Abstract
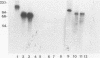
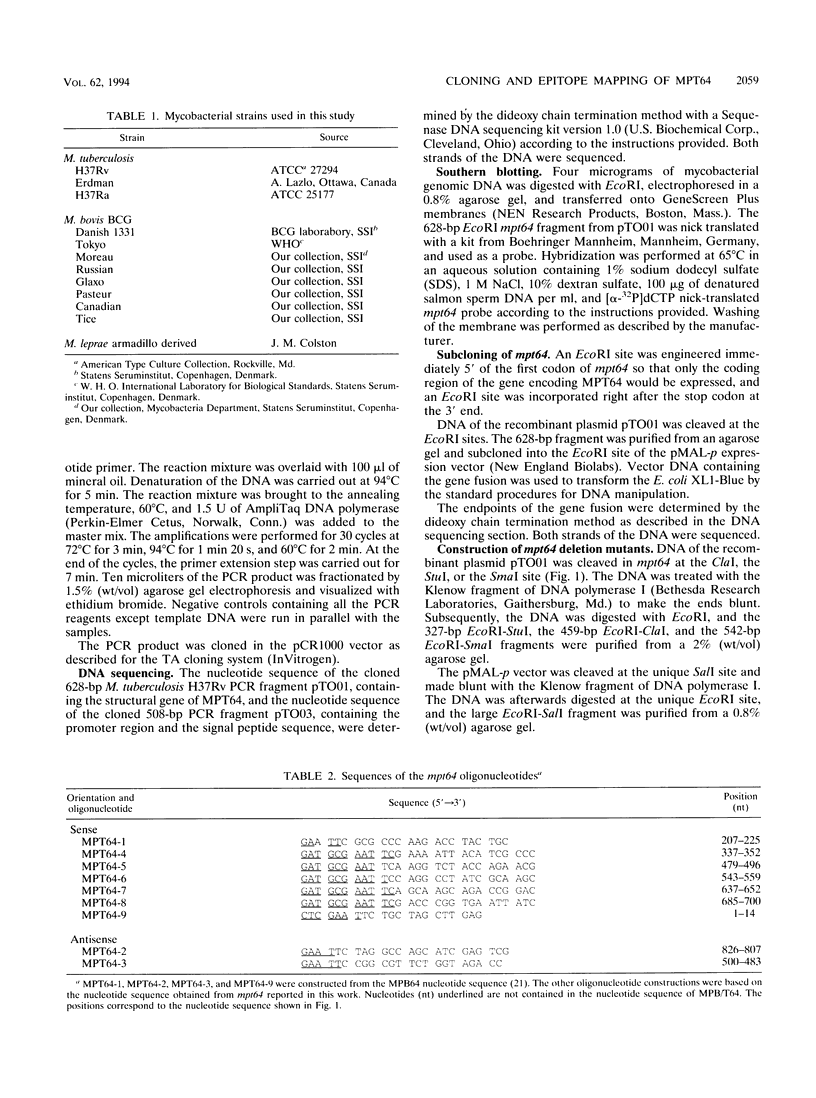
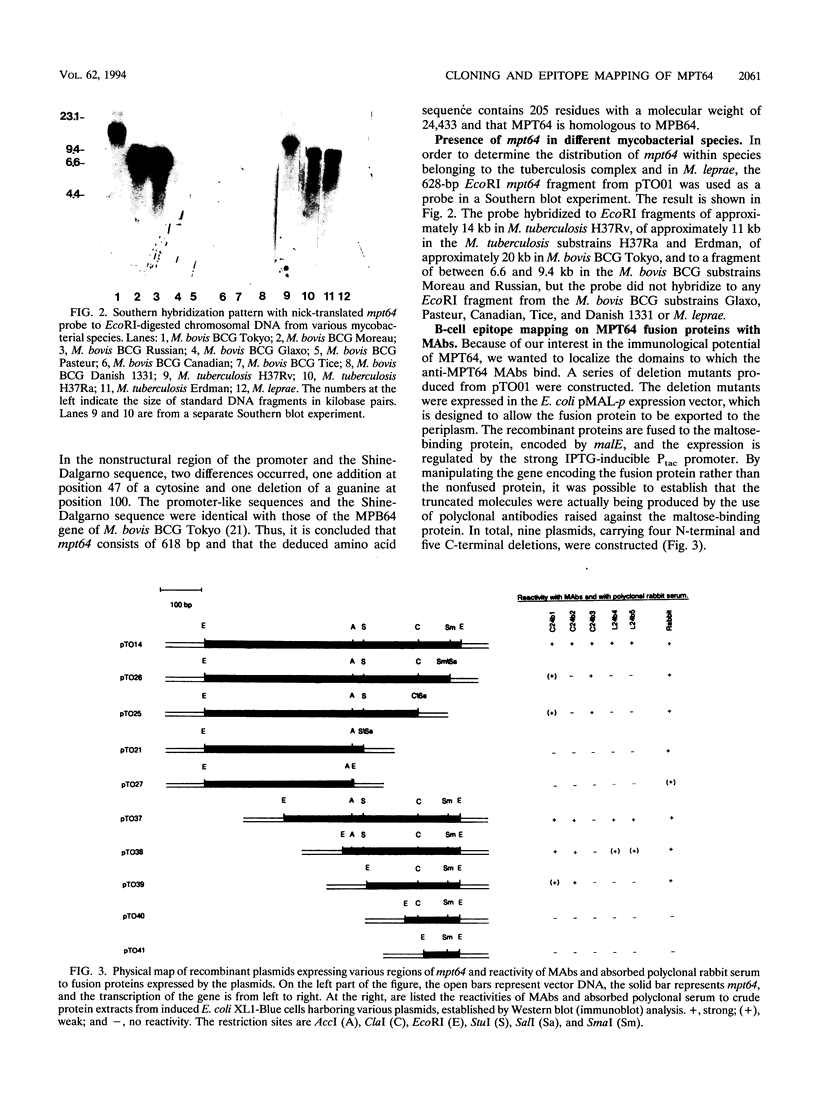
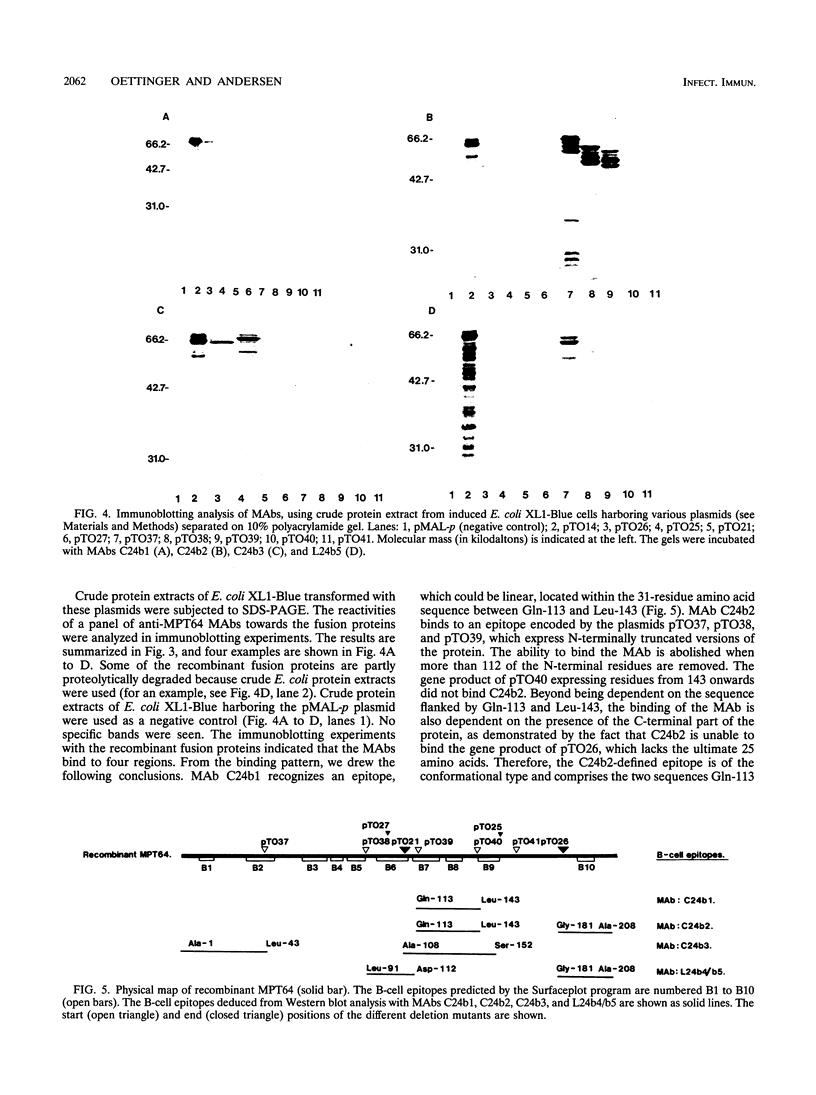
The gene of the immunogenic protein MPT64 found in culture filtrates of Mycobacterium tuberculosis H37Rv was cloned and sequenced. A comparison showed mpt64 and the gene encoding MPB64 from Mycobacterium bovis BCG Tokyo to be identical except for one silent mutation. The regions encoding the promoter and the signal peptide were also well conserved for the two sequences. Southern blot experiments on genomic mycobacterial DNA showed the presence of mpt64 in the M. tuberculosis substrains H37Rv, H37Ra, and Erdman and in the M. bovis BCG substrains Tokyo, Moreau, and Russian, whereas the M. bovis BCG substrains Glaxo, Pasteur, Canadian, Tice, and Danish 1331 and Mycobacterium leprae lack the gene. Southern blot analyses revealed differences in the restriction enzyme patterns within the M. tuberculosis substrains as well as within the M. bovis BCG substrains, indicating either different chromosomal localization of mpt64 or that mutations have occurred at different locations on the chromosomes. N-terminal and C-terminal deletion mutants were constructed for the mapping of B-cell epitopes on MPT64 with five monoclonal antibodies, C24b1, C24b2, C24b3, L24b4, and L24b5. Western blot (immunoblot) analysis revealed that the murine antibodies bind to one linear and three conformational epitopes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen A. B., Andersen P., Ljungqvist L. Structure and function of a 40,000-molecular-weight protein antigen of Mycobacterium tuberculosis. Infect Immun. 1992 Jun;60(6):2317–2323. doi: 10.1128/iai.60.6.2317-2323.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen A. B., Ljungqvist L., Hasløv K., Bentzon M. W. MPB 64 possesses 'tuberculosis-complex'-specific B- and T-cell epitopes. Scand J Immunol. 