Abstract
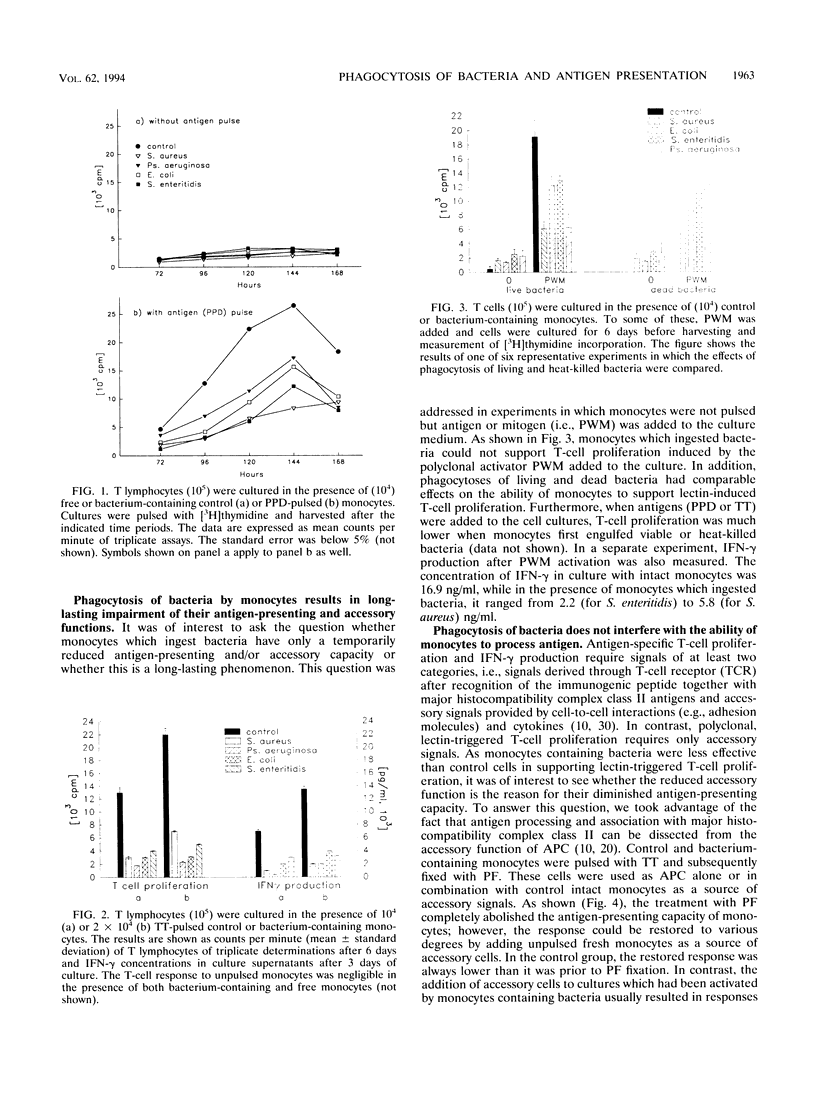
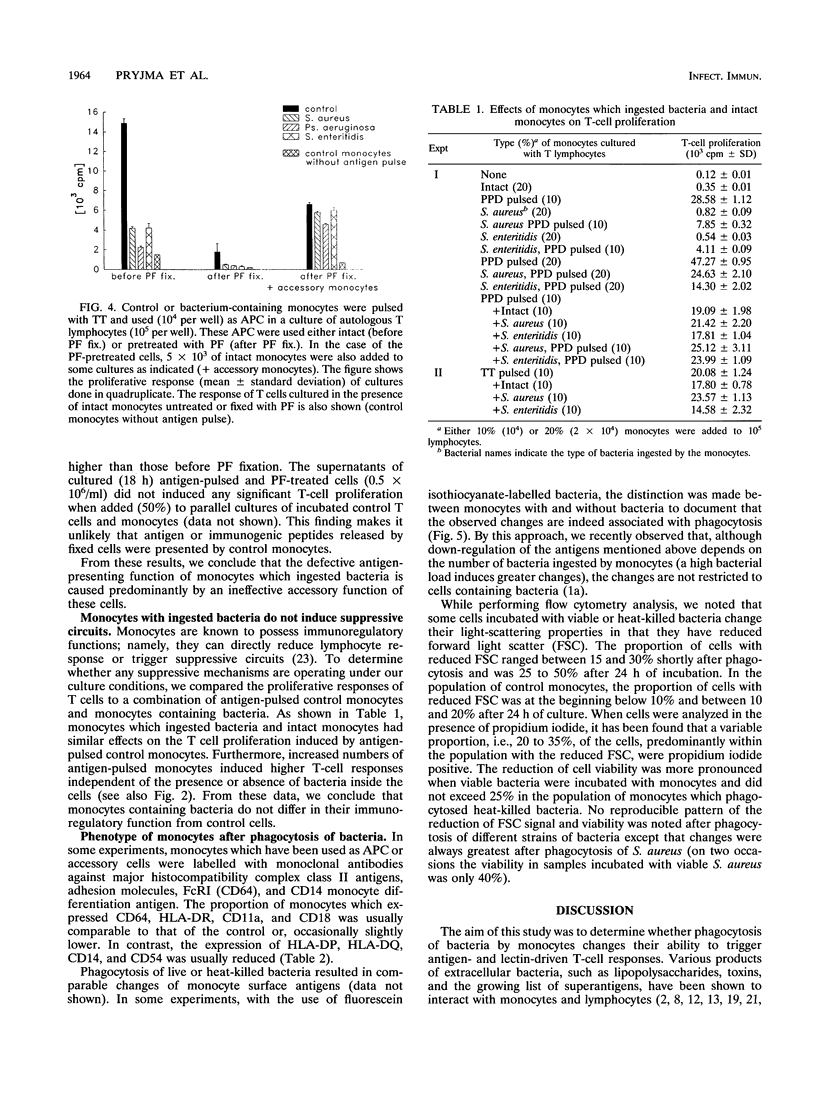
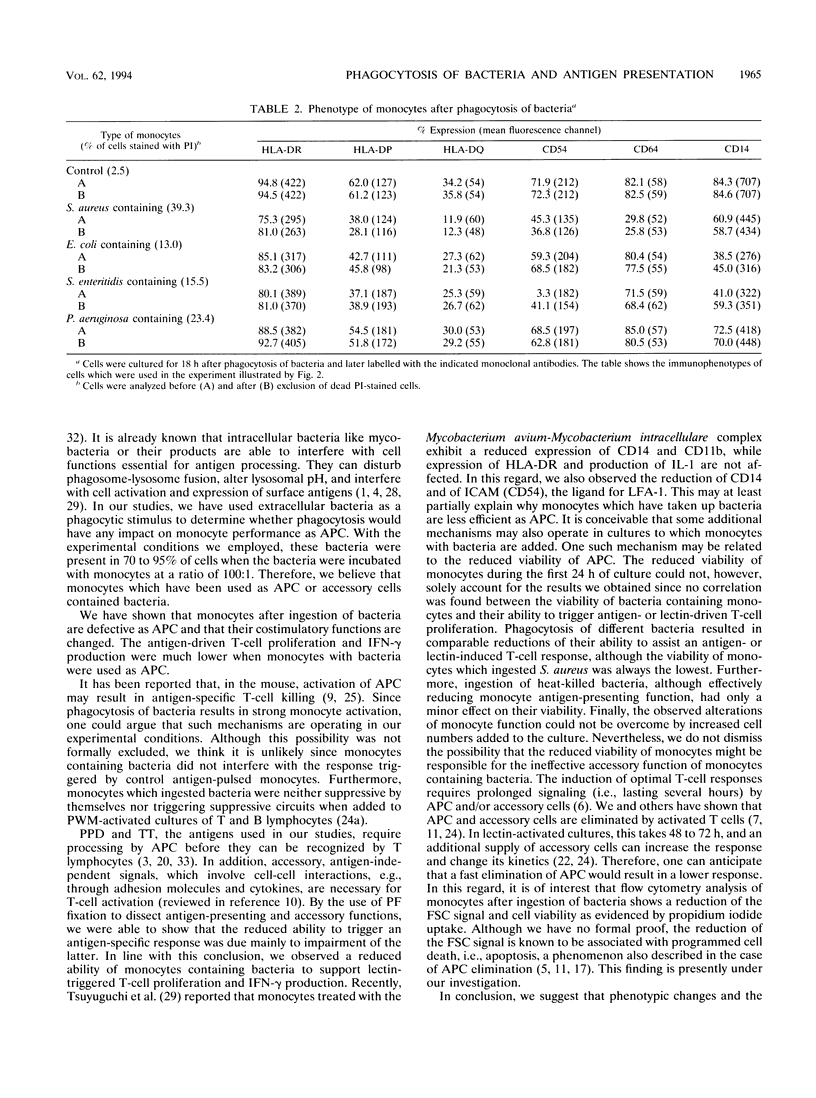
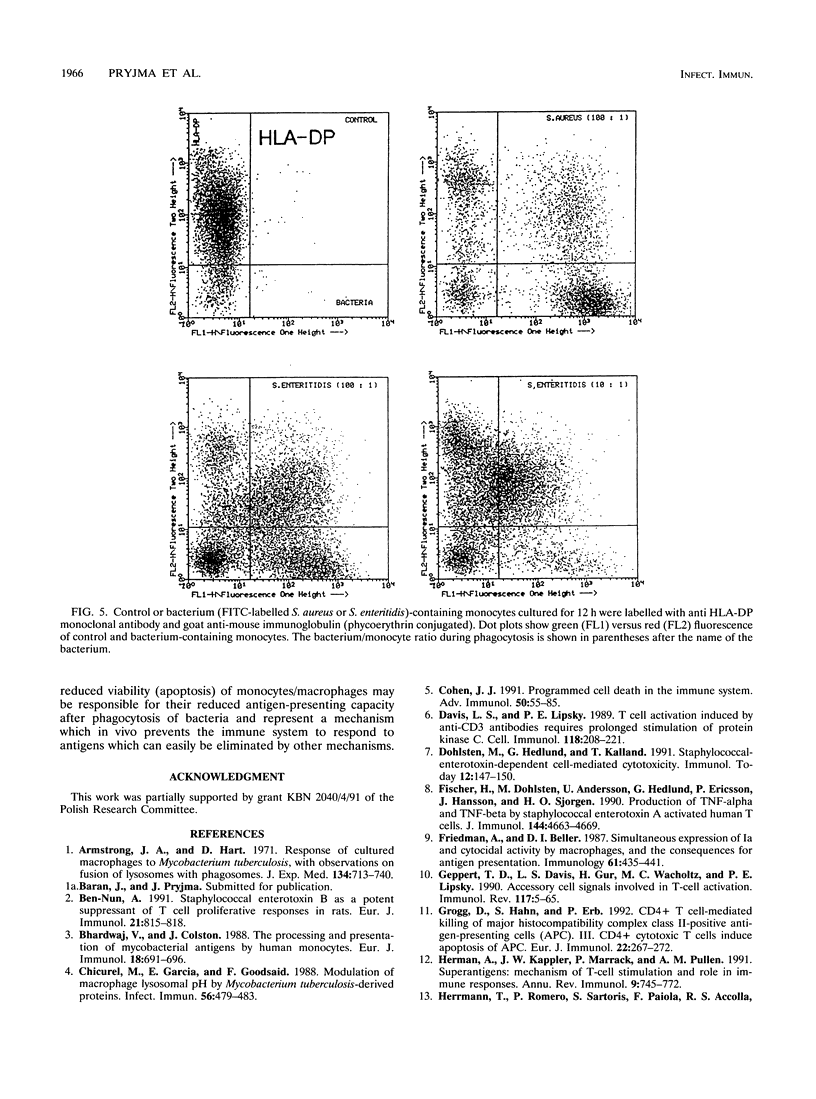
The antigen-presenting and accessory functions of monocytes were studied after phagocytosis of bacteria. Peripheral blood monocytes isolated from mononuclear cells by counterflow elutriation were incubated with suspensions of opsonized bacteria (Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, or Salmonella enteritidis) under conditions in which at least 80% of the monocytes engulfed microorganisms. Either the cells were pulsed with antigen (purified derivative of tuberculin or tetanus toxoid) and used as antigen-presenting cells for autologous T lymphocytes or the accessory function of the cells was examined in pokeweed mitogen-activated cultures of T cells. It has been found that phagocytosis of bacteria by monocytes reduces their ability to trigger antigen- and mitogen-induced proliferation. The reduced proliferative response of T lymphocytes was not due to a change of the kinetics of the response or triggering of suppressor mechanisms. Furthermore, antigen processing was not affected much after phagocytosis of bacteria since antigen-pulsed and paraformaldehyde-fixed cells containing bacteria were comparable to control cells in their antigen-presenting capacity. This phenomenon was observed after phagocytosis of both living and dead bacteria and was not correlated to the viability of monocytes, which were more affected after phagocytosis of living bacteria than of dead ones. As a result of phagocytosis of bacteria, reduced expression of CD54, CD14, and HLA-DQ, variable reduction of HLA-DP, CD58, and CD64, and reduced viability of monocytes were observed. In conclusion, phagocytosis of bacteria by monocytes affects their antigen-presenting and accessory functions presumably because of changes in the expression of molecules essential for monocyte-T-cell interactions and reduction of their viability.