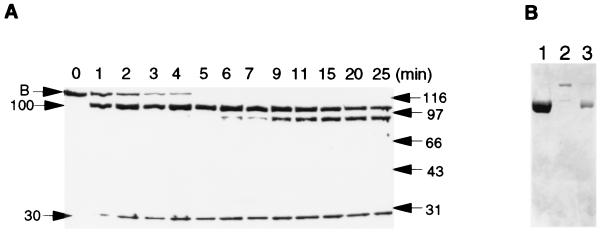
The RecB protein is susceptible to proteolysis during purification, producing two major polypeptides of about 100 kDa and 30 kDa on SDS/PAGE (data not shown). Subsequent limited proteolysis of the purified RecB protein with a nonspecific serine protease, subtilisin Carlsberg, produced two major fragments (Fig. 1A). Automated N-terminal sequencing of the two fragments revealed that cleavage occurred in the region of residues 928–933. The 100-kDa fragment was degraded further at longer digestion times, but the 30-kDa domain resisted further proteolysis. These results suggest that RecB is organized into a loosely folded 100-kDa N-terminal domain, with several subdomains, and a tightly folded 30-kDa C-terminal domain. An exposed linker segment (residues 928–933) connects the two domains.
Figure 1.
Limited proteolysis of the purified RecB protein (A) and purification of the RecB1–929 deletion mutant (B). (A) The RecB protein (100 μg) was digested by adding subtilisin (0.09 μg) in 0.2 M potassium phosphate, pH 6.8/5 mM DTT at 37°C. Samples were analyzed on a 10% SDS/PAGE gel. (B) SDS/PAGE analysis of the purified RecB1–929 protein (lane 1), partially digested RecB (lane 2), and the purified 100-kDa fragment from limited proteolysis (lane 3).
Chromatographic separation of the two fragments, generated by limited proteolysis, was achieved with a ssDNA-agarose column or a heparin-agarose column. The 30-kDa domain did not bind to these columns, whereas the 100-kDa fragment was eluted with an elution pattern identical to intact RecB, suggesting that the 100-kDa domain is responsible for binding DNA. Our initial assays contained the subtilisin-generated fragments. No activities were detected with the 30-kDa fragment, either by itself or in combination with RecC and RecD. The N-terminal 100-kDa domain has an ATP binding motif, as well as six other sequences conserved among DNA and RNA helicases (22–24). The 100-kDa fragment was found to be a helicase and an ATPase (data not shown) as predicted by these sequence alignment data. Because it was difficult to remove the remaining intact RecB by chromatography (Fig. 1B, lane 3), we constructed a plasmid that expressed the truncated RecB protein, RecB1–929 (Fig. 1B, lane 1). All in vitro assays presented in this paper were performed with the RecB1–929 deletion mutant.
Functions of the N-Terminal 100-kDa Domain of RecB.
The RecB1–929 protein is a ssDNA-dependent ATPase and an ATP-dependent helicase. Like RecB (35), the RecB1–929 protein is a poor ATPase using double-stranded calf thymus DNA cofactor (data not shown). However, 2 nM RecB1–929 protein hydrolyzed 70 μM ATP in 10 min using 100 μM total ATP, 200 μM (nucleotides) poly(dT) as the DNA cofactor, whereas 2 nM intact RecB protein hydrolyzed 62 μM ATP in 10 min in the identical assay conditions (data not shown). These findings establish two important points: (i) The RecB1–929 deletion mutant is as active as the intact RecB subunit, thereby eliminating the possibility that the deletion of its C-terminal domain caused any structural damage to the RecB1–929 protein. (ii) Both RecB and the deletion mutant are ssDNA-dependent ATPases. Helicases in general have higher affinity for ssDNA than dsDNA (36).
