Abstract
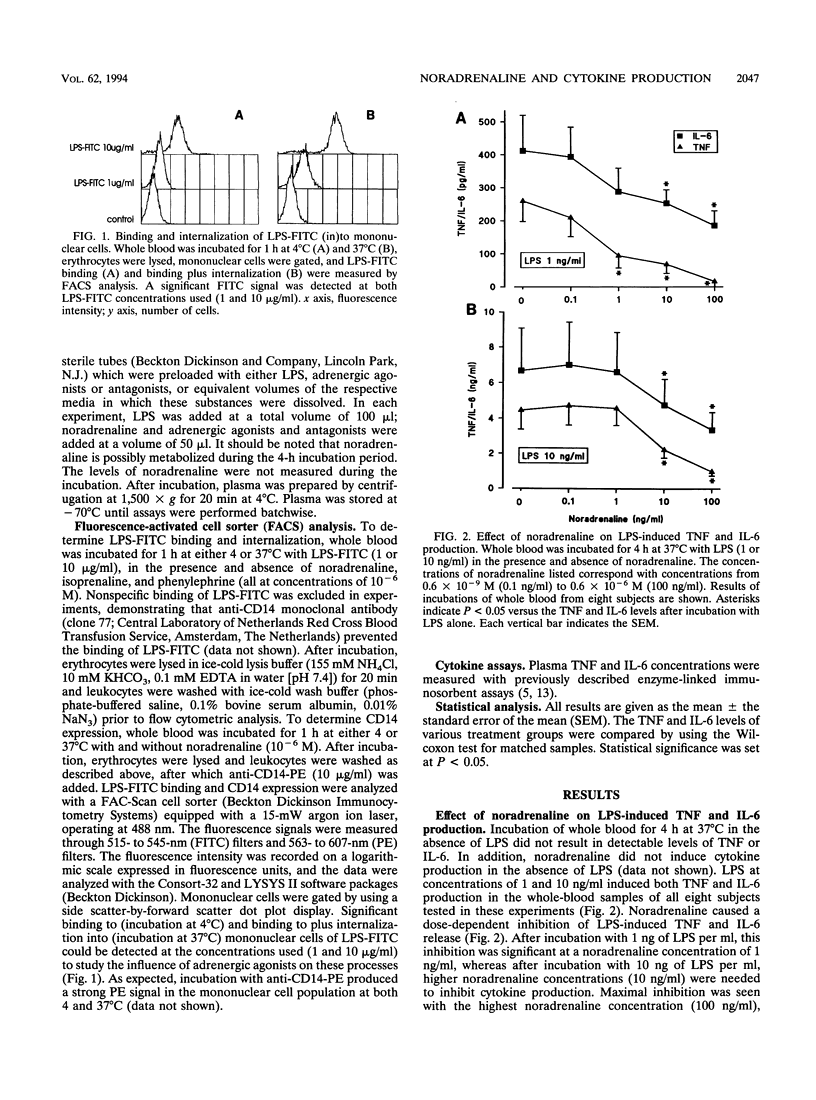
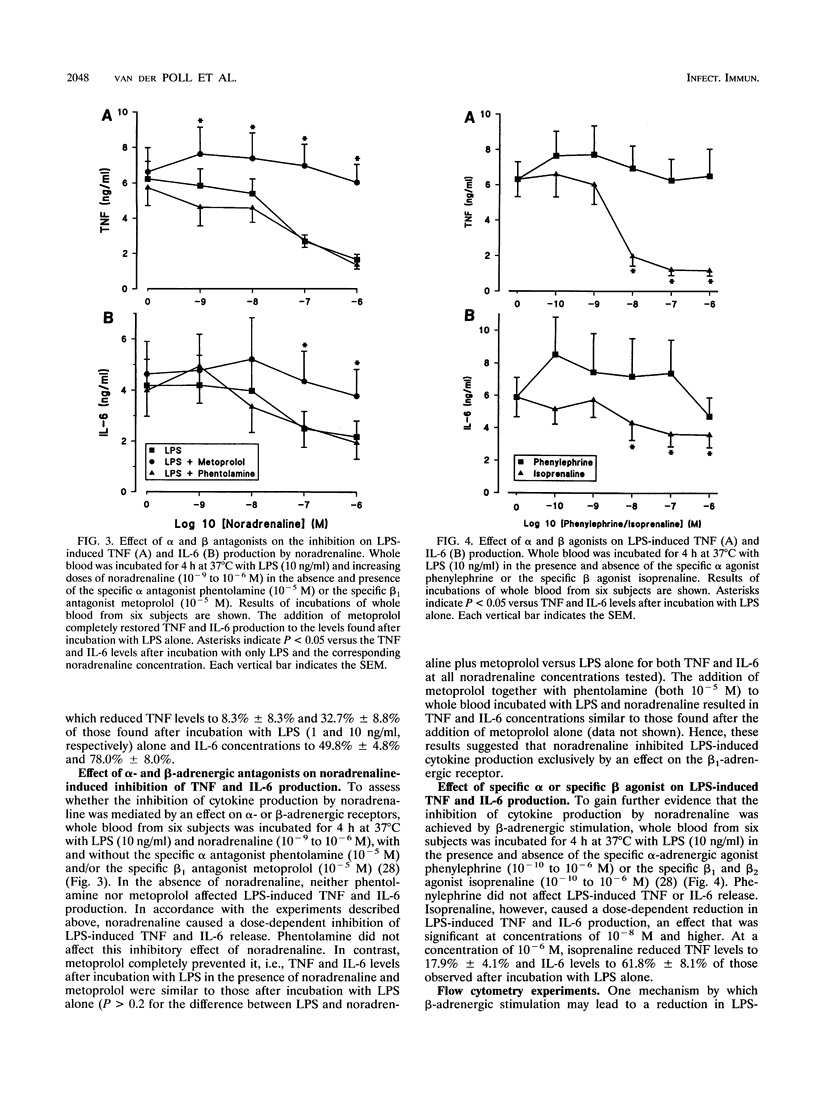
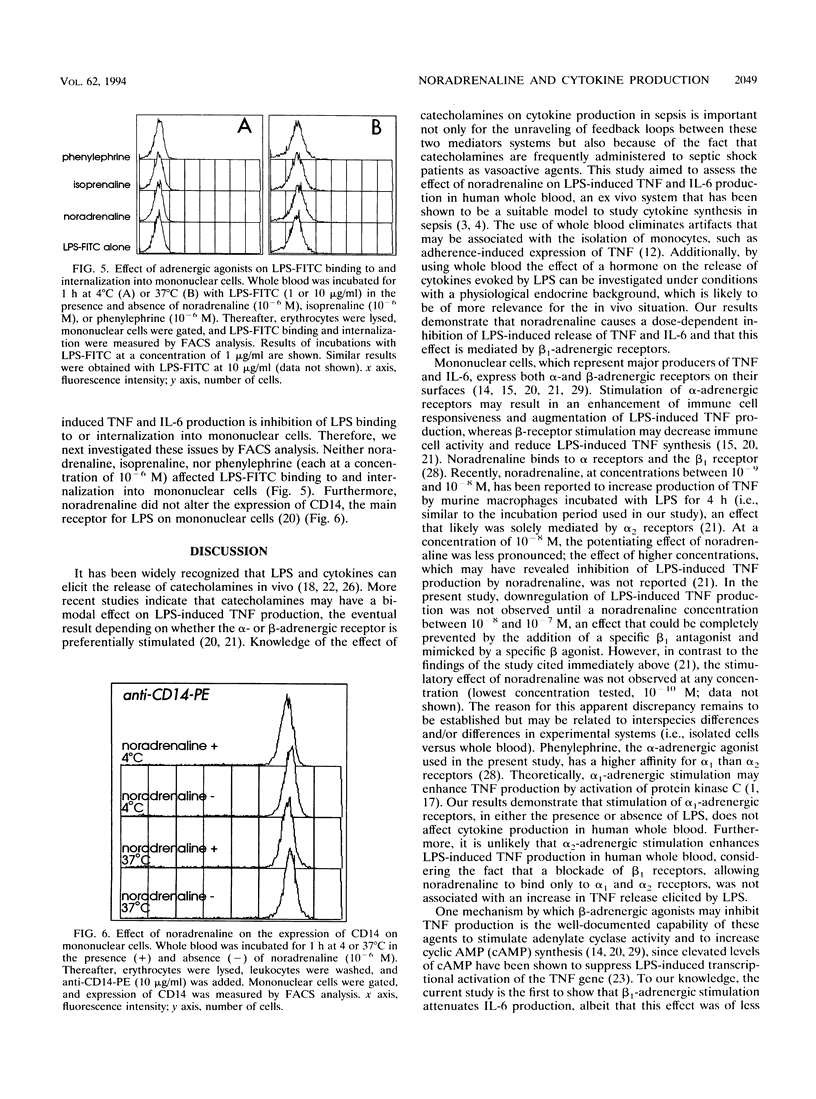
Sepsis and lipopolysaccharide (LPS) trigger the systemic release of both cytokines and catecholamines. Cytokines are known to be capable of eliciting a stress hormone response in vivo. The present study sought insight into the effect of noradrenaline on LPS-induced release of tumor necrosis factor alpha (TNF) and interleukin 6 (IL-6) in human whole blood. Whole blood was incubated with LPS for 4 h at 37 degrees C in the presence and absence of noradrenaline and/or specific alpha and beta antagonists and agonists. Noradrenaline caused a dose-dependent inhibition of LPS-induced TNF and IL-6 production. This effect could be completely prevented by addition of the specific beta 1, antagonist metoprolol, while it was not affected by the alpha antagonist phentolamine. Specific beta-adrenergic stimulation by isoprenaline mimicked the inhibiting effect of noradrenaline on LPS-evoked cytokine production, whereas alpha-adrenergic stimulation by phenylephrine had no effect. Fluorescence-activated cell sorter analysis demonstrated that beta-adrenergic stimulation had no effect on LPS binding to and internalization into mononuclear cells or on the expression of CD14, the major receptor for LPS on mononuclear cells. In acute sepsis, enhanced release of noradrenaline may be part of a negative feedback mechanism meant to inhibit ongoing TNF and IL-6 production.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bankey P., Carlson A., Ortiz M., Singh R., Cerra F. Tumor necrosis factor production by Kupffer cells requires protein kinase C activation. J Surg Res. 1990 Sep;49(3):256–261. doi: 10.1016/0022-4804(90)90130-t. [DOI] [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- DeForge L. E., Kenney J. S., Jones M. L., Warren J. S., Remick D. G. Biphasic production of IL-8 in lipopolysaccharide (LPS)-stimulated human whole blood. Separation of LPS- and cytokine-stimulated components using anti-tumor necrosis factor and anti-IL-1 antibodies. J Immunol. 1992 Apr 1;148(7):2133–2141. [PubMed] [Google Scholar]