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Nun A. Staphylococcal enterotoxin B as a potent suppressant of T cell proliferative responses in rats. Eur J Immunol. 1991 Mar;21(3):815–818. doi: 10.1002/eji.1830210341. [DOI] [PubMed] [Google Scholar]
- Bhardwaj V., Colston M. J. The processing and presentation of mycobacterial antigens by human monocytes. Eur J Immunol. 1988 May;18(5):691–696. doi: 10.1002/eji.1830180506. [DOI] [PubMed] [Google Scholar]
- Chicurel M., García E., Goodsaid F. Modulation of macrophage lysosomal pH by Mycobacterium tuberculosis-derived proteins. Infect Immun. 1988 Feb;56(2):479–483. doi: 10.1128/iai.56.2.479-483.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J. Programmed cell death in the immune system. Adv Immunol. 1991;50:55–85. doi: 10.1016/s0065-2776(08)60822-6. [DOI] [PubMed] [Google Scholar]
- Davis L. S., Lipsky P. E. T cell activation induced by anti-CD3 antibodies requires prolonged stimulation of protein kinase C. Cell Immunol. 1989 Jan;118(1):208–221. doi: 10.1016/0008-8749(89)90370-5. [DOI] [PubMed] [Google Scholar]
- Dohlsten M., Hedlund G., Kalland T. Staphylococcal-enterotoxin-dependent cell-mediated cytotoxicity. Immunol Today. 1991 May;12(5):147–150. doi: 10.1016/S0167-5699(05)80043-X. [DOI] [PubMed] [Google Scholar]
- Fischer H., Dohlsten M., Andersson U., Hedlund G., Ericsson P., Hansson J., Sjögren H. O. Production of TNF-alpha and TNF-beta by staphylococcal enterotoxin A activated human T cells. J Immunol. 1990 Jun 15;144(12):4663–4669. [PubMed] [Google Scholar]
- Friedman A., Beller D. I. Simultaneous expression of Ia and cytocidal activity by macrophages, and the consequences for antigen presentation. Immunology. 1987 Aug;61(4):435–441. [PMC free article] [PubMed] [Google Scholar]