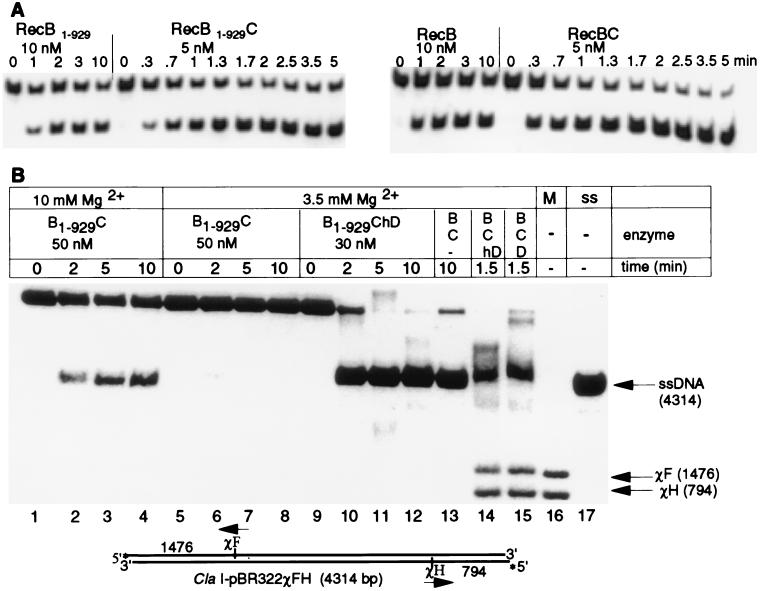
The purified RecB1–929 protein is able to unwind a 21-bp oligomer (Fig. 2A). The rate and extent of unwinding are comparable to that of intact RecB. The reconstituted RecB1–929C protein at a given enzyme concentration unwinds the 21-bp oligomer faster and to a greater extent than RecB1–929 does, indicating that RecB1–929 associates with RecC (Fig. 2A Left). Analysis of protein mixtures by native gel electrophoresis in imidazole/Hepes buffer at pH 7.5 also showed that the interaction between RecB1–929 and RecC occurs. The mobility of the 100-kDa fragment showed a distinctive shift when it was mixed with RecC, forming a new band with slightly greater mobility than RecBC (data not shown). No such shift occurred when the 30-kDa proteolytic fragment was mixed with RecC, but rather two distinct bands corresponding to RecC and 30-kDa domain, respectively, were observed. Furthermore, the ATPase activities of both RecB and RecB1–929 are low with 1 μM (dT)12 cofactor at 45 μM ATP concentration (Kmapp of (dT)12 = 4. 5 μM for RecBC at 1.2 mM ATP; ref. 37), but adding RecC to the RecB1–929 deletion mutant increases the activity 10-fold and adding RecC to the intact RecB protein increases the activity 2- to 3-fold (data not shown). These results show that the protein–protein interaction domain of the RecB subunit with RecC lies in the N-terminal domain of RecB.
Figure 2.
Helicase assays. (A) Unwinding of a 21-bp DNA fragment by RecB1–929 and reconstituted RecB1–929C (Left) and by RecB and reconstituted RecBC (Right). (B) Unwinding of ClaI-digested [5′-32P]pBR322χ+FH by using the indicated enzymes and 50 nM RecBC (lane 13), 10 nM RecBChD (lane 14), and 1 nM RecBCD (lane 15). Each reaction contained 2 nM DNA molecules and 5 mM ATP, with the indicated Mg2+ concentration in buffer A. Aliquots were analyzed by electrophoresis on a 1% agarose gel. Lane 16 (M), χ-markers: ClaI-digested [5′-32P]pBR322 χ+FH, cleaved again with AvaI and then heat-denatured. The AvaI sites are 75 nt away from χF and 13 nt away from χH. Lane 17 (ss), heat-denatured ClaI-digested [5′-32P]pBR322 χ+FH.