1991 Sep;34(3):365–372. doi: 10.1111/j.1365-3083.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Andersen P., Askgaard D., Ljungqvist L., Bennedsen J., Heron I. Proteins released from Mycobacterium tuberculosis during growth. Infect Immun. 1991 Jun;59(6):1905–1910. doi: 10.1128/iai.59.6.1905-1910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Askgaard D., Ljungqvist L., Bentzon M. W., Heron I. T-cell proliferative response to antigens secreted by Mycobacterium tuberculosis. Infect Immun. 1991 Apr;59(4):1558–1563. doi: 10.1128/iai.59.4.1558-1563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzofsky J. A. Intrinsic and extrinsic factors in protein antigenic structure. Science. 1985 Sep 6;229(4717):932–940. doi: 10.1126/science.2410982. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Murray C. J. Tuberculosis: commentary on a reemergent killer. Science. 1992 Aug 21;257(5073):1055–1064. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- Borremans M., de Wit L., Volckaert G., Ooms J., de Bruyn J., Huygen K., van Vooren J. P., Stelandre M., Verhofstadt R., Content J. Cloning, sequence determination, and expression of a 32-kilodalton-protein gene of Mycobacterium tuberculosis. Infect Immun. 1989 Oct;57(10):3123–3130. doi: 10.1128/iai.57.10.3123-3130.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit L., de la Cuvellerie A., Ooms J., Content J. Nucleotide sequence of the 32 kDa-protein gene (antigen 85 A) of Mycobacterium bovis BCG. Nucleic Acids Res. 1990 Jul 11;18(13):3995–3995. doi: 10.1093/nar/18.13.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M., Nagai S., Patarroyo M. E., Torres M. L., Ramirez C., Cruz N. Properties of proteins MPB64, MPB70, and MPB80 of Mycobacterium bovis BCG. Infect Immun. 1986 Apr;52(1):293–302. doi: 10.1128/iai.52.1.293-302.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Ulstrup J. C., Jonassen T. O., Melby K., Nagai S., Harboe M. Evidence for absence of the MPB64 gene in some substrains of Mycobacterium bovis BCG. Infect Immun. 