Abstract
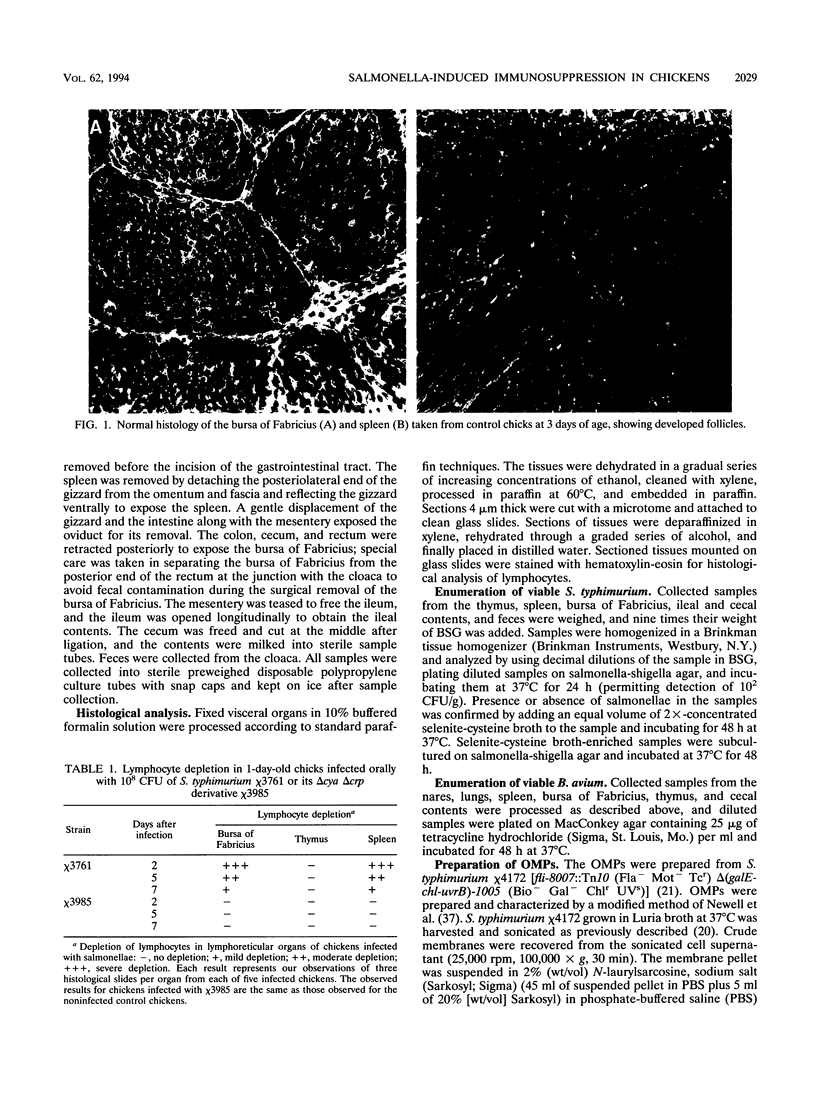
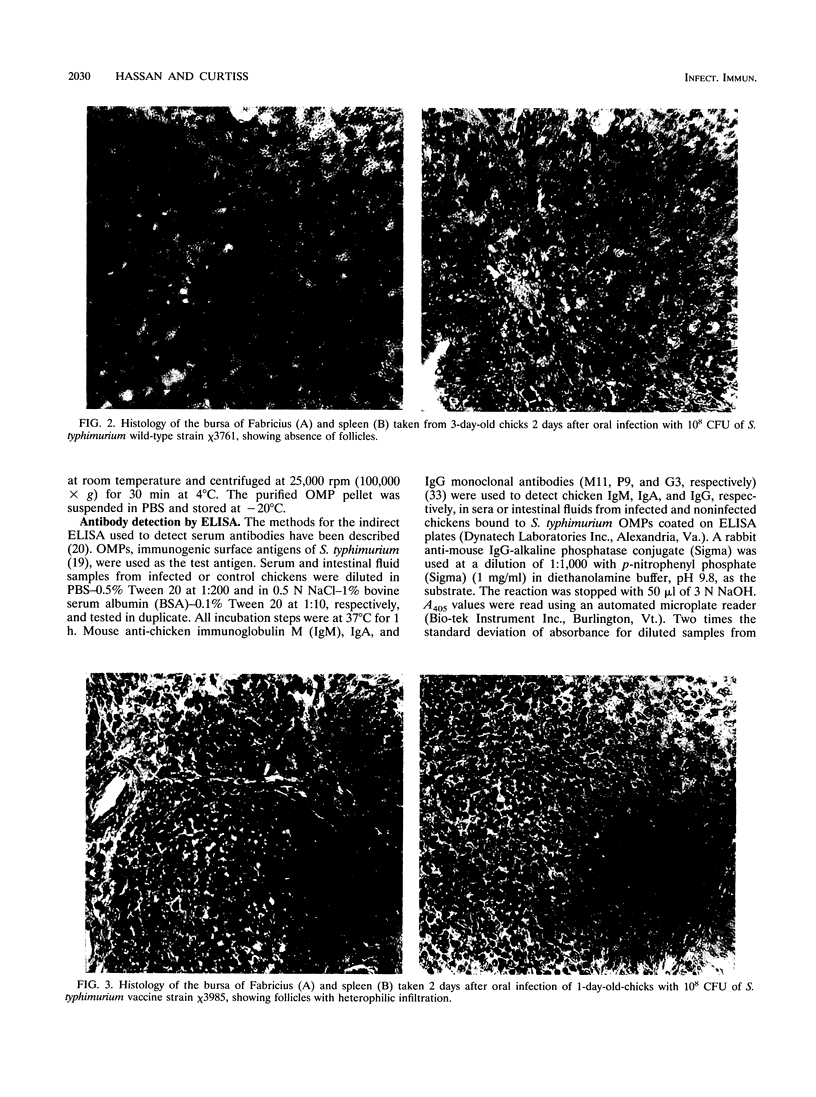
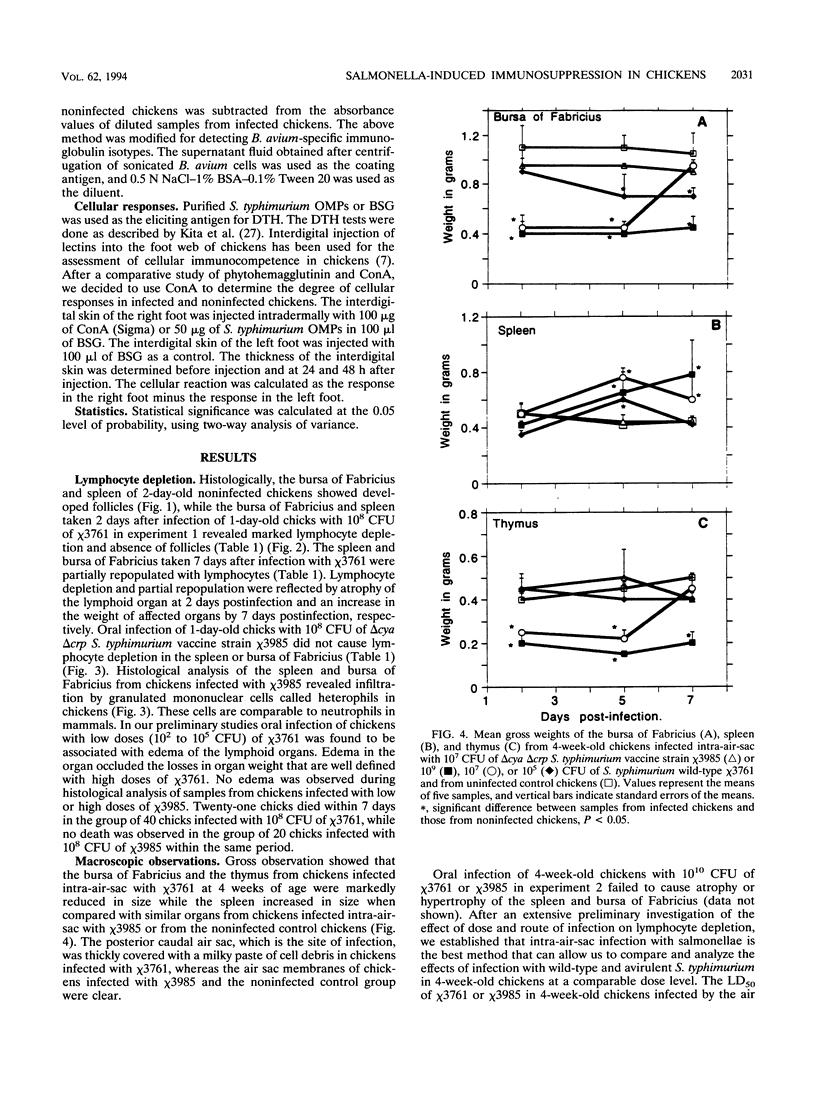
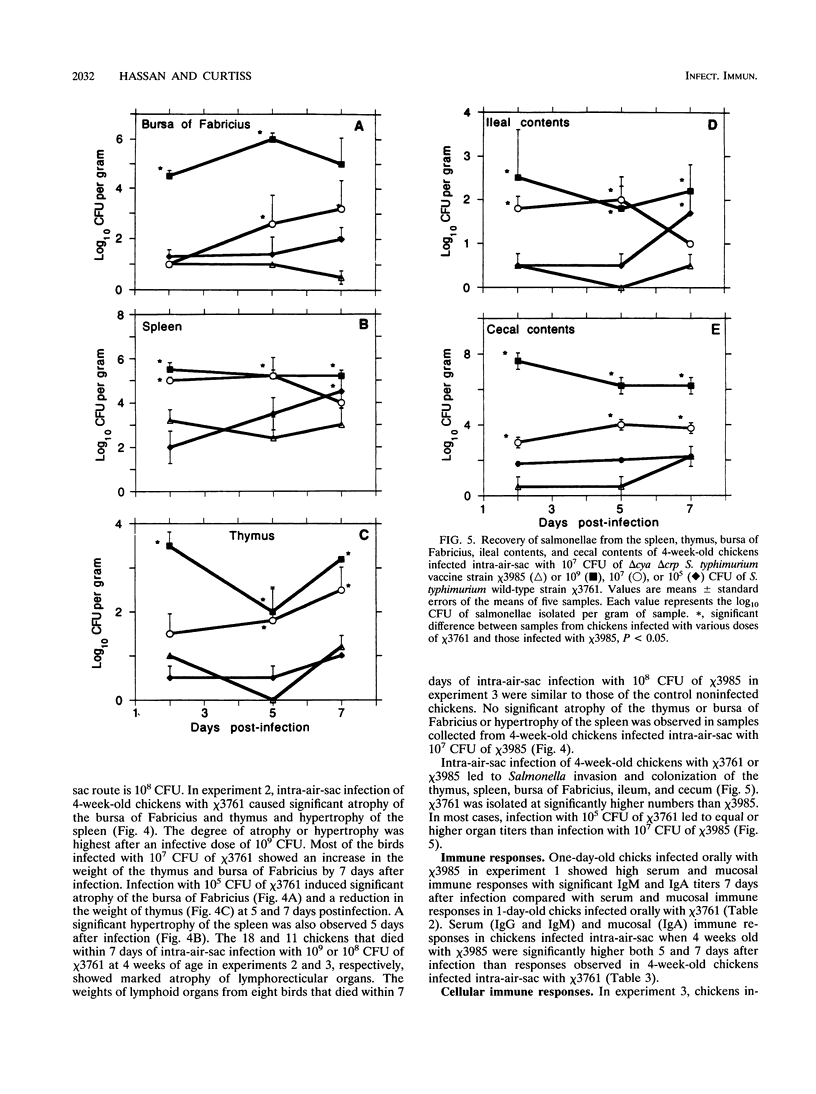
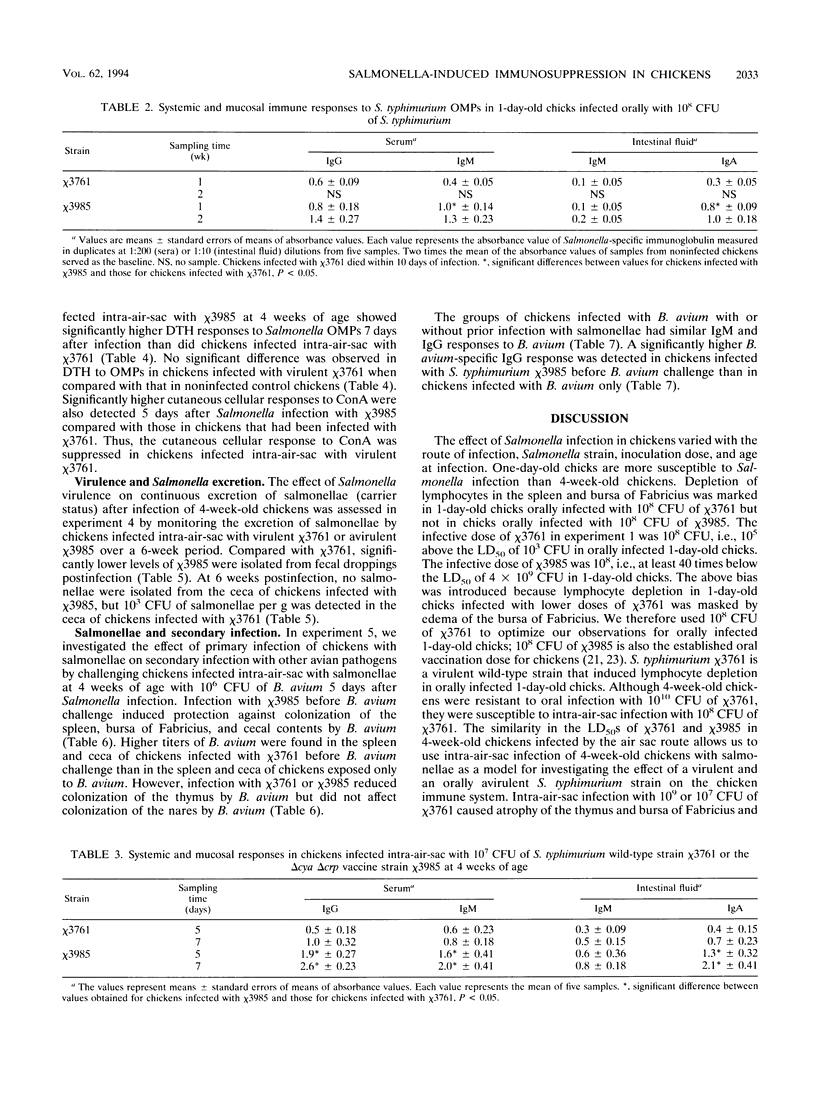
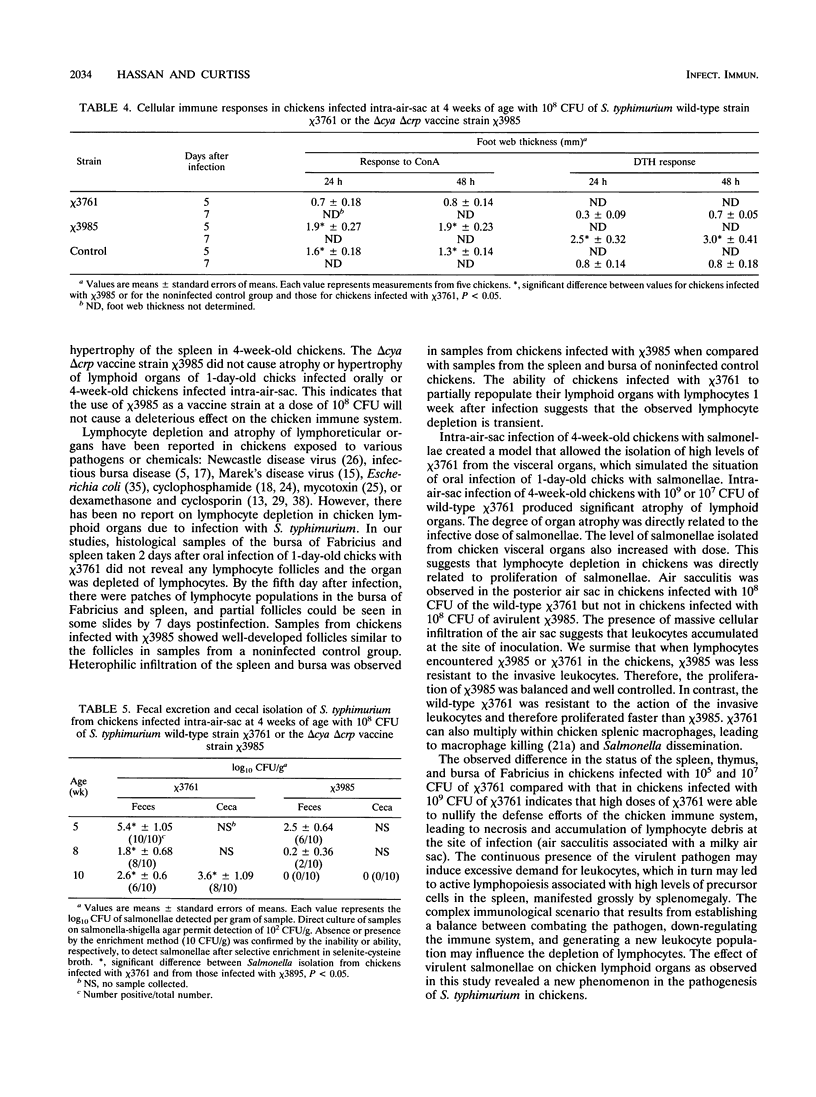
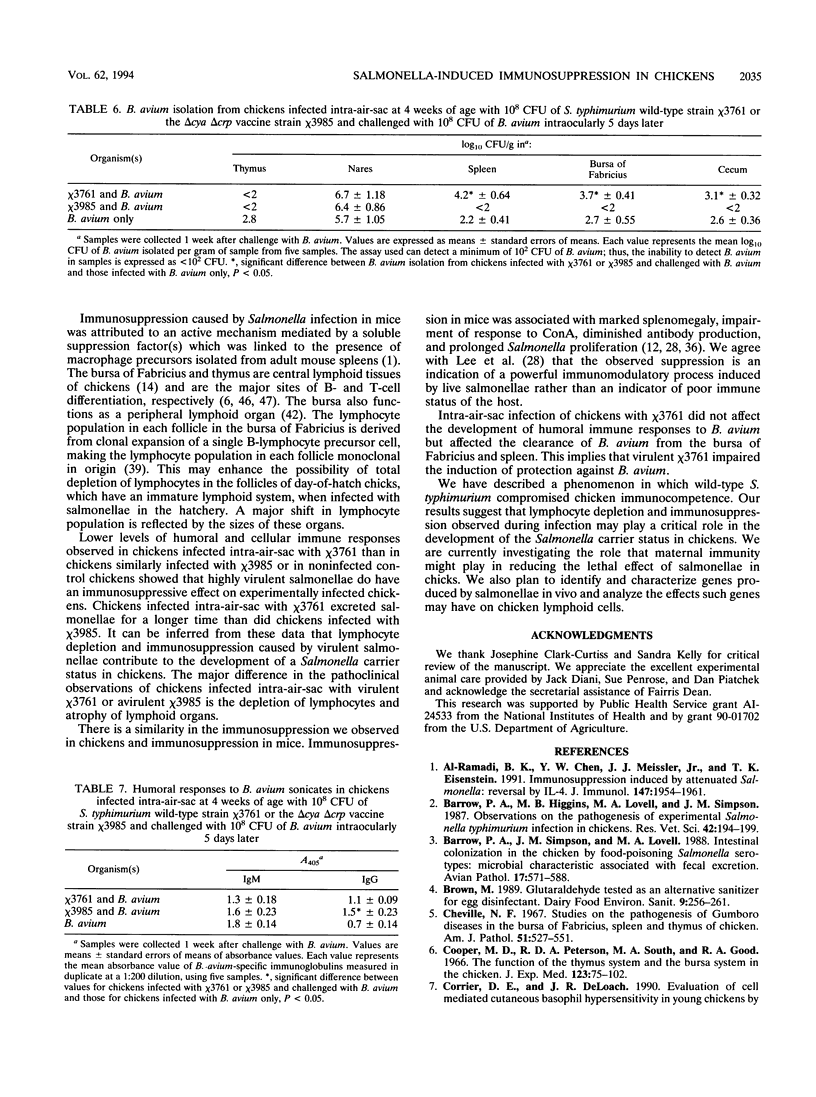
The effect of experimental Salmonella infection on chicken lymphoid organs, immune responses, and fecal shedding of salmonellae were assessed following oral inoculation of 1-day-old chicks or intra-air-sac infection of 4-week-old chickens with virulent S. typhimurium wild-type chi 3761 or avirulent S. typhimurium delta cya delta crp vaccine strain chi 3985. Some 4-week-old chickens infected intra-air-sac with chi 3761 or chi 3985 were challenged with Bordetella avium to determine the effect of Salmonella infection on secondary infection by B. avium. S. typhimurium chi 3761 caused lymphocyte depletion, atrophy of lymphoid organs, and immunosuppression 2 days after infection in 1-day-old chicks and 4-week-old chickens. The observed lymphocyte depletion or atrophy of lymphoid organs was transient and dose dependent. Lymphocyte depletion and immunosuppression were associated with prolonged fecal shedding of S. typhimurium chi 3761. No lymphocyte depletion, immunosuppression, or prolonged Salmonella