Abstract
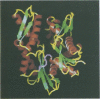
A cytoplasmic 75-kDa immunogen from Chlamydia trachomatis serovar L2 has previously been characterized as being similar to the Escherichia coli heat shock protein DnaK. We have localized a linear epitope for one monoclonal antibody specific for C. trachomatis DnaK. By use of a recombinant DNA technique, the epitope was limited to 14 amino acids. With synthetic peptides, the epitope was further limited to eight amino acids. Six of these amino acids are conserved in bovine HSP70, which has a known three-dimensional structure. The amino acid sequence homologous to the epitope is located in a linear part of the HSP70 molecule known as connect II.
Full text
PDF




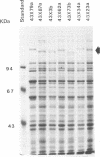
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehr W., Zhang Y. X., Joseph T., Su H., Nano F. E., Everett K. D., Caldwell H. D. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4000–4004. doi: 10.1073/pnas.85.11.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell J. C., Craig E. A. Major heat shock gene of Drosophila and the Escherichia coli heat-inducible dnaK gene are homologous. Proc Natl Acad Sci U S A. 1984 Feb;81(3):848–852. doi: 10.1073/pnas.81.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkelund S., Lundemose A. G., Christiansen G. Characterization of native and recombinant 75-kilodalton immunogens from Chlamydia trachomatis serovar L2. Infect Immun. 1989 Sep;57(9):2683–2690. doi: 10.1128/iai.57.9.2683-2690.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkelund S., Lundemose A. G., Christiansen G. Chemical cross-linking of Chlamydia trachomatis. Infect Immun. 1988 Mar;56(3):654–659. doi: 10.1128/iai.56.3.654-659.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkelund S., Lundemose A. G., Christiansen G. The 75-kilodalton cytoplasmic Chlamydia trachomatis L2 polypeptide is a DnaK-like protein. Infect Immun. 1990 Jul;58(7):2098–2104. doi: 10.1128/iai.58.7.2098-2104.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkelund S. The molecular biology and diagnostics of Chlamydia trachomatis. Dan Med Bull. 1992 Aug;39(4):304–320. [PubMed] [Google Scholar]
- Brunham R. C., Peeling R., Maclean I., McDowell J., Persson K., Osser S. Postabortal Chlamydia trachomatis salpingitis: correlating risk with antigen-specific serological responses and with neutralization. J Infect Dis. 1987 Apr;155(4):749–755. doi: 10.1093/infdis/155.4.749. [DOI] [PubMed] [Google Scholar]
- Conlan J. W., Clarke I. N., Ward M. E. Epitope mapping with solid-phase peptides: identification of type-, subspecies-, species- and genus-reactive antibody binding domains on the major outer membrane protein of Chlamydia trachomatis. Mol Microbiol. 1988 Sep;2(5):673–679. doi: 10.1111/j.1365-2958.1988.tb00076.x. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty K. M., DeLuca-Flaherty C., McKay D. B. Three-dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein. Nature. 1990 Aug 16;346(6285):623–628. doi: 10.1038/346623a0. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Meloen R. H., Barteling S. J. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3998–4002. doi: 10.1073/pnas.81.13.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Hearne C. M., Ellar D. J. Nucleotide sequence of a Bacillus subtilis gene homologous to the dnaK gene of Escherichia coli. Nucleic Acids Res. 1989 Oct 25;17(20):8373–8373. doi: 10.1093/nar/17.20.8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heegaard P. M., Holm A., Hagerup M. Practical multipeptide synthesis: dedicated software for the definition of multiple, overlapping peptides covering polypeptide sequences. Pept Res. 1993 Jan-Feb;6(1):7–9. [PubMed] [Google Scholar]