Neither the intact RecB protein nor the RecB1–929 protein by themselves (100 nM) unwound a 105-bp DNA fragment (no unwound product detected in 30 min; data not shown). However, both the reconstituted RecB1–929C enzyme (50 nM) and RecBC (50 nM) unwound linearized pBR322χ+FH plasmid DNA (Fig. 2B, lanes 1–4 and 13), suggesting that the role of RecC is to increase the processivity of unwinding by RecB. Comparison of the unwinding activity of reconstituted RecB1–929ChD (Fig. 2B, lanes 9–12) to that of RecB1–929C (Fig. 2B, lanes 5–8) at the same Mg2+ concentration (3.5 mM, with 5 mM ATP) clearly demonstrated that the hRecD associated with the RecB1–929C complex. RecB1–929ChD (30 nM) unwound the 4,300-bp linearized plasmid to near completion (93%, Fig. 2B, lane 12), whereas unwinding was barely detectable (3%) with 50 nM RecB1–929C under these conditions (Fig. 2B, lanes 5–8). This observation, in turn, suggests that protein–protein interactions occur between RecD and the N-terminal 100-kDa domain of RecB and/or RecC. Adding more Mg2+ (10 mM, with 5 mM ATP) to the reaction mixture with the RecB1–929C complex, however, led to processive unwinding of the plasmid (up to 35% unwound, Fig. 2B, lane 4). The higher Mg2+ concentration somewhat compensates for the lack of RecD and the C-terminal 30-kDa fragment of RecB in the reaction with the RecB1–929C complex (Fig. 2B, lanes 1–4). This observation indicates that RecD, the C-terminal 30-kDa domain of RecB, and Mg2+ may play similar role(s) in the processive unwinding of a long piece of dsDNA.
The most interesting observation in the assay is that the RecB1–929ChD complex (Fig. 2B, lanes 9–12) is functionally equivalent to the RecBC protein (Fig. 2B, lane 13). Both are competent helicases capable of unwinding the linearized plasmid to near completion. Neither generates the χ-specific fragments, but the RecBChD protein (Fig. 2B, lane 14) and the wild-type RecBCD (Fig. 2B, lane 15) produced both the full-length ssDNA and χ fragments. Therefore, χ-modified RecBCD in vivo could resemble RecBC as the RecD ejection model suggested (9, 10) or it could resemble RecB1–929ChD with the C-terminal 30-kDa domain of RecB sequestered away from the χ-containing DNA strand. The dsDNA exonuclease function of RecBCD is necessary for χ-dependent recombination (12). Because RecBC and RecB1–929ChD do not generate the χ fragments, they could not be a dsDNA exonuclease. Therefore, we conclude that there are two ways to suppress the dsDNA exonuclease function when RecBCD encounters a χ site: either by losing RecD or by losing the C-terminal 30-kDa domain of RecB.
What Role(s) Might the C-Terminal 30-kDa Domain of RecB Play in the Multiple RecBCD Enzymatic Activities?
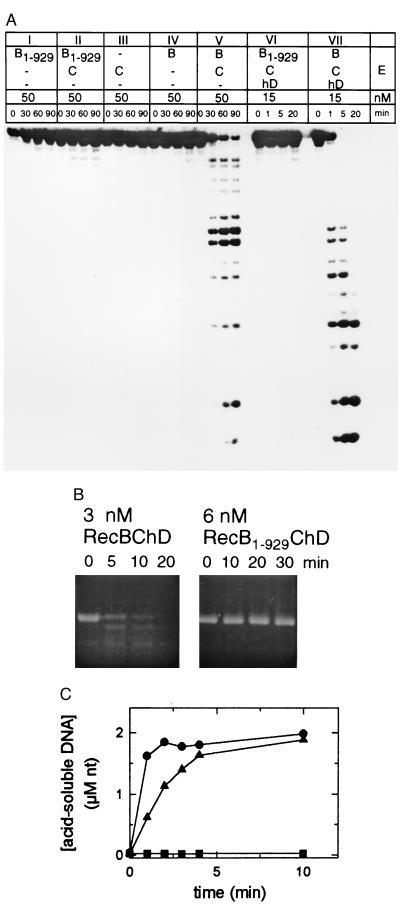
On recognition of χ, sequestering the C-terminal 30-kDa domain of RecB from the χ-containing DNA strand, instead of losing RecD, may be a better strategy for RecBCD to protect the long χ-specific 3′ single-stranded overhang necessary for homologous recombination. The highly processive helicase RecB1–929ChD shows no signs of DNA strand scission with either the single-stranded linear 25-mer (Fig. 3A, panel VI) or the single-stranded circular M13 phage DNA (Fig. 3B). Although the ssDNA exonuclease activity of reconstituted RecBC (Fig. 3A, panel V) is much slower than that of reconstituted RecBChD (Fig. 3A, panel VII), the DNA degradation, nevertheless, occurs in the absence of RecD. The degradation pattern of the linear single-stranded 25-mer by the RecBC enzyme (Fig. 3A, panel V) shows that RecBC cuts the substrate mostly in the middle. RecBC has been found to cleave single-stranded circular M13 phage DNA (21). Both the reconstituted RecBChD protein and the wild-type RecBCD enzyme rapidly degraded tritium-labeled linearized plasmid DNA, but the highly processive helicase RecB1–929ChD did not (Fig. 3C). RecBC (8 