Abstract
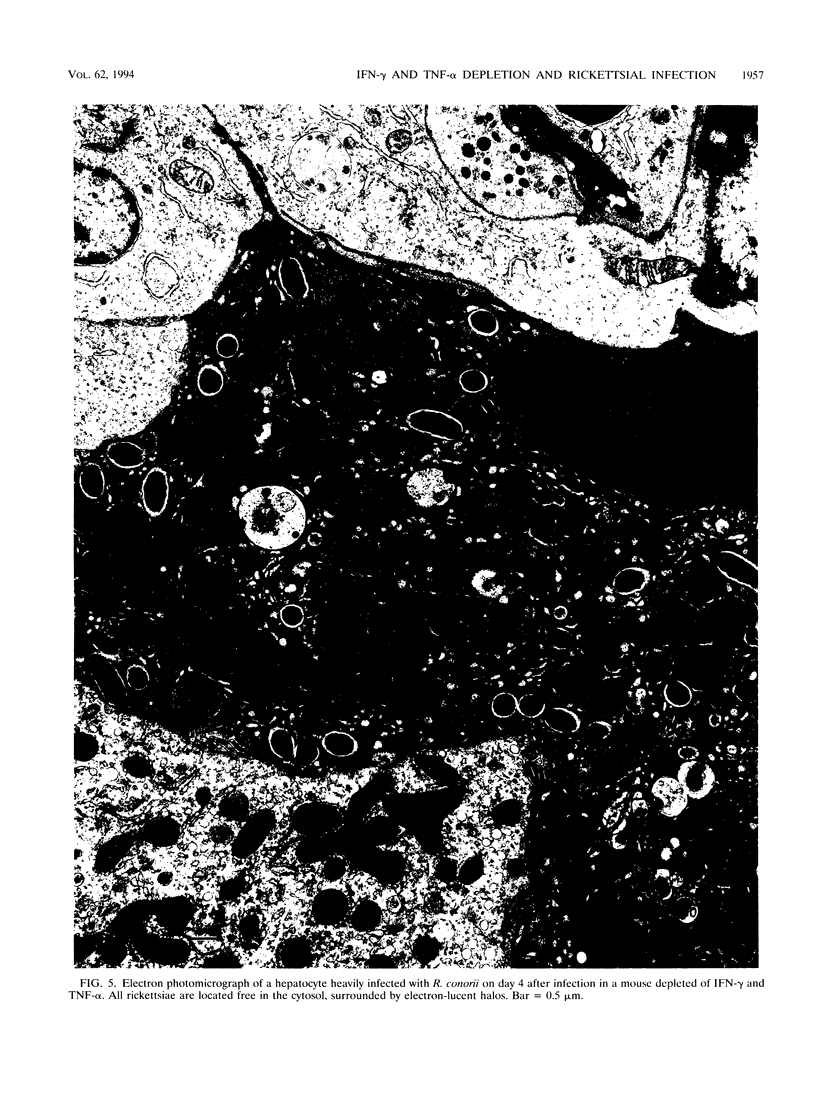
C3H/HeN mice infected intravenously with a dose of Rickettsia conorii (Malish 7 strain) that is sublethal for immunocompetent animals (1.1 x 10(3) PFU) developed disseminated infection of endothelial cells of the brain, lungs, heart, liver, kidney, testis, and testicular adnexa. In R. conorii-infected mice depleted of gamma interferon (IFN-gamma) and/or tumor necrosis factor alpha (TNF-alpha) by intravenous administration of neutralizing monoclonal antibodies on days 0, 2, and 4, the mortality rate was 100%. Death of the cytokine-depleted animals on days 5 and 6 was associated with overwhelming rickettsial infection documented by titration of rickettsial content in the brain and liver and by immunohistologic demonstration of massive quantities of R. conorii in endothelial cells of all organs examined, in macrophages of the liver and spleen, and in hepatocytes. Nondepleted, immunocompetent animals showed markedly reduced rickettsial content in the tissues on day 6, with rickettsial destruction in phagolysosomes not only in macrophages but also in endothelial cells and hepatocytes. All nondepleted, infected mice recovered and appeared completely healthy by day 9. Assay of liver infiltrated by lymphocytes and macrophages revealed mRNA of IFN-gamma and TNF-alpha, indicating that the host defenses were activated at the site of infection. Treatment of mice with an analog of L-arginine reduced the synthesis of nitric oxide and impaired rickettsial killing. Nitric oxide production was also impaired in cytokine-depleted infected mice. These observations support the hypothesis that IFN-gamma secreted by T lymphocytes and natural killer cells and TNF-alpha secreted by macrophages act in a synergistic, paracrine fashion on adjacent rickettsia-infected endothelial cells, hepatocytes, and macrophages to stimulate synthesis of nitric oxide, which kills intracellular R. conorii.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crist A. E., Jr, Wisseman C. L., Jr, Murphy J. R. Characteristics of lymphoid cells that adoptively transfer immunity to Rickettsia mooseri infection in mice. Infect Immun. 1984 Apr;44(1):55–60. doi: 10.1128/iai.44.1.55-60.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumler J. S., Gage W. R., Pettis G. L., Azad A. F., Kuhadja F. P. Rapid immunoperoxidase demonstration of Rickettsia rickettsii in fixed cutaneous specimens from patients with Rocky Mountain spotted fever. Am J Clin Pathol. 1990 Mar;93(3):410–414. doi: 10.1093/ajcp/93.3.410. [DOI] [PubMed] [Google Scholar]
- Feng H. M., Walker D. H. Interferon-gamma and tumor necrosis factor-alpha exert their antirickettsial effect via induction of synthesis of nitric oxide. Am J Pathol. 1993 Oct;143(4):1016–1023. [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Broadnax L. M. Urinary nitrate excretion in relation to murine macrophage activation. Influence of dietary L-arginine and oral NG-monomethyl-L-arginine. J Immunol. 1991 Feb 15;146(4):1294–1302. [PubMed] [Google Scholar]
- Green S. J., Nacy C. A., Schreiber R. D., Granger D. L., Crawford R. M., Meltzer M. S., Fortier A. H. Neutralization of gamma interferon and tumor necrosis factor alpha blocks in vivo synthesis of nitrogen oxides from L-arginine and protection against Francisella tularensis infection in Mycobacterium bovis BCG-treated mice. Infect Immun. 1993 Feb;61(2):689–698. doi: 10.1128/iai.61.2.689-698.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A. Evidence that tumor necrosis factor has an important role in antibacterial resistance. J Immunol. 1989 Nov 1;143(9):2894–2899. [PubMed] [Google Scholar]