- DeForge L. E., Remick D. G. Kinetics of TNF, IL-6, and IL-8 gene expression in LPS-stimulated human whole blood. Biochem Biophys Res Commun. 1991 Jan 15;174(1):18–24. doi: 10.1016/0006-291x(91)90478-p. [DOI] [PubMed] [Google Scholar]
- Engelberts I., Möller A., Schoen G. J., van der Linden C. J., Buurman W. A. Evaluation of measurement of human TNF in plasma by ELISA. Lymphokine Cytokine Res. 1991 Apr;10(1-2):69–76. [PubMed] [Google Scholar]
- Fong Y., Lowry S. F. Tumor necrosis factor in the pathophysiology of infection and sepsis. Clin Immunol Immunopathol. 1990 May;55(2):157–170. doi: 10.1016/0090-1229(90)90094-7. [DOI] [PubMed] [Google Scholar]
- Fong Y., Tracey K. J., Moldawer L. L., Hesse D. G., Manogue K. B., Kenney J. S., Lee A. T., Kuo G. C., Allison A. C., Lowry S. F. Antibodies to cachectin/tumor necrosis factor reduce interleukin 1 beta and interleukin 6 appearance during lethal bacteremia. J Exp Med. 1989 Nov 1;170(5):1627–1633. doi: 10.1084/jem.170.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayn K. N. Hormonal control of metabolism in trauma and sepsis. Clin Endocrinol (Oxf) 1986 May;24(5):577–599. doi: 10.1111/j.1365-2265.1986.tb03288.x. [DOI] [PubMed] [Google Scholar]
- Girardin E., Grau G. E., Dayer J. M., Roux-Lombard P., Lambert P. H. Tumor necrosis factor and interleukin-1 in the serum of children with severe infectious purpura. N Engl J Med. 1988 Aug 18;319(7):397–400. doi: 10.1056/NEJM198808183190703. [DOI] [PubMed] [Google Scholar]
- Glauser M. P., Zanetti G., Baumgartner J. D., Cohen J. Septic shock: pathogenesis. Lancet. 1991 Sep 21;338(8769):732–736. doi: 10.1016/0140-6736(91)91452-z. [DOI] [PubMed] [Google Scholar]