shedding was observed in groups of chickens infected orally or intra-air-sac with chi 3985. Infection of chickens with salmonellae before challenge with B. avium did not suppress the specific antibody response to B. avium. However, B. avium isolation was higher in visceral organs of chickens infected with chi 3761 and challenged with B. avium than in chickens infected with B. avium only. Infection of chickens with chi 3985 reduced B. avium colonization. We report a new factor in Salmonella pathogenesis and reveal a phenomenon which may play a critical role in the development of Salmonella carrier status in chickens. We also showed that 10(8) CFU of chi 3985, which is our established oral vaccination dose for chickens, did not cause immunosuppression or enhance the development of Salmonella carrier status in chickens.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrow P. A., Huggins M. B., Lovell M. A., Simpson J. M. Observations on the pathogenesis of experimental Salmonella typhimurium infection in chickens. Res Vet Sci. 1987 Mar;42(2):194–199. [PubMed] [Google Scholar]
- CURTIS S. R., 3rd CHROMOSOMAL ABERRATIONS ASSOCIATED WITH MUTATIONS TO BACTERIOPHAGE RESISTANCE IN ESCHERICHIA COLI. J Bacteriol. 1965 Jan;89:28–40. doi: 10.1128/jb.89.1.28-40.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheville N. F. Studies on the pathogenesis of Gumboro disease in the bursa of Fabricius, spleen, and thymus of the chicken. Am J Pathol. 1967 Oct;51(4):527–551. [PMC free article] [PubMed] [Google Scholar]
- Cooper M. D., Raymond D. A., Peterson R. D., South M. A., Good R. A. The functions of the thymus system and the bursa system in the chicken. J Exp Med. 1966 Jan 1;123(1):75–102. doi: 10.1084/jem.123.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrier D. E., DeLoach J. R. Evaluation of cell-mediated, cutaneous basophil hypersensitivity in young chickens by an interdigital skin test. Poult Sci. 1990 Mar;69(3):403–408. doi: 10.3382/ps.0690403. [DOI] [PubMed] [Google Scholar]
- Cox N. A., Bailey J. S., Mauldin J. M., Blankenship L. C. Presence and impact of Salmonella contamination in commercial broiler hatcheries. Poult Sci. 1990 Sep;69(9):1606–1609. doi: 10.3382/ps.0691606. [DOI] [PubMed] [Google Scholar]
- Deschenes M., Guenounou M., Ronco E., Vacheron F., Nauciel C. Impairment of lymphocyte proliferative responses and interleukin-2 production in susceptible (C57BL/6) mice infected with Salmonella typhimurium. Immunology. 1986 Jun;58(2):225–230. [PMC free article] [PubMed] [Google Scholar]