nM) produced 0.6 μM acid-soluble nucleotides in 10 min at 37°C (about 10 times the background level), whereas 0.2 nM RecBCD produced 6.2 μM acid-soluble nucleotides in reaction mixtures containing 10 mM MgCl2, 500 μM ATP, and 68 μM [3H]dsDNA, at pH 7.0 (data not shown). Although RecBC is an insignificant dsDNA exonuclease compared with RecBCD, RecBC has at least 10 times more dsDNA exonuclease activity than RecB1–929ChD; 9 nM RecB1–929ChD produced no acid-soluble DNA above background, 0.03 μM nucleotides (Fig. 3C). Thus, losing the C-terminal 30-kDa domain of RecB is a more efficient way of suppressing the dsDNA exonuclease activity of RecBCD than is losing RecD.
Figure 3.
Nuclease assays. All reaction mixtures contained buffer A and 10 mM MgCl2. (A) ssDNA exonuclease assay with 100 nM single-stranded linear 5′-32P-labeled 25-mer as the substrate. (B) ssDNA endonuclease assay using 6 nM single-stranded circular M13 phage DNA as the substrate. (C) dsDNA exonuclease assay using AvaI-digested [3H]pTZ19R. Each reaction contained 2.3 μM (nucleotides) double-stranded [3H]pTZ19R, 250 μM ATP, and 9 nM RecB1–929CD (•), 0.2 nM RecBCD (▴), or 9 nM RecBChD (▪).
DISCUSSION
We have presented evidence that the N-terminal 100-kDa domain of RecB is an ATP-dependent helicase and that it associates with RecC and hRecD. The reconstituted RecB1–929ChD enzyme is a highly processive helicase that is able to unwind a linear 4,300-bp plasmid (Fig. 2B). Deletion of the C-terminal 30-kDa domain of the RecB subunit, however, abolishes all nuclease activities of the RecBCD enzyme (Fig. 3). This observation is in complete agreement with the results obtained with the AddAB enzyme, an ATP-dependent nuclease from Bacillus subtilis. Deletion of the C-terminal region of the AddA protein, a RecB homologue, abolished the exonuclease activity of AddAB but left its helicase activity intact (38). Two C-terminal regions of AddA (residues 1153–1176 and 1189–1216) are homologous to two regions in the C-terminal 30-kDa domain of RecB (residues 1061–1084 and 1099–1126) (38).
The results presented in this paper (Fig. 3) imply that RecBCD has a single nuclease catalytic center that works on the three different forms of DNA. If RecBCD relied on three different sets of active site residues to cleave its three different substrates, then disabling one catalytic center by deletion would not abolish strand scission on all of the RecBCD substrates. There are, however, two other possible explanations for the total loss of the nuclease activity after deletion of the 30-kDa domain: (i) It is possible that the truncated RecB1–929 protein is unable to associate with RecC or RecD, which might have the nuclease active site(s). The helicase assays (Fig. 2), along with ATPase and native protein gel data, clearly argue against this notion. Also, the (dT)12-dependent ATPase activity at a low concentration of (dT)12 was higher with RecB1–929C than RecBC. (ii) The deletion of the C-terminal 30-kDa domain of RecB could have inactivated the nuclease active site(s) through a global conformational change. This possibility could be ruled out if we could locate the nuclease active site in the C-terminal 30-kDa domain of RecB. We suspect two reasons for the inactivity associated with the subtilisin-generated 30-kDa domain of RecB either by itself or with combinations of RecC or hRecD: (i) The C-terminal 30-kDa domain of RecB does not bind DNA, as indicated by its chromatographic behavior on the ssDNA-agarose column. The 30-kDa fragment flowed through the column, whereas the 100-kDa domain was bound to the column. (ii) The 30-kDa fragment may not assemble productively with the other subunits without the N-terminal 100-kDa domain. The protein–protein interactions of RecB with RecC and RecD occur through the N-terminal 100-kDa domain, as the helicase data (Fig. 2) and native protein gel indicated.