- Geppert T. D., Davis L. S., Gur H., Wacholtz M. C., Lipsky P. E. Accessory cell signals involved in T-cell activation. Immunol Rev. 1990 Oct;117:5–66. doi: 10.1111/j.1600-065x.1990.tb00566.x. [DOI] [PubMed] [Google Scholar]
- Grogg D., Hahn S., Erb P. CD4+ T cell-mediated killing of major histocompatibility complex class II-positive antigen-presenting cells (APC). III. CD4+ cytotoxic T cells induce apoptosis of APC. Eur J Immunol. 1992 Jan;22(1):267–272. doi: 10.1002/eji.1830220139. [DOI] [PubMed] [Google Scholar]
- Herman A., Kappler J. W., Marrack P., Pullen A. M. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- Herrmann T., Romero P., Sartoris S., Paiola F., Accolla R. S., Maryanski J. L., MacDonald H. R. Staphylococcal enterotoxin-dependent lysis of MHC class II negative target cells by cytolytic T lymphocytes. J Immunol. 1991 Apr 15;146(8):2504–2512. [PubMed] [Google Scholar]
- Kaufmann S. H. Immunity against intracellular bacteria: biological effector functions and antigen specificity of T lymphocytes. Curr Top Microbiol Immunol. 1988;138:141–176. [PubMed] [Google Scholar]
- Keller R., Gehri R., Keist R. The interaction of macrophages and bacteria: Escherichia coli species, bacterial lipopolysaccharide, and lipid A differ in their ability to induce tumoricidal activity and the secretion of reactive nitrogen intermediates in macrophages. Cell Immunol. 1992 Apr 15;141(1):47–58. doi: 10.1016/0008-8749(92)90126-a. [DOI] [PubMed] [Google Scholar]
- Madsen M., Johnsen H. E. A methodological study of E-rosette formation using AET-treated sheep red blood cells. J Immunol Methods. 1979 May 10;27(1):61–74. doi: 10.1016/0022-1759(79)90239-4. [DOI] [PubMed] [Google Scholar]
- Mangan D. F., Welch G. R., Wahl S. M. Lipopolysaccharide, tumor necrosis factor-alpha, and IL-1 beta prevent programmed cell death (apoptosis) in human peripheral blood monocytes. J Immunol. 1991 Mar 1;146(5):1541–1546. [PubMed] [Google Scholar]