- Edelman A. S., Sanchez P. L., Robinson M. E., Hochwald G. M., Thorbecke G. J. Primary and secondary wattle swelling response to phytohemagglutinin as a measure of immunocompetence in chickens. Avian Dis. 1986 Jan-Mar;30(1):105–111. [PubMed] [Google Scholar]
- Fujimoto Y., Okada K., Kakihata K., Matsui T., Narita M. Initial lesions in chickens infected with JM strain of Marek's disease virus. Jpn J Vet Res. 1974 Jul;22(3):80–94. [PubMed] [Google Scholar]
- Gast R. K., Beard C. W. Age-related changes in the persistence and pathogenicity of Salmonella typhimurium in chicks. Poult Sci. 1989 Nov;68(11):1454–1460. doi: 10.3382/ps.0681454. [DOI] [PubMed] [Google Scholar]
- Giambrone J. J., Donahoe J. P., Dawe D. L., Eidson C. S. Specific suppression of the bursa-dependent immune system of chicks with infectious bursal disease virus. Am J Vet Res. 1977 May;38(5):581–583. [PubMed] [Google Scholar]
- Glick B. Morphological changes and humoral immunity in cyclophosphamide-treated chicks. Transplantation. 1971 May;11(5):433–439. doi: 10.1097/00007890-197105000-00001. [DOI] [PubMed] [Google Scholar]
- Hassan J. O., Barrow P. A., Mockett A. P., Mcleod S. Antibody response to experimental Salmonella typhimurium infection in chickens measured by ELISA. Vet Rec. 1990 May 26;126(21):519–522. [PubMed] [Google Scholar]