RecBCD is an unusual class of enzyme with many contradictory functions. It functions as a nonspecific endonuclease and exonuclease but at the same time recognizes a χ site. The χ-activated RecBCD (similar to RecBC) in vitro is reversible by adding more Mg2+ to the reaction mixture (18). The data indicated that the χ-modified RecBCD enzyme becomes a nonspecific dsDNA exonuclease in the presence of 10 mM Mg2+; the subsequent unwinding of the leftover substrate did not lead to accumulation of fully ssDNA (18). This finding indicates that Mg2+ and χ have opposite effects on the conformational change of RecBCD to regulate its nuclease and helicase functions. Is it possible that in the in vitro experiment (18) χ may have broken an electrostatic interaction between RecD and the C-terminal domain of RecB instead of causing ejection of RecD? Reconstitution of RecBC is easily done on ice or at 37°C, but reconstituting hRecD to the RecBC complex has been a challenging problem; it usually takes overnight incubation at room temperature (31). Therefore, it is highly unlikely that the ejected RecD subunit could reassociate with the RecBC complex in the in vitro experiment at the given time and temperature (18).
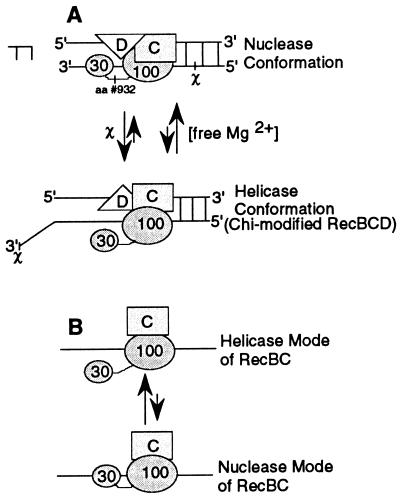
We have reported in this paper that the reconstituted RecB1–929ChD protein is a better helicase than reconstituted RecBC under identical in vitro assay conditions (Fig. 2B). However, our observation indicated that the reconstituted RecBC protein is a better dsDNA exonuclease than reconstituted RecB1–929ChD (Fig. 3C). Thus, we propose an alternate model, the C-terminal 30-kDa domain swing model, to explain the event leading to suppression of the nuclease function of RecBCD by χ (Fig. 4A). In the 30-kDa domain swing model, RecBCD scans the DNA looking for a χ site, while processively degrading it at the same time. On finding χ, a global conformational change in the RecBCD holoenzyme forces the C-terminal cleavage domain of RecB to swing out of the χ-containing DNA strand, around the flexible linker segment (residues 928–933) that was prone to proteolysis (Fig. 1A). This “swing” of the cleavage domain may cause transformation of RecBCD from a 3′ → 5′ nuclease to a helicase, as suggested by the RecD ejection model (9, 10), or to a 5′ → 3′ nuclease as more recent in vitro studies suggested (2, 3). In the C-terminal 30-kDa domain swing model, the C-terminal 30-kDa domain of RecB simply swings in and out of the DNA around the linker segment, depending on the Mg2+ concentration, explaining the low nonspecific nuclease activity when Mg2+ is low.
Figure 4.
C-terminal 30-kDa domain swing model. (A) The effects of χ and Mg2+ on the conformation of RecBCD. The helicase form, in which the 30-kDa domain is removed from the DNA, is promoted by χ and by low concentrations of free Mg2+. The nuclease conformation, in which the 30-kDa domain swings back to the DNA, is favored at high concentrations of free Mg2+. (B) The possible role of RecD in the processive exonuclease activity by RecBCD. The helicase form of RecBC is favored in the absence of RecD, but the presence of RecD shifts the equilibrium to processive nuclease mode of RecBCD (A) in which rapid degradation of DNA occurs.