- Mannhalter J. W., Zlabinger G. J., Ahmad R., Eibl M. M. Human T cell proliferation in response to E. coli presented by autologous macrophages is antigen specific. Clin Exp Immunol. 1983 Oct;54(1):95–102. [PMC free article] [PubMed] [Google Scholar]
- Misfeldt M. L., Legaard P. K., Howell S. E., Fornella M. H., LeGrand R. D. Induction of interleukin-1 from murine peritoneal macrophages by Pseudomonas aeruginosa exotoxin A. Infect Immun. 1990 Apr;58(4):978–982. doi: 10.1128/iai.58.4.978-982.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno J., Lipsky P. E. Differential ability of fixed antigen-presenting cells to stimulate nominal antigen-reactive and alloreactive T4 lymphocytes. J Immunol. 1986 May 15;136(10):3579–3587. [PubMed] [Google Scholar]
- Newcomb J. R., Lin Y. S., Rogers T. J. Requirement for accessory cells in suppression of MOPC-315 IgA secretion by staphylococcal enterotoxin B-induced T-suppressor cells. Cell Immunol. 1990 Sep;129(2):528–537. doi: 10.1016/0008-8749(90)90227-i. [DOI] [PubMed] [Google Scholar]
- Pryjma J., Ernst M., Fetting R., Woloszyn M., Zembala M., Flad H. D. The role of monocytes in the induction and regulation of IFN-gamma production by lectin-activated human T lymphocytes. Eur Cytokine Netw. 1991 Aug-Sep;2(4):273–279. [PubMed] [Google Scholar]
- Pryjma J., Zembala M., Pituch-Noworolska A., Ernst M., Van der Bosch J., Flad H. D. Monocyte-T-cell interactions in pokeweed mitogen-activated cultures. Immunology. 1990 Nov;71(3):397–403. [PMC free article] [PubMed] [Google Scholar]
- Rao A., Faas S. J., Miller L. J., Riback P. S., Cantor H. Lysis of inducer T cell clones by activated macrophages and macrophage-like cell lines. J Exp Med. 1983 Oct 1;158(4):1243–1258. doi: 10.1084/jem.158.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson R. J., Shepperdson R. T., Vatter A. E., Talmage D. W. Isolation and enumeration of peripheral blood monocytes. J Immunol. 1977 Apr;118(4):1409–1414. [PubMed] [Google Scholar]