- Hassan J. O., Curtiss R., 3rd Control of colonization by virulent Salmonella typhimurium by oral immunization of chickens with avirulent delta cya delta crp S. typhimurium. Res Microbiol. 1990 Sep-Oct;141(7-8):839–850. doi: 10.1016/0923-2508(90)90119-b. [DOI] [PubMed] [Google Scholar]
- Hassan J. O., Mockett A. P., Catty D., Barrow P. A. Infection and reinfection of chickens with Salmonella typhimurium: bacteriology and immune responses. Avian Dis. 1991 Oct-Dec;35(4):809–819. [PubMed] [Google Scholar]
- Hassan J. O., Porter S. B., Curtiss R., 3rd Effect of infective dose on humoral immune responses and colonization in chickens experimentally infected with Salmonella typhimurium. Avian Dis. 1993 Jan-Mar;37(1):19–26. [PubMed] [Google Scholar]
- Hiraga T., Sugimura M., Kudo N. Effect of cyclophosphamide on the thymus and the bursa of Fabricius in chickens. Jpn J Vet Res. 1976 Oct;24(3-4):87–98. [PubMed] [Google Scholar]
- Hoerr F. J., Carlton W. W., Yagen B. Mycotoxicosis caused by a single dose of T-2 toxin or diacetoxyscirpenol in broiler chickens. Vet Pathol. 1981 Sep;18(5):652–664. doi: 10.1177/030098588101800510. [DOI] [PubMed] [Google Scholar]