The recB2109 mutation abolishes χ recognition by RecB2109CD but leaves nonspecific nuclease activity intact (39), suggesting either the N-terminal 100-kDa domain or the C-terminal 30-kDa domain of RecB has the recognition domain for χ sequence. We speculate that the recognition of χ occurs through interaction of residues in the N-terminal 100-kDa domain of RecB and a χ sequence. If the recognition and the cleavage of specific DNA sequences occur by the cleavage domain like most type II restriction endonucleases, such as BamHI or EcoRI (40), then the cleavage should occur within the recognition sequence, not 4, 5, or 6 nt away from the recognition site.
The recently published crystal structure of FokI restriction endonuclease shows that FokI regulates its nonspecific nuclease activity by “sequestration” of the C-terminal cleavage domain until it is needed (41). This sequestration is made possible by extensive electrostatic interactions between the C-terminal cleavage domain and the N-terminal recognition domain. FokI recognizes a specific DNA sequence and cleaves DNA phosphodiester bonds 9 and 13 bp away from the recognition site; RecBCD recognizes a χ sequence and cleaves the DNA phosphodiester bonds 4–6 nt away from the recognition site. Does the difference in the cutting sites away from the recognition sites (9–13 bp for FokI and 4–6 nt for RecBCD) reflect the differences in the lengths of their linker segments (14 residues for FokI and 6 residues for RecBCD)?
If RecB carries out both the nuclease and the helicase activities, what roles do the other two subunits, RecC and RecD, play in RecBCD enzymatic activities? We speculate, in the absence of any structural data, that RecD might be an accessory factor that helps the C-terminal cleavage domain of RecB position properly on the DNA for processive cleavage to occur. We were able to detect a low level of nuclease activity with RecBC by using a linear 25-mer, indicating that RecD is not essential for cleavage of phosphodiester bonds of linear DNA (Fig. 3A). Reconstitution of hRecD to RecBC, however, allows the nuclease activity to proceed much faster with less enzyme. In RecBC, without RecD, the 30-kDa domain of RecB swings in and out of the DNA around the flexible exposed linker segment, favoring the helicase mode where the cleavage domain is away from the DNA (Fig. 4B). When RecD is present, the equilibrium shifts to the processive nuclease mode (Fig. 4A). RecC might be a clamp factor that allows RecB to unwind a DNA substrate processively. We observed that both RecBC and RecB1–929C unwound the 4,300-bp linear DNA, whereas RecB was not able to unwind a 105-bp DNA fragment.
The data that we have presented to develop a new model, the C-terminal 30-kDa domain swing model, do not contradict the in vivo and the in vitro data on which the RecD ejection model is based: (i) The dsDNA exonuclease function of RecBCD is the prerequisite for χ-dependent recombination (12): only the efficient dsDNA exonucleases (RecBChD and RecBCD, Fig. 3C) generated the χ fragment (Fig. 2B). (ii) χ-activated RecBCD is “phenotypically equivalent to RecBC(D−) (17).” Both RecBC and RecB1–929ChD are competent helicases (Fig. 2B). The evidence presented in this paper has shown that RecBCD could swing its C-terminal cleavage domain in and out of the DNA, around the linker segment, to regulate its degradative (nuclease) and recombinogenic (helicase) functions. RecBCD, which has survived the vigorous evolutionary selection process, must have developed an efficient mechanism to suppress its nuclease activities after encountering a χ site. We have presented evidence in this paper suggesting that sequestering the C-terminal 30-kDa domain of RecB, from DNA, is a more efficient way to suppress the three different nuclease activities of RecBCD than ejecting RecD. Ejecting RecD (RecBC) still leaves the ssDNA endonuclease and the ssDNA exonuclease functions of RecBCD (Fig. 3), but sequestering the C-terminal 30-kDa domain of RecB (RecB1–929CD) abolishes all nuclease activities of RecBCD. If RecD were ejected after RecBCD encounters a χ site, rather than the C-terminal 30-kDa domain of RecB being sequestered, a long χ-specific 3′ ssDNA overhang necessary for recombination would have suffered from ssDNA exonuclease attack by RecBC (Fig. 3A, panel V).