- Shenker B. J., Vitale L., Slots J. Immunosuppressive effects of Prevotella intermedia on in vitro human lymphocyte activation. Infect Immun. 1991 Dec;59(12):4583–4589. doi: 10.1128/iai.59.12.4583-4589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley L. D., Hunter S. W., Brennan P. J., Krahenbuhl J. L. Mycobacterial lipoarabinomannan inhibits gamma interferon-mediated activation of macrophages. Infect Immun. 1988 May;56(5):1232–1236. doi: 10.1128/iai.56.5.1232-1236.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuyuguchi I., Kawasumi H., Takashima T., Tsuyuguchi T., Kishimoto S. Mycobacterium avium-Mycobacterium intracellular complex-induced suppression of T-cell proliferation in vitro by regulation of monocyte accessory cell activity. Infect Immun. 1990 May;58(5):1369–1378. doi: 10.1128/iai.58.5.1369-1378.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Seventer G. A., Shimizu Y., Horgan K. J., Luce G. E., Webb D., Shaw S. Remote T cell co-stimulation via LFA-1/ICAM-1 and CD2/LFA-3: demonstration with immobilized ligand/mAb and implication in monocyte-mediated co-stimulation. Eur J Immunol. 1991 Jul;21(7):1711–1718. doi: 10.1002/eji.1830210719. [DOI] [PubMed] [Google Scholar]
- Weir D. M., Blackwell C. C. Interaction of bacteria with the immune system. J Clin Lab Immunol. 1983 Jan;10(1):1–12. [PubMed] [Google Scholar]
- Wells A. F., Hightower J. A., Parks C., Kufoy E., Fox A. Systemic injection of group A streptococcal peptidoglycan-polysaccharide complexes elicits persistent neutrophilia and monocytosis associated with polyarthritis in rats. Infect Immun. 1989 Feb;57(2):351–358. doi: 10.1128/iai.57.2.351-358.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler H. K., Orlin C. A., Cluff C. W. Differential requirements for the processing and presentation of soluble and particulate bacterial antigens by macrophages. Eur J Immunol. 1987 Sep;17(9):1287–1296. doi: 10.1002/eji.1830170911. [DOI] [PubMed] [Google Scholar]