- Kita E., Matsuda Y., Matsuda K., Kashiba S. Separate transfer of mouse protection and delayed-type hypersensitivity with Salmonella typhimurium transfer factor. Cell Immunol. 1984 Sep;87(2):528–537. doi: 10.1016/0008-8749(84)90021-2. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., Gibson C. W., Eisenstein T. K. Macrophage-mediated mitogenic suppression induced in mice of the C3H lineage by a vaccine strain of Salmonella typhimurium. Cell Immunol. 1985 Mar;91(1):75–91. doi: 10.1016/0008-8749(85)90033-4. [DOI] [PubMed] [Google Scholar]
- Lillehoj H. S. Effects of immunosuppression on avian coccidiosis: cyclosporin A but not hormonal bursectomy abrogates host protective immunity. Infect Immun. 1987 Jul;55(7):1616–1621. doi: 10.1128/iai.55.7.1616-1621.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILNER K. C., SHAFFER M. F. Bacteriologic studies of experimental Salmonella infections in chicks. J Infect Dis. 1952 Jan-Feb;90(1):81–96. doi: 10.1093/infdis/90.1.81. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Imada Y., Maeda M. Lymphocytic depletion of bursa of Fabricius and thymus in chickens inoculated with Escherichia coli. Vet Pathol. 1986 Nov;23(6):712–717. doi: 10.1177/030098588602300610. [DOI] [PubMed] [Google Scholar]
- Nauciel C., Vilde F., Ronco E. Host response to infection with a temperature-sensitive mutant of Salmonella typhimurium in a susceptible and a resistant strain of mice. Infect Immun. 