1993 May;61(5):1730–1734. doi: 10.1128/iai.61.5.1730-1734.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J. M., Guo D., Hodges R. S. New hydrophilicity scale derived from high-performance liquid chromatography peptide retention data: correlation of predicted surface residues with antigenicity and X-ray-derived accessible sites. Biochemistry. 1986 Sep 23;25(19):5425–5432. doi: 10.1021/bi00367a013. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Sweetser D., Thole J., van Embden J., Young R. A. The etiologic agents of leprosy and tuberculosis share an immunoreactive protein antigen with the vaccine strain Mycobacterium bovis BCG. Infect Immun. 1987 Aug;55(8):1932–1935. doi: 10.1128/iai.55.8.1932-1935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987 Mar;169(3):1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., Keulen W. J., De Bruyn J., Kolk A. H., Groothuis D. G., Berwald L. G., Tiesjema R. H., van Embden J. D. Characterization, sequence determination, and immunogenicity of a 64-kilodalton protein of Mycobacterium bovis BCG expressed in escherichia coli K-12. Infect Immun. 1987 Jun;55(6):1466–1475. doi: 10.1128/iai.55.6.1466-1475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi R., Matsuo K., Yamazaki A., Abe C., Nagai S., Terasaka K., Yamada T. Cloning and characterization of the gene for immunogenic protein MPB64 of Mycobacterium bovis BCG. Infect Immun. 1989 Jan;57(1):283–288. doi: 10.1128/iai.57.1.283-288.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. B., Kaufmann S. H., Hermans P. W., Thole J. E. Mycobacterial protein antigens: a compilation. Mol Microbiol. 1992 Jan;6(2):133–145. doi: 10.1111/j.1365-2958.1992.tb01994.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. How signal sequences maintain cleavage specificity. J Mol Biol. 1984 Feb 25;173(2):243–251. doi: 10.1016/0022-2836(84)90192-x. [DOI] [PubMed] [Google Scholar]