Acknowledgments
We thank Dr. Brian Martin of the Natonal Institutes of Health for the N-terminal sequencing. We also thank Dr. Sarah Woodson and Dr. Jason Kahn for the careful reading of this manuscript. This work was supported by funding from the National Institute of Health Grant GM39777.
ABBREVIATIONS
- hRecD
histidine-tagged RecD protein
- ssDNA
single-stranded DNA
- dsDNA
double-stranded DNA
References
- 1.Smith G R. Microbiol Rev. 1988;52:1–28. doi: 10.1128/mr.52.1.1-28.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson D G, Kowalczykowski S C. Cell. 1997;90:77–86. doi: 10.1016/s0092-8674(00)80315-3. [DOI] [PubMed] [Google Scholar]
- 3.Anderson D G, Churchill J J, Kowalczykowski S C. Genes to Cells. 1997;2:117–128. doi: 10.1046/j.1365-2443.1997.1130311.x. [DOI] [PubMed] [Google Scholar]
- 4.Dixon D A, Kowalczykowski S C. Cell. 1993;73:87–96. doi: 10.1016/0092-8674(93)90162-j. [DOI] [PubMed] [Google Scholar]
- 5.Ponticelli A S, Schultz D W, Taylor A F, Smith G R. Cell. 1985;41:145–151. doi: 10.1016/0092-8674(85)90069-8. [DOI] [PubMed] [Google Scholar]
- 6.Myers R S, Stahl F W. Annu Rev Genet. 1994;28:49–70. doi: 10.1146/annurev.ge.28.120194.000405. [DOI] [PubMed] [Google Scholar]
- 7.Taylor A F, Schultz D W, Ponticelli A S, Smith G R. Cell. 1985;41:153–163. doi: 10.1016/0092-8674(85)90070-4. [DOI] [PubMed] [Google Scholar]
- 8.Kowalczykowski S C, Dixon D A, Eggleston S D, Lauder S D. Microbiol Rev. 1994;58:401–465. doi: 10.1128/mr.58.3.401-465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thaler D S, Sampson E, Siddiqi I, Rosenberg S M, Stahl F W, Stahl M. In: Mechanisms and Consequences of DNA Damage Processing. Friedberg E, Hanawalt P, editors. New York: Liss; 1988. pp. 413–422. [Google Scholar]
- 10.Thaler D S, Sampson E, Siddiqi I, Rosenberg S M, Thomason L C, Stahl F W, Stahl M M. Genome. 1989;31:53–67. doi: 10.1139/g89-013. [DOI] [PubMed] [Google Scholar]
- 11.Stahl F W, Thomason L C, Siddiqi I, Stahl M M. Genetics. 1990;126:519–533. doi: 10.1093/genetics/126.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaudhury A M, Smith G R. Proc Natl Acad Sci USA. 1984;81:7850–7854. doi: 10.1073/pnas.81.24.7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amundsen S K, Taylor A F, Chaudhury A M, Smith G R. Proc Natl Acad Sci USA. 1986;83:5558–5562. doi: 10.1073/pnas.83.15.5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaudhury A M, Smith G R. Mol Gen Genet. 1985;201:525–528. doi: 10.1007/BF00331350. [DOI] [PubMed] [Google Scholar]
- 15.Rinken R, Thoms B, Wackernagel W. J Bacteriol. 1992;174:5424–5429. doi: 10.1128/jb.174.16.5424-5429.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Köppen A, Krobitsch S, Thomas B, Wackernagel W. Proc Natl Acad Sci USA. 1995;92:6249–6253. doi: 10.1073/pnas.92.14.6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myers R S, Kuzminov A, Stahl F W. Proc Natl Acad Sci USA. 1995;92:6244–6248. doi: 10.1073/pnas.92.14.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dixon D A, Churchill J J, Kowalczykowski S C. Proc Natl Acad Sci USA. 1994;91:2980–2984. doi: 10.1073/pnas.91.8.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixon D A, Kowalczykowski S C. Cell. 1991;66:361–371. doi: 10.1016/0092-8674(91)90625-9. [DOI] [PubMed] [Google Scholar]