1985 Sep;49(3):523–527. doi: 10.1128/iai.49.3.523-527.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell D. G., McBride H., Pearson A. D. The identification of outer membrane proteins and flagella of Campylobacter jejuni. J Gen Microbiol. 1984 May;130(5):1201–1208. doi: 10.1099/00221287-130-5-1201. [DOI] [PubMed] [Google Scholar]
- Nowak J. S., Kai O., Peck R., Franklin R. M. The effects of cyclosporin A on the chicken immune system. Eur J Immunol. 1982 Oct;12(10):867–876. doi: 10.1002/eji.1830121013. [DOI] [PubMed] [Google Scholar]
- Pink J. R., Vainio O., Rijnbeek A. M. Clones of B lymphocytes in individual follicles of the bursa of Fabricius. Eur J Immunol. 1985 Jan;15(1):83–87. doi: 10.1002/eji.1830150116. [DOI] [PubMed] [Google Scholar]
- Popiel I., Turnbull P. C. Passage of Salmonella enteritidis and Salmonella thompson through chick ileocecal mucosa. Infect Immun. 1985 Mar;47(3):786–792. doi: 10.1128/iai.47.3.786-792.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner T., Mueller J., Hess M. W., Cottier H., Sordat B., Ropke C. The bursa of Fabricius: a central organ providing for contact between the lymphoid system and intestinal content. Cell Immunol. 1974 Aug;13(2):304–312. doi: 10.1016/0008-8749(74)90247-0. [DOI] [PubMed] [Google Scholar]