- Herrero-Herrero J. I., Walker D. H., Ruiz-Beltran R. Immunohistochemical evaluation of the cellular immune response to Rickettsia conorii in taches noires. J Infect Dis. 1987 Apr;155(4):802–805. doi: 10.1093/infdis/155.4.802. [DOI] [PubMed] [Google Scholar]
- Jerrells T. R., Turco J., Winkler H. H., Spitalny G. L. Neutralization of lymphokine-mediated antirickettsial activity of fibroblasts and macrophages with monoclonal antibody specific for murine interferon gamma. Infect Immun. 1986 Jan;51(1):355–359. doi: 10.1128/iai.51.1.355-359.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon R. H., Pedersen C. E., Jr Immune responses to Rickettsia akari infection in congenitally athymic nude mice. Infect Immun. 1980 May;28(2):310–313. doi: 10.1128/iai.28.2.310-313.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokorin I. N., Kabanova E. A., Shirokova E. M., Abrosimova G. E., Rybkina N. N., Pushkareva V. i. Role of T lymphocytes in Rickettsia conorii infection. Acta Virol. 1982 Jan;26(1-2):91–97. [PubMed] [Google Scholar]
- Li H., Jerrells T. R., Spitalny G. L., Walker D. H. Gamma interferon as a crucial host defense against Rickettsia conorii in vivo. Infect Immun. 1987 May;55(5):1252–1255. doi: 10.1128/iai.55.5.1252-1255.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor E., Sarov I. Inhibition of Rickettsia conorii growth by recombinant tumor necrosis factor alpha: enhancement of inhibition by gamma interferon. Infect Immun. 1990 Jun;58(6):1886–1890. doi: 10.1128/iai.58.6.1886-1890.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor E., Sarov I. Tumor necrosis factor alpha and prostaglandin E2 production by human monocyte-derived macrophages infected with spotted fever group rickettsiae. Ann N Y Acad Sci. 1990;590:157–167. doi: 10.1111/j.1749-6632.1990.tb42218.x. [DOI] [PubMed] [Google Scholar]
- Manor E. The effect of monocyte-derived macrophages on the growth of Rickettsia conorii in permissive cells. Acta Virol. 1992 Jan;36(1):13–18. [PubMed] [Google Scholar]
- Murphy J. R., Wisseman C. G., Jr, Fiset P. Mechanisms of immunity in typhus infection: adoptive transfer of immunity to Rickettsia mooseri. Infect Immun. 1979 May;24(2):387–393. doi: 10.1128/iai.24.2.387-393.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R., Wisseman C. L., Jr, Fiset P. Mechanisms of immunity in typhus infection: analysis of immunity to Rickettsia mooseri infection of guinea pigs. Infect Immun. 1980 Mar;27(3):730–738. doi: 10.1128/iai.27.3.730-738.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoult D., de Micco C., Gallais H., Toga M. Laboratory diagnosis of Mediterranean spotted fever by immunofluorescent demonstration of Rickettsia conorii in cutaneous lesions. J Infect Dis. 1984 Jul;150(1):145–148. doi: 10.1093/infdis/150.1.145. [DOI] [PubMed] [Google Scholar]
- Salvemini D., Pistelli A., Mollace V., Anggård E., Vane J. The metabolism of glyceryl trinitrate to nitric oxide in the macrophage cell line J774 and its induction by Escherichia coli lipopolysaccharide. Biochem Pharmacol. 1992 Jul 7;44(1):17–24. doi: 10.1016/0006-2952(92)90032-e. [DOI] [PubMed] [Google Scholar]