- Hack C. E., De Groot E. R., Felt-Bersma R. J., Nuijens J. H., Strack Van Schijndel R. J., Eerenberg-Belmer A. J., Thijs L. G., Aarden L. A. Increased plasma levels of interleukin-6 in sepsis. Blood. 1989 Oct;74(5):1704–1710. [PubMed] [Google Scholar]
- Haskill S., Johnson C., Eierman D., Becker S., Warren K. Adherence induces selective mRNA expression of monocyte mediators and proto-oncogenes. J Immunol. 1988 Mar 1;140(5):1690–1694. [PubMed] [Google Scholar]
- Helle M., Boeije L., de Groot E., de Vos A., Aarden L. Sensitive ELISA for interleukin-6. Detection of IL-6 in biological fluids: synovial fluids and sera. J Immunol Methods. 1991 Apr 8;138(1):47–56. doi: 10.1016/0022-1759(91)90063-l. [DOI] [PubMed] [Google Scholar]
- Higgins T. J., David J. R. Effect of isoproterernol and aminiphylline on cyclic AMP levels of guinea pig macrophages. Cell Immunol. 1976 Nov;27(1):1–10. doi: 10.1016/0008-8749(76)90147-7. [DOI] [PubMed] [Google Scholar]
- Koff W. C., Dunegan M. A. Modulation of macrophage-mediated tumoricidal activity by neuropeptides and neurohormones. J Immunol. 1985 Jul;135(1):350–354. [PubMed] [Google Scholar]
- Le J. M., Vilcek J. Interleukin 6: a multifunctional cytokine regulating immune reactions and the acute phase protein response. Lab Invest. 1989 Dec;61(6):588–602. [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Revhaug A., Michie H. R., Manson J. M., Watters J. M., Dinarello C. A., Wolff S. M., Wilmore D. W. Inhibition of cyclo-oxygenase attenuates the metabolic response to endotoxin in humans. Arch Surg. 1988 Feb;123(2):162–170. doi: 10.1001/archsurg.1988.01400260042004. [DOI] [PubMed] [Google Scholar]
- Schandené L., Vandenbussche P., Crusiaux A., Alègre M. L., Abramowicz D., Dupont E., Content J., Goldman M. Differential effects of pentoxifylline on the production of tumour necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6) by monocytes and T cells. Immunology. 1992 May;76(1):30–34. [PMC free article] [PubMed] [Google Scholar]