- Keat A., Thomas B. J., Taylor-Robinson D. Chlamydial infection in the aetiology of arthritis. Br Med Bull. 1983 Apr;39(2):168–174. doi: 10.1093/oxfordjournals.bmb.a071811. [DOI] [PubMed] [Google Scholar]
- Kornak J. M., Kuo C. C., Campbell L. A. Sequence analysis of the gene encoding the Chlamydia pneumoniae DnaK protein homolog. Infect Immun. 1991 Feb;59(2):721–725. doi: 10.1128/iai.59.2.721-725.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung T. K., Rajendran M. Y., Monfries C., Hall C., Lim L. The human heat-shock protein family. Expression of a novel heat-inducible HSP70 (HSP70B') and isolation of its cDNA and genomic DNA. Biochem J. 1990 Apr 1;267(1):125–132. doi: 10.1042/bj2670125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie K. R., Adams E., Britton W. J., Garsia R. J., Basten A. Sequence and immunogenicity of the 70-kDa heat shock protein of Mycobacterium leprae. J Immunol. 1991 Jul 1;147(1):312–319. [PubMed] [Google Scholar]
- Mehra V., Sweetser D., Young R. A. Efficient mapping of protein antigenic determinants. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7013–7017. doi: 10.1073/pnas.83.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldal M., Holm C. B., Bojesen G., Jakobsen M. H., Holm A. Multiple column peptide synthesis, Part 2 (1, 2). Int J Pept Protein Res. 1993 Mar;41(3):250–260. doi: 10.1111/j.1399-3011.1993.tb00333.x. [DOI] [PubMed] [Google Scholar]
- Mouritsen S., Meldal M., Rubin B., Holm A., Werdelin O. The T-lymphocyte proliferative response to synthetic peptide antigens of defined secondary structure. Scand J Immunol. 1989 Dec;30(6):723–730. doi: 10.1111/j.1365-3083.1989.tb02482.x. [DOI] [PubMed] [Google Scholar]
- Peake P. W., Britton W. J., Davenport M. P., Roche P. W., McKenzie K. R. Analysis of B-cell epitopes in the variable C-terminal region of the Mycobacterium leprae 70-kilodalton heat shock protein. Infect Immun. 1993 Jan;61(1):135–141. doi: 10.1128/iai.61.1.135-141.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippmann F., Taylor W. R., Rothbard J. B., Green N. M. A hypothetical model for the peptide binding domain of hsp70 based on the peptide binding domain of HLA. EMBO J. 1991 May;10(5):1053–1059. doi: 10.1002/j.1460-2075.1991.tb08044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Stanley K. K., Luzio J. P. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 1984 Jun;3(6):1429–1434. doi: 10.1002/j.1460-2075.1984.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson K., Inglis N. F., Rae B., Donachie W., Sharp J. M. Complete nucleotide sequence of a gene encoding the 70 kd heat shock protein of Mycobacterium paratuberculosis. Nucleic Acids Res. 1991 Aug 25;19(16):4552–4552. doi: 10.1093/nar/19.16.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K., Hauser R., Campbell J., Ostheimer G. J. Isolation of dnaJ, dnaK, and grpE homologues from Borrelia burgdorferi and complementation of Escherichia coli mutants. Mol Microbiol. 1993 Feb;7(3):359–369. doi: 10.1111/j.1365-2958.1993.tb01128.x. [DOI] [PubMed] [Google Scholar]
- Wetzstein M., Dedio J., Schumann W. Complete nucleotide sequence of the Bacillus subtilis dnaK gene. Nucleic Acids Res. 1990 Apr 25;18(8):2172–2172. doi: 10.1093/nar/18.8.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G., Brunham R. C. Antigenic analysis of the chlamydial 75-kilodalton protein. Infect Immun. 1992 Mar;60(3):1221–1224. doi: 10.1128/iai.60.3.1221-1224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylicz M., LeBowitz J. H., McMacken R., Georgopoulos C. The dnaK protein of Escherichia coli possesses an ATPase and autophosphorylating activity and is essential in an in vitro DNA replication system. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6431–6435. doi: 10.1073/pnas.80.21.6431. [DOI] [PMC free article] [PubMed] [Google Scholar]