- 20.Masterson C, Boehmer P E, McDonald F, Chaudhuri S, Hickson I D, Emmerson P T. J Biol Chem. 1992;267:13564–13572. [PubMed] [Google Scholar]
- 21.Palas K M, Kushner S R. J Biol Chem. 1990;265:3447–3454. [PubMed] [Google Scholar]
- 22.Gorbalenya A E, Koonin E V, Donchenko A P, Blinov V M. FEBS Lett. 1988;235:16–24. doi: 10.1016/0014-5793(88)81226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorbalenya A E, Koonin E V, Donchenko A P, Blinov V M. Nucleic Acids Res. 1989;17:4713–4730. doi: 10.1093/nar/17.12.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodgman T C. Nature (London) 1988;333:22–23. doi: 10.1038/333022b0. [DOI] [PubMed] [Google Scholar]
- 25.Rosamond J, Telander K M, Linn S. J Biol Chem. 1979;254:8646–8652. [PubMed] [Google Scholar]
- 26.Friedman E A, Smith H O. J Biol Chem. 1972;247:2846–2853. [PubMed] [Google Scholar]
- 27.Wright M, Buttin G, Hurwitz J. J Biol Chem. 1971;246:6543–6555. [PubMed] [Google Scholar]
- 28.Muskavitch K M T, Linn S. J Biol Chem. 1982;257:2641–2648. [PubMed] [Google Scholar]
- 29.Taylor A, Smith G R. Cell. 1980;22:447–457. doi: 10.1016/0092-8674(80)90355-4. [DOI] [PubMed] [Google Scholar]
- 30.Lieberman R P, Oishi M. Proc Natl Acad Sci USA. 1974;71:4816–4820. doi: 10.1073/pnas.71.12.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen H, Ruan B, Yu M, Wang J, Julin D A. J Biol Chem. 1997;272:10072–10079. doi: 10.1074/jbc.272.15.10072. [DOI] [PubMed] [Google Scholar]
- 32.Boehmer P E, Emmerson P T. Gene (Amst) 1991;102:1–6. doi: 10.1016/0378-1119(91)90529-k. [DOI] [PubMed] [Google Scholar]
- 33.Chaudhury A M, Smith G R. J Bacteriol. 1984;160:788–791. doi: 10.1128/jb.160.2.788-791.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korangy F, Julin D A. J Biol Chem. 1992;267:1727–1732. [PubMed] [Google Scholar]
- 35.Korangy F, Julin D A. Biochemistry. 1993;32:4873–4880. doi: 10.1021/bi00069a024. [DOI] [PubMed] [Google Scholar]
- 36.Lohman T M, Bjornson K P. Annu Rev Biochem. 1996;65:169–214. doi: 10.1146/annurev.bi.65.070196.001125. [DOI] [PubMed] [Google Scholar]
- 37.Chamberlin M, Julin D A. Biochemistry. 1996;35:15949–15961. doi: 10.1021/bi961643n. [DOI] [PubMed] [Google Scholar]
- 38.Haijema B, Venema G, Kooistra J. J Bacteriol. 1996;178:5086–5091. doi: 10.1128/jb.178.17.5086-5091.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eggleston A K, Kowalczykowski S C. J Mol Biol. 1993;231:605–620. doi: 10.1006/jmbi.1993.1313. [DOI] [PubMed] [Google Scholar]
- 40.Newman M, Strzelecka T, Dorner L F, Schildkraut I, Aggarwal A K. Nature (London) 1994;368:660–664. doi: 10.1038/368660a0. [DOI] [PubMed] [Google Scholar]
- 41.Wah D A, Hirsch J A, Dorner L F, Schildkraut I, Aggarwal A K. Nature (London) 1997;388:97–100. doi: 10.1038/40446. [DOI] [PubMed] [Google Scholar]