- Schleifer J. H., Juven B. J., Beard C. W., Cox N. A. The susceptibility of chicks to Salmonella montevideo in artificially contaminated poultry feed. Avian Dis. 1984 Apr-Jun;28(2):497–503. [PubMed] [Google Scholar]
- Soerjadi A. S., Rufner R., Snoeyenbos G. H., Weinack O. M. Adherence of salmonellae and native gut microflora to the gastrointestinal mucosa of chicks. Avian Dis. 1982 Jul-Sep;26(3):576–584. [PubMed] [Google Scholar]
- Toivanen P., Toivanen A. Bursal and postbursal stem cells in chicken. Functional characteristics. Eur J Immunol. 1973 Sep;3(9):585–595. doi: 10.1002/eji.1830030912. [DOI] [PubMed] [Google Scholar]
- WARNER N. L., SZENBERG A., BURNET F. M. The immunological role of different lymphoid organs in the chicken. I. Dissociation of immunological responsiveness. Aust J Exp Biol Med Sci. 1962 Oct;40:373–387. doi: 10.1038/icb.1962.42. [DOI] [PubMed] [Google Scholar]
- Williams Smith H., Tucker J. F. The virulence of salmonella strains for chickens: their excretion by infected chickens. J Hyg (Lond) 1980 Jun;84(3):479–488. doi: 10.1017/s0022172400027017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Ramadi B. K., Chen Y. W., Meissler J. J., Jr, Eisenstein T. K. Immunosuppression induced by attenuated Salmonella. Reversal by IL-4. J Immunol. 1991 Sep 15;147(6):1954–1961. [PubMed] [Google Scholar]