- Spitalny G. L., Havell E. A. Monoclonal antibody to murine gamma interferon inhibits lymphokine-induced antiviral and macrophage tumoricidal activities. J Exp Med. 1984 May 1;159(5):1560–1565. doi: 10.1084/jem.159.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Cloned mouse interferon-gamma inhibits the growth of Rickettsia prowazekii in cultured mouse fibroblasts. J Exp Med. 1983 Dec 1;158(6):2159–2164. doi: 10.1084/jem.158.6.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Inhibition of the growth of Rickettsia prowazekii in cultured fibroblasts by lymphokines. J Exp Med. 1983 Mar 1;157(3):974–986. doi: 10.1084/jem.157.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Isolation of Rickettsia prowazekii with reduced sensitivity to gamma interferon. Infect Immun. 1989 Jun;57(6):1765–1772. doi: 10.1128/iai.57.6.1765-1772.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Role of the nitric oxide synthase pathway in inhibition of growth of interferon-sensitive and interferon-resistant Rickettsia prowazekii strains in L929 cells treated with tumor necrosis factor alpha and gamma interferon. Infect Immun. 1993 Oct;61(10):4317–4325. doi: 10.1128/iai.61.10.4317-4325.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. H., Burday M. S., Folds J. D. Laboratory diagnosis of Rocky Mountain spotted fever. South Med J. 1980 Nov;73(11):1443-6, 1449. doi: 10.1097/00007611-198011000-00007. [DOI] [PubMed] [Google Scholar]
- Walker D. H., Cain B. G., Olmstead P. M. Laboratory diagnosis of Rocky Mountain spotted fever by immunofluorescent demonstration of Rickettsia in Cutaneous lesions. Am J Clin Pathol. 1978 Jun;69(6):619–623. doi: 10.1093/ajcp/69.6.619. [DOI] [PubMed] [Google Scholar]
- Walker D. H., Gear J. H. Correlation of the distribution of Rickettsia conorii, microscopic lesions, and clinical features in South African tick bite fever. Am J Trop Med Hyg. 1985 Mar;34(2):361–371. doi: 10.4269/ajtmh.1985.34.361. [DOI] [PubMed] [Google Scholar]
- Walker D. H., Henderson F. W. Effect of immunosuppression on Rickettsia rickettsii infection in guinea pigs. Infect Immun. 1978 Apr;20(1):221–227. doi: 10.1128/iai.20.1.221-227.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. H., Mattern W. D. Acute renal failure in Rocky Mountain spotted fever. Arch Intern Med. 1979 Apr;139(4):443–448. [PubMed] [Google Scholar]
- Walker D. H., Occhino C., Tringali G. R., Di Rosa S., Mansueto S. Pathogenesis of rickettsial eschars: the tache noire of boutonneuse fever. Hum Pathol. 1988 Dec;19(12):1449–1454. doi: 10.1016/s0046-8177(88)80238-7. [DOI] [PubMed] [Google Scholar]
- Walker D. H., Parks F. M., Betz T. G., Taylor J. P., Muehlberger J. W. Histopathology and immunohistologic demonstration of the distribution of Rickettsia typhi in fatal murine typhus. Am J Clin Pathol. 1989 Jun;91(6):720–724. doi: 10.1093/ajcp/91.6.720. [DOI] [PubMed] [Google Scholar]
- Walker D. H., Popov V. L., Wen J., Feng H. M. Rickettsia conorii infection of C3H/HeN mice. A model of endothelial-target rickettsiosis. Lab Invest. 1994 Mar;70(3):358–368. [PubMed] [Google Scholar]
- Walker D. H., Staiti A., Mansueto S., Tringali G. Frequent occurrence of hepatic lesions in boutonneuse fever. Acta Trop. 1986 Jun;43(2):175–181. [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. Interferonlike factors from antigen- and mitogen-stimulated human leukocytes with antirickettsial and cytolytic actions on Rickettsia prowazekii. Infected human endothelial cells, fibroblasts, and macrophages. J Exp Med. 1983 Jun 1;157(6):1780–1793. doi: 10.1084/jem.157.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]