- Severn A., Rapson N. T., Hunter C. A., Liew F. Y. Regulation of tumor necrosis factor production by adrenaline and beta-adrenergic agonists. J Immunol. 1992 Jun 1;148(11):3441–3445. [PubMed] [Google Scholar]
- Spengler R. N., Allen R. M., Remick D. G., Strieter R. M., Kunkel S. L. Stimulation of alpha-adrenergic receptor augments the production of macrophage-derived tumor necrosis factor. J Immunol. 1990 Sep 1;145(5):1430–1434. [PubMed] [Google Scholar]
- Tannenbaum C. S., Hamilton T. A. Lipopolysaccharide-induced gene expression in murine peritoneal macrophages is selectively suppressed by agents that elevate intracellular cAMP. J Immunol. 1989 Feb 15;142(4):1274–1280. [PubMed] [Google Scholar]
- Tobias P. S., Ulevitch R. J. Lipopolysaccharide binding protein and CD14 in LPS dependent macrophage activation. Immunobiology. 1993 Apr;187(3-5):227–232. doi: 10.1016/S0171-2985(11)80341-4. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Van der Poll T., Romijn J. A., Endert E., Borm J. J., Büller H. R., Sauerwein H. P. Tumor necrosis factor mimics the metabolic response to acute infection in healthy humans. Am J Physiol. 1991 Oct;261(4 Pt 1):E457–E465. doi: 10.1152/ajpendo.1991.261.4.E457. [DOI] [PubMed] [Google Scholar]
- Verghese M. W., Snyderman R. Hormonal activation of adenylate cyclase in macrophage membranes is regulated by guanine nucleotides. J Immunol. 1983 Feb;130(2):869–873. [PubMed] [Google Scholar]
- Zhang Y., Lin J. X., Vilcek J. Synthesis of interleukin 6 (interferon-beta 2/B cell stimulatory factor 2) in human fibroblasts is triggered by an increase in intracellular cyclic AMP. J Biol Chem. 1988 May 5;263(13):6177–6182. [PubMed] [Google Scholar]
- van Deventer S. J., Büller H. R., ten Cate J. W., Aarden L. A., Hack C. E., Sturk A. Experimental endotoxemia in humans: analysis of cytokine release and coagulation, fibrinolytic, and complement pathways. Blood. 1990 Dec 15;76(12):2520–2526. [PubMed] [Google Scholar]



